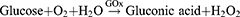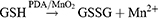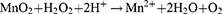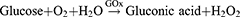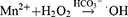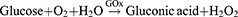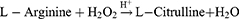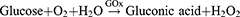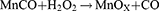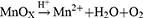Back to Journals » International Journal of Nanomedicine » Volume 17
Glucose Metabolism Intervention-Facilitated Nanomedicine Therapy
Authors Li Z, Li X, Ai S, Liu S, Guan W
Received 3 March 2022
Accepted for publication 27 May 2022
Published 17 June 2022 Volume 2022:17 Pages 2707—2731
DOI https://doi.org/10.2147/IJN.S364840
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Zhiyan Li,* Xianghui Li,* Shichao Ai,* Song Liu, Wenxian Guan
Department of Gastrointestinal Surgery, Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, Nanjing, 210008, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Song Liu; Wenxian Guan, Department of Gastrointestinal Surgery, Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, 321 Zhongshan Road, Nanjing, 210008, People’s Republic of China, Tel +86-25-68182222 ext. 60930 ; +86-25-68182222 ext. 60931, Email [email protected]; [email protected]
Abstract: Ordinarily, cancer cells possess features of abnormally increased nutrient intake and metabolic pathways. The disorder of glucose metabolism is the most important among them. Therefore, starvation therapy targeting glucose metabolism specifically, which results in metabolic disorders, restricted synthesis, and inhibition of tumor growth, has been developed for cancer therapy. However, issues such as inadequate targeting effectiveness and drug tolerance impede their clinical transformation. In recent years, nanomaterial-assisted starvation treatment has made significant progress in addressing these challenges, whether as a monotherapy or in combination with other medications. Herein, representative researches on the construction of nanosystems conducting starvation therapy are introduced. Elaborate designs and interactions between different treatment mechanisms are meticulously mentioned. Not only are traditional treatments based on glucose oxidase involved, but also newly sprung small molecule agents targeting glucose metabolism. The obstacles and potential for advancing these anticancer therapies were also highlighted in this review.
Keywords: nanomedicine, starvation therapy, combined therapy, cancer metabolism
Introduction
As one of the most threatening health risks, cancer causes tens of millions of new cases and deaths each year.1 Moreover, the incidence, and mortality of cancer are rising rapidly worldwide.2 However, traditional oncology treatments, including surgical excision, chemotherapy, and radiotherapy, cannot eradicate malignancies.3,4 Tumor metastasis and recurrence remain the major reasons for unfavorable prognosis, in which tumor metabolism displays an immense role.
Starvation therapy (ST) deprives critical nutrients and intervenes in tumor metabolism. It has gained the attention of scientists as an emerging treatment in past years. Malignant cells demand additional rates of catabolite absorption, transport, and usage than their normal counterparts. They remodel their metabolism to promote growth, proliferation, and even metastasis.4,5 Since curing heterogeneous cancers based on distinctive genetic mutations has been proven to be complex and challenging, targeting the common metabolism phenotype shared by tumors is considered an extensive and sensitive anticancer strategy.6
The