Back to Journals » International Journal of Nanomedicine » Volume 14
Glucose-Sensitive Nanoparticles Based On Poly(3-Acrylamidophenylboronic Acid-Block-N-Vinylcaprolactam) For Insulin Delivery
Authors Wu J , Yang Y, Li S, Shi A , Song B, Niu S, Chen W, Yao Z
Received 26 June 2019
Accepted for publication 11 September 2019
Published 4 October 2019 Volume 2019:14 Pages 8059—8072
DOI https://doi.org/10.2147/IJN.S220936
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Jun-zi Wu,1,* Yuqing Yang,1,* Shude Li,2 Anhua Shi,1 Bo Song,1 Shiwei Niu,3 WenHui Chen,1 Zheng Yao1
1Yunnan Provincial Key Laboratory of Molecular Biology for Sinomedicine, School of Basic Medical, Yunnan University of Chinese Medicine, Kunming, Yunnan 650500, People’s Republic of China; 2Department of Biochemistry and Molecular Biology, College of Basic Medicine, Kunming Medical University, Kunming, Yunnan 650500, People’s Republic of China; 3Department of Biotechnology, College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai 201620, People’s Republic of China
*These authors contributed equally to this work
Correspondence: WenHui Chen; Zheng Yao
Yunnan Provincial Key Laboratory of Molecular Biology for Sinomedicine, School of Basic Medical, Yunnan University of Chinese Medicine, No. 1076, Yuhua Road, Chenggong District, Kunming, Yunnan 650500, People’s Republic of China
Tel/fax +86 871 8855 7524
Email [email protected]; [email protected]
Background: Compared with random copolymers, block copolymerization is easier to prepare for nanoparticles with core-shell structure, and they will have better glucose sensitivity and higher insulin loading.
Purpose: In our study, insulin-loaded poly (3-acrylamidophenylboronic acid-block-N-vinyl caprolactam) p(AAPBA-b-NVCL) nanoparticles were successfully prepared and were glucose-sensitive, which could effectively lower the blood sugar levels within 72 hrs.
Methods: The polymer of p(AAPBA-b-NVCL) was produced by reversible addition-fragmentation chain transfer polymerization based on different ratios of 3-acrylamidophenylboronic acid (AAPBA) and N-vinylcaprolactam (NVCL), and its structure was discussed by Fourier transform infrared spectroscopy and 1H-nuclear magnetic resonance . Next, the polymer was manufactured into the nanoparticles, and the characteristics of nanoparticles were detected by dynamic light scattering, lower critical solution temperature, and transmission electron microscopy. After that, the cell and animal toxicity of nanoparticles were also investigated.
Results: The results demonstrated that p(AAPBA-b-NVCL) was successfully synthesized, and can be easily self-assembled to form nanoparticles. The new nanoparticles included monodisperse submicron particles, with the size of the nanoparticle ranged between 150 and 300nm and are glucose- and temperature-sensitive. Meanwhile, insulin can be easily loaded by p(AAPBA-b-NVCL) nanoparticles and an effective sustained release of insulin was observed when the nanoparticles were placed in physiological saline. Besides, MTT assay revealed that cell viability was more than 80%, and mice demonstrated no negative impact on blood biochemistry and heart, liver, spleen, lung, and kidney after intraperitoneal injection of 10 mg/kg/d of nanoparticles. This suggested that the nanoparticles were low-toxic to both cells and animals. Moreover, they could lower the blood sugar level within 72h.
Conclusion: Our research suggested that these p(AAPBA-b-NVCL) nanoparticles might have the potential to be applied in a delivery system for insulin or other hypoglycemic proteins.
Keywords: N-vinylcaprolactam, NVCL, 3-acrylamidophenylboronic acid, AAPBA, nanoparticles, glucose-sensitive, temperature-sensitive
Introduction
Recently, chronic non-communicable diseases such as diabetes have become the most serious public health problem all over the world.1 In 2014, 9.3% of Americans have diabetes (i.e., approximately 29.1 million persons) and normal individuals are at 40% risk of developing diabetes.2 In People’s Republic of China, the prevalence of type 2 diabetes mellitus has been significantly increasing and has become the leading cause of morbidity. The prevalence of diabetes in People’s Republic of China in 1980, 1994, and 2001 was 0.67%, 2.5%, and 5.5%, respectively.3 There is no doubt that diabetes has become a serious epidemic in producing a sizable burden on both the individual as well as the society. Diabetes mellitus is a chronic disease that is associated with long-term complications of brain, kidney, and heart,4 followed by destruction and loss of pancreatic ß-cells, causing insulin deficiency. This might also allow the abnormalities to arise due to insulin resistance.5 Unlike pancreatic secretion of insulin, the standard pharmacological administration of insulin is not regulated by an endogenous feedback mechanism. Even with carefully designed dosing regimens or with the use of innovative insulin products (i.e., engineered basal and rapid-acting insulin analogs) and even in the context of strict dietary and lifestyle adherence by patients, diabetic patients often experience periods of hyperglycemia or hypoglycemia.6 Although there are numerous hydrogels, polymers, liposomes and nano-insulin formulations for targeted and controlled delivery, they still could not solve the above problem. Therefore,7 it is very important to develop a closed-loop drug delivery system that is similar to “artificial islets”.
Intelligent polymer hydrogels (such as temperature-sensitive polymer,8 pH-sensitive polymer,9 magnetic-sensitive polymer,10 etc.) can sense and respond the environmental stimuli, and then change their volume and morphology accordingly. This has attracted much attention of the researchers in the field of drug delivery. For diabetes, the glucose-sensitive intelligent drug sustained-controlled carrier material can be determined by introducing phenylboronic acid (PBA) or its derivatives into the polymer system, which has broad application prospects in this field.11–13 Currently, the nanoparticles made by 3-acrylamidophenylboronic acid (AAPBA) and temperature-sensitive monomers such as insulin smart slow-release sensitive materials have been widely reported. Shiomori et al14 have prepared a temperature-sensitive and glucose-sensitive hydrogel based on PBA derivatives and N-isopropylacrylamide (NIPAAm). The research also found that PBA easily forms a hydrophilic compound with glucose, and insulin could regulate the amount released by changing the concentration of glucose. In addition, Rahman et al15 synthesized a monodispersed glucose-sensitive p (NIPAAm-AA) microgel based on NIPAAm, AAPBA, and acrylic acid (AA), and found that the microgels in the contraction state swell with increasing glucose concentration at room temperature. Meanwhile, the study also found that the glucose sensitivity of this polymer is related to environmental pH, ionic strength, and PBA content in the polymer. When the pH value is 9 and the ionic strength remained low, making the glucose stimulation responsiveness very obvious.
N-vinylcaprolactam (NVCL) is a kind of temperature-sensitive material,16 and few studies17,18 have reported that compared to the most widely used temperature-sensitive monomers such as NIPAAm, NVCL is associated with low toxicity, and when manufactured it into nanoparticles, its structure and function is easy to regulate. So, it has become a hot research topic in recent years. In addition to the good plasticity of NVCL, it can also be used in the preparation of water gels, nanoparticles, hybrid particles, etc. Recent studies on the construction of insulin-loaded nanoparticles based on copolymerization of NVCL and PBA have been reported in the year 2012. Ahmad Bitar et al19 have manufactured a glucose-sensitive microgel based on NVCL and 4-vinylphenylboronic acid (VPBA), and the results revealed that this new p(VPBA-NVCL) microgel has an excellent glucose sensitivity at pH=7.4, but the study lacked the drug loading, the drug cumulative release, and the toxicology research. Besides the synthesis of a new type of nanoparticle named p(NVCL-co-AAPBA) based on glucose and temperature sensitivity via a random copolymer by Wu et al,20 and they also found that these new nanoparticles demonstrated good glucose sensitivity and ideal temperature sensitivity. Furthermore, insulin can change its release dosage by changing the glucose concentration. Moreover, the nanoparticles have almost no side effects, but the performance of poly (NVCL-co-AAPBA) nanoparticles is not very ideal.
Compared with random copolymers, block copolymerization is more easily prepared for nanoparticles with core-shell structure, and will they have better glucose sensitivity and higher insulin loading? Can they produce better glucose sensitivity? To answer these questions, a new p(AAPBA-b-NVCL) polymer was synthesized by reversible addition-fragmentation chain transfer polymerization (RAFT) copolymerization, and then its characteristics were evaluated. Next, the p(AAPBA-b-NVCL) polymer was manufactured by using the p(AAPBA-b-NVCL) nanoparticles (Scheme 1 is the schematic presentation of p(AAPBA-b-NVCL) nanoparticles). Next, the insulin loading and release characteristics of nanoparticles were studied in vitro and their toxicology was also examined both in vitro and in vivo. Furthermore, insulin-loaded p(AAPBA-b-NVCL) nanoparticles were injected into mice with type 2 diabetes for observing its therapeutic effects. Our research provided insights in effectively enhancing diabetic control.
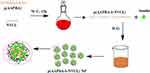 |
Scheme 1 Schematic representation of temperature- and glucose-sensitive poly (3-acrylamidophenylboronic acid-block-N-vinylcaprolactam) p(AAPBA-b-NVCL) nanoparticles. |
Materials And Methods
Materials
AAPBA was obtained from the Beijing Pure Chem. Co., Ltd. (Beijing, People’s Republic of China), NVCL was supplied by Hubei Xinyuan shun Pharmaceutical Chemical Co., Ltd. (Wuhan, People’s Republic of China), 2,2-azobisisobutyronitrile (AIBN) and S-1- dodecyl-S0-(a, a0- dimethyl-a00-acetic acid) trithiocarbonate (DDATC) were purchased from Zhengzhou Alpha Chemical Co., Ltd. (Zhengzhou, People’s Republic of China). Dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) were purchased from the Shandong Deyan Chemical Co., Ltd. (Jinan, People’s Republic of China). Insulin was purchased from Shanghai Hengyuan Biotechnology Co., Ltd. (Shanghai, People’s Republic of China). Water was distilled before use. All solvents used in these experiments were of analytical grade.
Poly(3-Acrylamidophenyl Boronic Acid-b-Diethylene Glycol Dimethacrylate) [p(AAPBA-b-NVCL)]
Synthesis Of Poly (3-Acrylamidophenylboronic Acid) [p(AAPBA)]
p(AAPBA) was synthesized as described in a previous study21 by RAFT polymerization. AAPBA (1000 mg), DDATC (10 mg), and AIBN (1 mg) were weighed, placed them into a 50 mL reaction tube, mixed, and dissolved them with DMF (1 mL). After purging with nitrogen for 30 mins, the tube was transferred into an oil bath at 70ºC for 12 hrs. The tube was recovered by rapid cooling in ice water for 5 mins. This was followed by the precipitation of the product by diethyl ether, washed with acetone, dried under vacuum for 75 hrs, and finally the p(AAPBA) was obtained (Scheme 2).
 |
Scheme 2 Synthesis of p(AAPBA). |
Synthesis Of p(AAPBA-B-NVCL)
The p(AAPBA), DEGMA and AIBN (1 mg) were weighed, placed them into a 50 mL reaction tube, mixed and then dissolved in DMF (1 mL). After purging with nitrogen for 30 mins, the tube was transferred into an oil bath at 70ºC for 12 hrs. The tube was then recovered by rapid cooling in ice water for 5 mins, followed by precipitation of the product by diethyl ether, washed with acetone, dried under vacuum for 72 hrs, and finally the p(AAPBA-b-NVCL) was obtained (Scheme 3). By changing the ratio of p(AAPBA) to DEGMA, 5 polymers were prepared as detailed in Table 1, which were as follows: p(AAPBA), p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4.
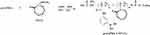 |
Scheme 3 Synthesis of p(AAPBA-b-NVCL). |
 |
Table 1 LCST, The Molecular Weights (Mw And Mn), Molecular Weight Distributions And Size Distribution Of Self-Assembled Nanomicelles With Different Ratios |
Characterization Of Polymers
Characterization of p(AAPBA-b-NVCL) including 1H-nuclear magnetic resonance (1H-NMR) spectra, Fourier transform infrared spectroscopy (FT-IR) spectra, molecular weight (Mw and Mn) and lower critical solution temperature (LCST) were achieved as reported by previous methods.22
1H-NMR spectra: The purified p(AAPBA-b-NVCL) samples (3–5mg) were dissolved in d6-DMSO (1mL), and then their 1H-NMR spectra were recorded by using a Bruker DRX 400 MHz spectrometer (Bruker, Rheinstetten, Germany).
FT-IR spectra: FT-IR spectra of p(AAPBA-b-NVCL) samples were recorded by using an FTS-6000 instrument (Bio-Rad Co., Hercules, CA, USA) in 4000–400 cm−1 range using a KBr tablet (sample: KBr mass ratio 1:100) at a resolution of 2 cm−1.
Mw and Mn: The gel permeation chromatography measurements were used on a Waters LS system to obtain the molecular weights (Mw and Mn) and molecular weight distributions (Mw/Mn, PDI) of p(AAPBA-b-NVCL) polymers.
LCST: The LCST of p(AAPBA-b-NVCL) polymers was determined using UV-vis spectrophotometer (Lambda 35, Perkin Elmer, USA) at a heating rate of 0.5°C/min.
Preparation Of Nanoparticles (Nanoparticles)
Ten milligrams p(AAPBA-b-NVCL) was dissolved in a mixed solvent containing DMSO and water (1mL, v/v, 1:1), followed by dropwise addition of the solution into 20 mL of water, and continuously stirred for 500r/min. After 30 mins, the solution was transferred into a dialysis tube (molecular weight cut off was 3500) for 72 hrs to dialyze against water (during the dialyzing process replaced water every 3 hrs). Next, freeze-drying by using a vacuum freeze dryer (18NS, Beijing YaXing Instrument Company), the pure p(AAPBA-b-NVCL) nanoparticles were obtained.
The insulin-loaded p(AAPBA-b-NVCL) nanoparticles were prepared according to a similar method by dissolving 500 µg insulin in a mixed solvent containing DMSO and water (1mL, v/v, 1:1). As described above, after freeze-drying, the insulin-loaded p(AAPBA-b-NVCL) nanoparticles were obtained. The nanoparticles then underwent filtered treatment using 0.45μm organic nylon filter membrane [Emerson Technology Co., Ltd (Shanghai, People’s Republic of China)]. The amount of free insulin in the supernatant was determined using a UV spectrometer (Shimadzu UV-2550) at 595 nm. The following equations were used to calculate the insulin encapsulation efficiency (EE) and loading capacity (LC):
EE%=(total insulin-free insulin)/total insulin×100
LC%=(total insulin-free insulin)/nanoparticles weight×100
Characterization Of The Nanoparticles
The nanoparticles of p(AAPBA-b-NVCL) were characterized by dynamic light scattering (DLS) and transmission electron microscopy (TEM) as reported previously.23
DLS: The change in the characteristics of particle size was decided by DLS measurements at different glucose concentrations, pH, and temperature.
TEM: The morphology of p(AAPBA-b-NVCL) nanoparticles was recorded by TEM (JEM-2100, JEOL, Japan). The samples were prepared by 1 mg/mL p(AAPBA-b-NVCL) aqueous solution onto a copper grid, followed by thin films of formvar and carbon at 36°C.
In Vitro Release Behavior
Different p(AAPBA-b-NVCL) nanoparticles in vitro release characteristics of the insulin at different glucose concentrations, temperature, and pH were investigated.
For different glucose concentrations, insulin-loaded nanoparticles (5 mg) were added into 0, 1, 2, and 3 mg/mL glucose concentrations at 37°C with 20 mL of pH 7.4 phosphate buffer solution (0.1 M) by shaking (100 r/min) for 120 hrs.
For different temperatures, the insulin-loaded nanoparticles (5 mg) were added into 0mg/mL glucose concentrations at 17°C, 22°C, 27°C, 32°C, 37°C, 42°C with 20 mL of pH 7.4 PBS (0.1 M) by shaking (100 r/min) for 120 hrs.
For pH, the insulin-loaded nanoparticles (5 mg) were added into 0mg/mL glucose concentrations at 37°C with 20 mL of pH 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, and 9.0 PBS (0.1 M) by continuous shaking (100 r/min) for 120 hrs.
At the determined time, 1mL supernatant was withdrawn and a fresh buffer solution was added, the insulin was monitored using UV spectrophotometry and the results are reported as mean±standard deviation (n=3).
Toxicity Assay
Cell Viability Study And MTT Assays
The cell viability of nanoparticles was evaluated using NIH 3T3 cells [purchased from Shanghai Hengfei Biotechnology Co., Ltd (Shanghai, People’s Republic of China)]. The cell lines were grown in DMEM (Gibco) with O2:CO2=95%:5%. The cells (10,000 cells per well) were seeded into 96-well plates and were incubated for 24 hrs. Then, a range of concentrations from 25 to 125 ug/mL nanoparticle suspensions (100 mL) was added into the plates. After 24 hrs, 20 mL MTT solution (5 mg/mL in PBS buffer) was added into each well. After 4 hrs, the medium was removed and the samples were air dried. DMSO (200 mL) was added to dissolve the formed crystals. Finally, a microplate reader (Thermo Multiskan MK3, Thermo Scientific Company, Waltham, UK) was used to measure the optical density of the solution at 570 nm, and NIH 3T3 cells without any treatment were used as controls.
Toxicological Experiments
Thirty Kunming mice (15 males and 15 females, weighing around 19–23 g) were randomly divided into control group and experimental groups [p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4], with 6 in each group. The mice in the experimental groups were injected with 10mg/kg/d p(AAPBA-b-NVCL) nanoparticles and the mice in the control group received 1 mL/kg/d saline solution. After 60 days, the mice were sacrificed and their blood was collected. Serum creatinine, serum glutathione, total cholesterol, glucose, uric acid, aspartate amino transferase, and glutamine transaminase were analyzed using an automated Abbott C8000 biochemistry analyzer (Abbott, Chicago, USA). Meanwhile, their livers, kidneys, spleens, hearts, and lungs were collected and underwent HE staining.
In Vivo Hypoglycemic Experiments
The diabetic mice model was successfully constructed by STZ injection (65mg/kg) method. After that, they were randomly divided into diabetic group (n=6), insulin injection group (n=6) and p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4 injection groups (n=6). In addition, 6 healthy mice were selected as a control group. The mice then received treatment accordingly, and mice in the diabetic group and control group were given distilled water (0.5 mL) injections daily, the insulin injection group mice received insulin injections (0.16 mg/d), and the p(AAPBA-b-NVCL) group mice received insulin-loaded p(AAPBA-b-NVCL) nanoparticles (the injection formulation was given UV lamp sterilization treatment). The blood glucose levels were determined using a Blood Glucose Meter (SXT-1) (Sinocare Biosensor Co., Ltd) within 96 hrs and their blood was collected from the tail veins. In our research, all animal experimental procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 8523, revised 1985) and all the experiments were approved by the Animal Care and Use Committee of Yunnan University of Chinese Medicine (No. R-062018067, 25.02.2018).
Results And Discussion
FT-IR Spectroscopy And 1H-NMR Spectroscopy
FT-IR and 1H-NMR assist in accurately detecting whether a polymer is synthesized or not, and so the structure of p(AAPBA-b-NVCL) was observed by FT-IR and 1H-NMR. Figure 1 shows the FT-IR spectra of AAPBA, NVCL, p(AAPBA), and p(AAPBA-b-NVCL)3. AAPBA showed four characteristic absorption bands: C=O str. (1660 cm−1), C=C str. (1640 cm−1), O-B-O (1350 cm−1), and B-O at 1015 cm−1. The absorption of the meta-substituted benzene was at 790 cm−1 and 700 cm−1. NVCL demonstrated C=O str. at 1660 cm−1, C=C str. at 1620 cm−1; CH2 str, between 2850 and 3000 cm−1 and C=CH str. at 3110 cm−1. In p(AAPBA) and p(AAPBA-b-NVCL) 3, the absorption due to C=C disappeared, which indicated the occurrence of successful polymerization. In FT-IR spectra of p(AAPBA-b-NVCL), there exists an O-B-O str. at 1340 cm−1, and the meta-substituted benzene absorption was at 790 cm−1 and 700 cm−1, which proved that AAPBA has been incorporated into the polymer. This was consistent with the previous reports,20 but the meta-substituted benzene absorption in p(AAPBA-co-NVCL) cannot be seen, but it can be clearly seen in p(AAPBA-b-NVCL), which might be caused due to the content of AAPBA.
 |
Figure 1 FT-IR spectra of NVCL, AAPBA, pAAPBA, and p(AAPBA-b-NVCL)3. |
Figure 2 demonstrates 1H-NMR spectra of NVCL, AAPBA, p(AAPBA), and p(AAPBA-b-NVCL). The spectrum of NVCL (Figure 2A) (DMSO-d6) δ: 7.23 (1H, 3-H), 5.65 (2H, 2-H), 4.52 (2H, 4-H), 3.30 (2H, 1-H), 2.50 (2H, 5-H), 1.62 (2H, 6-H), and 1.14 (2H, 7-H). AAPBA (Figure 2B) showed the following assignments: 1H NMR (DMSO-d6): δ: 10.09 (1H, 4-H), 8.10-7.25 (the H of benzene ring), 6.47 (1H, 2-H), 6.26 (1H, 3-H), 5.75 (2H, 1-H) and 1.60 (1H, 5-H). p(AAPBA) (Figure 2C; pH= 9.5, NaOD/D2O)δ: 8.35-6.33 (the H of benzene ring), 2.85 (2H, 6-H), 2.25 (1H, 4-H), and the p(AAPBA-b-NVCL) (Figure 2D; pH= 9.5, NaOD/D2O) δ: 8.45 (1H, 1-H), 8.17-7.32 (the H of benzene ring), 7.22 (1H, 2-H), 3.90 (1H, 2-H), 2.55 (2H, 5-H), 1.65 (1H, 4-H), and 1.35 (6H, 3-H). Compared with the spectra of monomers AAPBA and NVCL, it was obvious that the peaks of the ethylene group in p(AAPBA) and p(AAPBA-b-NVCL) were disappeared and the peaks of these protons can be assigned in the structure of p(AAPBA-b-NVCL).24,25
 |
Figure 2 1H-NMR spectra of (A) NVCL, (B) AAPBA, (C) pAAPBA, and (D) p(AAPBA-b-NVCL)3. |
LCST, Zeta Potential, pH, Glucose And Temperature Sensitivity
Based on the results of FT-IR and 1HNMR, the polymer of P (AAPBA-b-NVCL) that was successfully synthesized can be obtained. Next, these were manufactured into the nanoparticles to observe the LCST of the nanoparticles by UV-vis spectroscopy. In general, the temperature-sensitive material acts as a drug carrier, and the LCST of nanoparticles was close to the body temperature to easily access the self-driving properties of the materials.26,27 As seen from Table 2, p(AAPBA) has no LCST value, and the values of p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, p(AAPBA-b-NVCL)4 were increased, respectively 33.5°C, 35.5°C, 37.5°C, and 40.5°C, which are all near to the body temperature. This confirmed that the nanoparticles were able to effectively perform the characteristic of temperature-sensitivity within the scope of body temperature. After that, the stability of the nanoparticles was investigated by the system zeta potential. The results showed that the absolute values of all the nanoparticles are between 30 and 40, confirming that the nanoparticles in this study are very stable. In addition, the size data and zeta potential (Figure S1F) of these nanoparticles in aqueous solution were seen in Figure S1 (Figure S1A–E were p(AAPBA), p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4, respectively), and the results indicated that the p(AAPBA-b-NVCL) nanoparticles have good particle size distribution and were very stable.
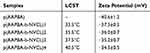 |
Table 2 The LCST And Zeta Potential Of p(AAPBA-b-NVCL) Nanoparticles With Different Ratios |
Next, the sensitivity changes of nanoparticles to changes in pH, glucose concentration, and temperature were examined. Figure 3 shows the hydrodynamic diameters changes of p(AAPBA-b-NVCL) in different pH, temperature, and glucose concentrations.
The pH of human physiological is 7.4, and the PBA and its derivatives have a high pKa (of about 8–9) generally.28 Under normal physiological environment, PBA polymer has poor water solubility and weak glucose responsiveness.29 To reduce the pKa value of PBA, two main methods have been adopted: one is to introduce a pull electron group on the benzene ring of PBA, and the other is to introduce benzenoboric acid into the copolymer.30 In our research, the pH sensitivity suspended the action of pure nanoparticles (1 mg) into PBS (1 mL) at pH 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5 and 9.0 for 0.5 hr. The results (Figure 3A) showed that when the pH ranged from 5.0 to 7.0, the diameters of p(AAPBA) nanoparticles and the four kinds of p(AAPBA-b-NVCL) nanoparticles almost demonstrated no increase, and when the pH value was above 7.0, the four kinds of p(AAPBA-b-NVCL) nanoparticles demonstrated a significant increase, and the diameters of p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4 were increased accordingly at 50, 51, 47, and 46nm (pH from 7.0 to 9.0). For p(AAPBA), the particle size was increased obviously when the pH was above 8.0. Our study further confirmed that NCVL and AAPBA copolymerization reduced the pKa value of PBA. This was consistent with the study by Farooqi et al,31 in which a new type of microgels were synthesized based on poly [(N-isopropylacrylamide)-co-acrylamide-co-(acrylic acid), and obtained similar results.
Currently, poly N-isopropyl acrylamide (PNIPAM) has been extensively studied due to its temperature sensitivity,32–34 but the research regarding its biodegradability and biocompatibility remained poor. poly NVCL is also a temperature-sensitive material that contains a lactam structure, and its LCST value is also close to the human body temperature value (between 30–40°C). In addition, compared with PNIPAM, the structure of the ring lactam also allows it to produce good coordination properties. Besides, it has no toxic hydrolysates with amino derivatives formed, and so there is no rejection from the body.35 Prabaharan et al36 in the study conducted on NVCL showed manufacturing of nanoparticles by grafting an amino group of chitosan and an amide bond of poly-N-vinylcaprolactam. They also found that NVCL not only enhances the temperature sensitivity of the polymer, but also improves the water solubility of chitosan. Finally, NVCL achieved its use in the field of drug loading and drug release and has potential application value in the field of biomedicine. In our research, temperature sensitivity involves suspending of blank nanoparticles (1 mg) in PBS (1 mL, pH 7.4) for 0.5 hr and the size of the nanoparticles was measured by DLS at 12°C, 17°C, 22°C, 27°C, 32°C, 37°C, and 42°C. Figure 3B shows the changes of p(AAPBA-b-NVCL) nanoparticle sizes with increasing temperature and the results demonstrated that p(AAPBA) nanoparticles were not changed under different temperature conditions. For p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b -NVCL)3, and p(AAPBA-b-NVCL)4, their diameters were hardly changed between 12°C and 27°C. When the temperature was higher than 27°C, the nanoparticles were decreased with increasing temperature, and the diameters of p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4 were correspondingly decreased by 65, 60, 61, and 58nm, respectively.
In our study, the glucose sensitivity was determined by treating the blank nanoparticles with 0, 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg/mL glucose in PBS (1 mL) for 0.5 hr and the size of nanoparticles was measured by DLS at 37°C. Figure 3C shows the changes of p(AAPBA-b-NVCL) nanoparticle sizes with increasing glucose concentration and demonstrated that the p(AAPBA) nanoparticles have no change under different glucose concentrations. For p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4, their diameters were significantly increased, and p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4 were correspondingly increased by 55, 65, 61, and 60 nm at 0 mg/mL glucose to 3 mg/mL glucose, respectively. This may be linked to high proportion of AAPBA in p(AAPBA-b-NVCL). Our findings are in agreement with most of the studies, such as Wang et al37, in which poly(ethylene glycol)-b-poly(acrylic acid-co-acrylamidophenylboronic acid) copolymer was synthesized by RAFT. The copolymer was fluorescent and self-assembled to form glucose-sensitive micelles, and exhibited good glucose sensitivity. In addition, Wang et al38 used APBA to partially modify polyethylene glycol-b-acrylic acid (PEG-b-PAA) to synthesize a block copolymer PEG-b-(PAA-co-PAAPBA) that can be self-assembled into micelles. They also found that the copolymer has good glucose sensitivity in an aqueous solution at pH=7.4. These results demonstrated that introduction of PBA into the copolymer allows the copolymer to exert its glucose sensitivity under physiological conditions.
Figure 3D presents the reversible glucose sensitivity of p(AAPBA-b-NVCL) nanoparticles at pH =7.4, and the results showed that after addition and removal of glucose, the diameters of p(AAPBA-b-NVCL) nanoparticles remained well glucose sensitivity. This indicated excellent stability and reversibility of this polymer and hence its potential application as a drug carrier.
 |
Figure 3 Hydrodynamic diameters at different: (A) pH, (B) temperatures, (C) glucose concentration, and (D) reversible glucose sensitivity. |
The TEM photographs of p(AAPBA-b-NVCL)3 nanoparticles illustrated that the nanoparticles are spherical in shape with good dispersion (Figure 4A). Figure 4B shows that the nanoparticles remained spherical after treatment with 3 mg/mL glucose for 4d indicating the stability of the prepared nanoparticles. In addition, TEM photographs of p(AAPBA) (Figure S2A), p(AAPBA-b-NVCL)1 (Figure S2B), p(AAPBA-b-NVCL)2 (Figure S2C), and p(AAPBA-b-NVCL)4 (Figure S2D) nanoparticles in aqueous solution are presented in Figure S2. These indicated that the p(AAPBA-b-NVCL) nanoparticles are spherical in aqueous solution at pH=7.4.
 |
Figure 4 TEM of p(AAPBA-b-NVCL)3 nanoparticles (A) before and (B) after treatment with 3 mg/mL glucose. |
Insulin Loading And Release
In the previous work, when the insulin concentration was 1.5 mg/mL, wrapping of 20 mg of p(AAPBA-co-NVCL) nanoparticles can effectively balance the drug loading rate and EE.20 So, our study also determined the insulin concentration to be 1.5 mg/mL and the nanoparticles used were 20mg. The results revealed that the LC(%) in p(AAPBA), p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4 nanoparticles group showed no differences. For the EE%, the p(AAPBA) demonstrated the highest, i.e., up to 80%, and p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4 nanoparticles demonstrated no differences (Table 3), i.e., up to 70%. The EE% was consistent with our previous reports,20 but they were significantly higher compared to Guo et al's39 study. Guo et al's study found a new type of glucose-sensitive nanoparticles p(AAPBA-b-GAMA). Their research also found that the drug loading rate was only about 60%, which may be due to that the hydrophilic properties of p(AAPBA-b-NVCL) are not as high as p(AAPBA-b-GAMA), while the polymers with high hydrophilicity reduce the drug loading rate of insulin.
 |
Table 3 Insulin Loading Capacity And Encapsulation Efficiency Of p(AAPBA-B-NVCL) Nanoparticles |
After successful packaging of insulin, the insulin release characteristics of nanoparticles under physiological conditions were investigated. From Figure 5, p(AAPBA-b-NVCL) 1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4 nanoparticles demonstrated good glucose sensitivity. For insulin-loaded p(AAPBA) nanoparticles, the release was increased only by 8% when the glucose concentration ranged from 0mg/mL to 3mg/mL, and p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4 were increased by 25%, 30%, 32%, and 29%, respectively, further confirming that NVCL and AAPBA copolymerization was beneficial for glucose sensitivity. In addition, Figure 5A–C demonstrates the effective drug release time of p(AAPBA-b-NVCL)1, p(AAPBA-b-NVCL)2, p(AAPBA-b-NVCL)3, and p(AAPBA-b-NVCL)4 nanoparticles as only 12 hrs with 0mg/mL glucose concentration, and 24 hrs with 1mg/mL, and 48 hrs with 3mg/mL. This indicated that 3mg/mL glucose concentration was conducive to the release of insulin in the nanoparticles. This also confirmed that the nanoparticles have good glucose sensitivity. Figure 5D demonstrates insulin-loaded P(AAPBA-b-NVCL)3 nanoparticles at 0, 1, and 3 mg/mL glucose concentrations. The release characteristics were similar to the results conducted by Guo et al,40 and it can be seen that the nanoparticles have very good glucose sensitivity.
Cytotoxicity Testing And Animal Toxicity
Next, the cytotoxic effects of p(AAPBA-b-NVCL) nanoparticles on multiple cell lines were determined. At present, many studies underwent copolymerization of AAPBA and other functional monomers as drug carriers to investigate their cytotoxicity. Xing et al41 synthesized p(NIPAM-AAPBA) microgels and found that the nanogels did not affect the cell activity. In addition, Guo et al24 synthesized a new insulin-loaded nanoparticles of p(AAPBA-b-AGA), which also proved that these nanoparticles did not cause cytotoxicity. In our study, the cytotoxic effect of p(AAPBA-b-NVCL) nanoparticles and p(AAPBA-b-NVCL) nanoparticles on NIH 3T3 cells was determined by MTT method. The cells were exposed to suspensions of nanoparticles from 20 to 100 mg/mL and cells without pretreatment were used as negative control group (the negative control group was 100%). Figure 6 shows that the cell survival rate of all nanoparticles was higher than 80%, and this indicated that the nanoparticles are safe. Thus, potential application of these for insulin delivery was observed, suggesting that the nanoparticles based on AAPBA and functional monomers have weak cytotoxicity and good cytocompatibility.
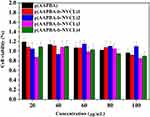 |
Figure 6 Cell viability of p(AAPBA-b-NVCL) nanoparticles by MTT assay at 37°C after incubation for 24 hrs. All values are presented as mean±SD (n = 5). |
Animal toxicity tests are necessary to determine whether the materials are safe in vivo and the results are reported in Figure 7 and Table 4. The toxicity was tested in mice by intraperitoneally injecting with 10 mg/kg/d of nanoparticles according to the weight of the mice. The results for over 60 days revealed that compared with control group, the four p(AAPBA-b-NVCL) groups showed no obvious damage. This was similar to that of our previous study findings,20 in which the p(AAPBA-b-NVCL) nanoparticles showed no significant effect on the visceral tissue. As there was no significant change in the p(AAPBA) particle size at different glucose concentrations at pH=7.4, there was no effective role played by them in hypoglycemic conditions after administrating into the body, and so this part was not added in the experiment. The results showed that the materials are safe and have no negative impact on the blood biochemical values, which indicated that the materials have no toxicity in 60 days.
 |
Table 4 Effect Of Administration By Injection Of Nanoparticles On The Biochemical Parameters Of Rats After 10 d (n=5, mean±SD) |
In Vivo Hypoglycemic Studies
To effectively observe the hypoglycemic effect of insulin-loaded p(AAPBA-b-NVCL) nanoparticles, a diabetic mouse model was constructed by one-time injection of 65mg/kg STZ, and this model showed obvious pancreatic damage, which was consistent with our design. After injection, the blood of the mice was periodically obtained from the tail vein for analyzing the hypoglycemic effect using a blood glucose meter (GA-6, Sinocare). The results (Figure 8) showed that the blood glucose in the control group and model group remained stable during the treatment period (it was about 6.5mmol/L in the control group, and was about 23.5mmol/L in the model group). However, there are some differences in the four P(AAPBA-b-NVCL) nanoparticle groups. All P(AAPBA-b-NVCL) nanoparticles have similar glucose levels from 0 hr to 60 hrs, but p(AAPBA-b-NVCL)4 after 60 hrs, p(AAPBA-b-NVCL)3 after 72 hrs, p(AAPBA-b-NVCL)2 after 84 hrs, and p(AAPBA-b-NVCL)1 after 96 hrs showed no effective lowering of blood glucose. This suggested that insulin-loaded p(AAPBA-b-NVCL) nanoparticles demonstrated hypoglycemic effect, but different ratios of AAPBA and NVCL showed different hypoglycemic time. Compared to the hypoglycemic time in vitro, the release of insulin was finished within 12 hrs (Figure 5). On the other hand, the regulation of blood glucose was observed over 60 hrs, and the reason for this might be due to that after the injection of p(AAPBA-b-NVCL) nanoparticles, insulin changes the release amount according to the change of the blood glucose concentration, which prolonged the action time of insulin-loaded nanoparticles. In addition, this also proved that insulin-loaded p(AAPBA-b-NVCL) nanoparticles successfully induced glucose sensitivity when compared to insulin injection products that are currently used in the market.42 Moreover, compared to our previous research,20 p(AAPBA-b-NVCL) was longer than p(AAPBA-co-NVCL), and it also was significantly higher than most others reported in the effective hypoglycemic time.43,44 The reasons for these warrant further study.
 |
Figure 8 Blood glucose concentration after injection (A over 96 hrs, B over 6 hrs, because A did not show hypoglycemic effect within the first 6 hrs, and so we placed it separately, that is as B). |
Conclusions
A new amphiphilic block copolymer named p(AAPBA-b-NVCL) was synthesized in our study. This polymer can be easily self-assembled to form a spherical shape that is well dispersed, and have thermal- and glucose-sensitive properties under physiological conditions. Besides, this new nanoparticle can effectively encapsulate insulin, and the EE is approximately 12.5% due to hydrophilic-hydrophobic interactions. Also, the amount of insulin released significantly increases with increased glucose concentration, and showed no toxicity towards cells and animals. This nanoparticle also effectively reduced the blood sugar in diabetic mice within 72 hrs. In conclusion, our research suggested that p(AAPBA-b-NVCL) nanoparticles might have the potential for use as insulin delivery systems in future. However, there are still some limitations in our research, such as how to improve EE, how to reduce the size of the nanoparticles, how to improve the stability, and how to enhance the controlled release of insulin. These should be resolved in our future work.
Acknowledgments
This investigation was supported by the National Natural Science Foundation of China (no. 81860812).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Min J, Zhao Y, Slivka L, et al. Double burden of diseases worldwide: coexistence of undernutrition and overnutrition-related non-communicable chronic diseases. Obes Rev. 2017;19(1):49–61. doi:10.1111/obr.12605
2. Marathe PH, Gao HX, Close KL. American Diabetes Association Standards of Medical Care in diabetes 2017. J Diabetes. 2017;9(4):320–324. doi:10.1111/1753-0407.12524
3. Hu C, Jia W. Diabetes in China: epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes. 2018;67(1):3–11. doi:10.2337/dbi17-0013
4. Ferreiro A, Lombardi R, Burdmann EA. Acute kidney injury after cardiac surgery is associated with mid-term but not long-term mortality: a cohort-based study. PLoS One. 2017;12(7):e0181158. doi:10.1371/journal.pone.0181158
5. Xu T, Dainelli L, Yu K, et al. The short-term health and economic burden of gestational diabetes mellitus in China: a modelling study. BMJ Open. 2017;7(12):e018893. doi:10.1136/bmjopen-2017-018893
6. Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1–18. doi:10.1016/j.colsurfb.2009.09.001
7. Mccoy RG, Herrin J, Lipska KJ, Shah ND. Recurrent hospitalizations for severe hypoglycemia and hyperglycemia among U.S. adults with diabetes. J Diabetes Complications. 2018;32(7):693–701. doi:10.1016/j.jdiacomp.2018.04.007
8. Song N, Chen GH, Cong HL, Yu B, Feng YM. The synthesis and application of dual temperature/pH-sensitive polymer nanoparticles. Integr Ferroelectr. 2017;181(1):151–155. doi:10.1080/10584587.2017.1352407
9. Zhao M, Lee SH, Song JG, et al. Enhanced oral absorption of sorafenib via the layer-by-layer deposition of a pH-sensitive polymer and glycol chitosan on the liposome. Int J Pharm. 2018;544(1):14–20. doi:10.1016/j.ijpharm.2018.04.020
10. Zhang S, Chen H, Liu S, et al. Superabsorbent polymer with high swelling ratio, and temperature-sensitive and magnetic properties employed as an efficient dewatering medium of fine coal. Energy Fuel. 2017;31(2):1825–1831. doi:10.1021/acs.energyfuels.6b03083
11. Yetisen AK, Jiang N, Fallahi A, et al. Glucose‐sensitive hydrogel optical fibers functionalized with phenylboronic acid. Adv Mater. 2017;29(15):1606380. doi:10.1002/adma.201606380
12. Sato K, Shimizu S, Awaji K, et al. Lactate-induced decomposition of layer-by-layer films composed of phenylboronic acid-modified poly(allylamine) and poly(vinyl alcohol) under extracellular tumor conditions. J Colloid Interface Sci. 2018;510:302–307. doi:10.1016/j.jcis.2017.09.075
13. Bajgrowiczcieslak M, Alqurashi Y, Elshereif MI, et al. Optical glucose sensors based on hexagonally-packed 2.5-dimensional photonic concavities imprinted in phenylboronic acid functionalized hydrogel films. RSC Adv. 2017;7(85):53916–53924. doi:10.1039/c7ra11184c
14. Shiomori K, Ivanov AE, Galaev IY, et al. Thermoresponsive properties of sugar sensitive copolymer of N‐Isopropylacrylamide and 3‐(acrylamido)phenylboronic acid. Macromol Chem Phys. 2004;205(1):27–34. doi:10.1002/macp.200300019
15. Rahman M, Nahar Y, Ullah W, et al. Incorporation of iron oxide nanoparticles into temperature-responsive poly (N-isopropylacrylamide-co-acrylic acid) P (NIPAAm-AA) polymer hydrogel. J Polym Res. 2015;22(3):1–9. doi:10.1007/s10965-015-0673-y
16. Gaballa HA, Geever LM, Killion JA, et al. Synthesis, characterisation and drug release studies of pH and temperature sensitive chemically crosslinked N-vinylcaprolactam, acrylic acid, methacrylic acid, N,N-dimethylacrylamide and PEGDMA hydrogels. J Hydrogels. 2015;1(1):3–11. doi:10.1166/jh.2015.1002
17. Mishra S. Dispora and the difficult art of dying. Am J Nurs. 2004;14(6):421–424. doi:10.2307/3404866
18. Shatalov GV, Churilina EV, Kuznetsov VA, Verezhnikov VN. Copolymerization of N-vinylcaprolactam with N-vinyl(benz)imidazoles and the properties of aqueous solutions of the copolymers. Polym Sci. 2007;49(3–4):57–60. doi:10.1134/S1560090407030013
19. Bitar A, Fessi H, Elaissari A. Synthesis and characterization of thermally and glucose-sensitive poly N-vinylcaprolactam-based microgels. J Biomed Nanotechnol. 2012;8(5):709–719. doi:10.1166/jbn.2012.1439
20. J Z W, Bremner DH, Li HY, Sun X-Z, Zhu L-M. Synthesis and evaluation of temperature- and glucose-sensitive nanoparticles based on phenylboronic acid and N -vinylcaprolactam for insulin delivery. Mater Sci Eng C Mater Biol Appl. 2016;69(1):1026–1035. doi:10.1016/j.msec.2016.07.078
21. Wu JZ, Williams GR, Li HY, et al. Glucose- and temperature-sensitive nanoparticles for insulin delivery. Int J Nanomedicine. 2017;12:4037–4057. doi:10.2147/IJN.S132984
22. Wu JZ, Bremner DH, Li HY, Niu S-W, Li S-D, Zhu L-M. Phenylboronic acid-diol crosslinked 6-O-vinylazeloyl-d-galactose nanocarriers for insulin delivery. Mater Sci Eng C Mater Biol Appl. 2017;76(7):845–855. doi:10.1016/j.msec.2017.03.139
23. Li Z, Qiongwei H, Yangyang L, et al. Boronic acid as glucose-sensitive agent regulates drug delivery for diabetes treatment. Materials. 2017;10(2):170. doi:10.3390/ma10020170
24. Guo Q, Zhang T, An J, et al. Block versus random amphiphilic glycopolymer nanopaticles as glucose-responsive vehicles. Biomacromolecules. 2015;16(10):3345–3356. doi:10.1021/acs.biomac.5b01020
25. Zhang X, Wang Y, Zheng C, Li C. Phenylboronic acid-functionalized glycopolymeric nanoparticles for biomacromolecules delivery across nasal respiratory. Eur J Pharm Biopharm. 2012;82(1):76–84. doi:10.1016/j.ejpb.2012.05.013
26. Ji Y, Zhu M, Gong Y, Tang H, Li J, Cao Y. Thermoresponsive polymers with lower critical solution temperature‐ or upper critical solution temperature‐type phase behaviour do not induce toxicity to human endothelial cells. Basic Clin Pharmacol Toxicol. 2017;120(1):79–85. doi:10.1111/bcpt.12643
27. Zheng B, Luo Z, Deng Y, Zhang Q, Gao L, Dong S. A degradable low molecular weight monomer system with lower critical solution temperature behaviour in water. Chem Commun. 2019;55(6):782–785. doi:10.1039/c8cc09160a
28. Kitano S, Hisamitsu I, Koyama Y, Kataoka K, Okano T, Sakurai Y. Effect of the incorporation of amino groups in a glucose‐responsive polymer complex having phenylboronic acid moieties. Polym Adv Technol. 2010;2(5):261–264. doi:10.1002/pat.1991.220020508
29. Na W, Gao C, Lü S, et al. Novel amphiphilic glucose-responsive modified starch micelles for insulin delivery. RSC Adv. 2017;7(73):45978–45986. doi:10.1039/c7ra08291f
30. Vrbata D, Uchman M. Preparation of lactic acid- and glucose-responsive poly(ε-caprolactone)-b-poly(ethylene oxide) block copolymer micelles using phenylboronic ester as a sensitive block linkage. Nanoscale. 2018;10(18):8428–8442. doi:10.1039/c7nr09427b
31. Farooqi ZH, Khan A, Siddiq M. Temperature-induced volume change and glucose sensitivity of poly [(N-isopropylacry-lamide)-co-acrylamide-co-(phenylboronic acid)] microgels. Polym Int. 2011;60(10):1481–1486. doi:10.1002/pi.3106
32. Xue S, Wu Y, Wang J, et al. Boron nitride nanosheets/PNIPAM hydrogels with improved thermo-responsive performance. Materials. 2018;11(7):1069. doi:10.3390/ma11071069
33. Khine YY, Ganda S, Stenzel MH. Covalent tethering of temperature responsive pNIPAm onto TEMPO-oxidized cellulose nanofibrils via three-component passerini reaction. ACS Macro Lett. 2018;7(4):412–418. doi:10.1021/acsmacrolett.8b00051
34. Aravopoulou D, Kyriakos K, Miasnikova A, Laschewsky A, Papadakis CM, Kyritsis A. Comparative investigation of the thermoresponsive behavior of two diblock copolymers comprising PNIPAM and PMDEGA Blocks. J Phys Chem B. 2018;122(9):2655–2668. doi:10.1021/acs.jpcb.7b09647
35. Qian W, Xu P, Lu G, Huang X. Synthesis of PAA-g-PNVCL graft copolymer and studies on its loading of ornidazole. Chin J Chem. 2015;32(10):1049–1056. doi:10.1002/cjoc.201400472
36. Prabaharan M, Grailer JJ, Steeber DA, Gong S. Thermosensitive micelles based on folate-conjugated poly(N-vinylcaprolactam)-block-poly(ethylene glycol) for tumor-targeted drug delivery. Macromol Biosci. 2010;9(8):744–753. doi:10.1002/mabi.200800366
37. Wang B, Ma R, Liu G, et al. Glucose-responsive micelles from self-assembly of poly(ethylene glycol)-b-poly(acrylic acid-co-acrylamidophenylboronic acid) and the controlled release of insulin. Langmuir. 2009;25(21):12522–12528. doi:10.1021/la901776a
38. Wang B, Ma R, Liu G, et al. Effect of coordination on the glucose-responsiveness of PEG-b-(PAA-co-PAAPBA) micelles. Macromol Rapid Commun. 2010;31(18):1628–1634. doi:10.1002/marc.201000164
39. Guo Q, Wu Z, Zhang X, Sun L, Li C. Phenylboronate-diol crosslinked glycopolymeric nanocarriers for insulin delivery at physiological pH. Soft Matter. 2014;10(6):911–920. doi:10.1039/c3sm52485j
40. Guo H, Li H, Gao J, et al. Phenylboronic acid-based amphiphilic glycopolymeric nanocarriers for in vivo insulin delivery. Polym Chem. 2016;7(18):3189–3199. doi:10.1039/c6py00131a
41. Xing S, Ying Y, Zhang Y. Kinetics of glucose-induced swelling of P(NIPAM-AAPBA) microgels. Macromolecules. 2011;44(11):4479–4486. doi:10.1021/ma200586w
42. Zhuo T, Ying G, Zhang Y. The synthesis of a contraction-type glucose-sensitive microgel working at physiological temperature guided by a new glucose-sensing mechanism. Polym Chem. 2018;9(8):1012–1021. doi:10.1039/C8PY00072G
43. Miki R, Takei C, Ohtani Y, et al. Glucose responsive rheological change and drug release from a novel worm-like micelle gel formed in cetyltrimethylammonium bromide/phenylboronic acid/water system. Mol Pharm. 2018;15(3):1097–1114. doi:10.1021/acs.molpharmaceut.7b00988
44. Guo H, Guo Q, Chu T, Zhang X, Wu Z, Yu D. Glucose-sensitive polyelectrolyte nanocapsules based on layer-by-layer technique for protein drug delivery. J Mater Sci Mater Med. 2014;25(1):121–129. doi:10.1007/s10856-013-5055-6
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.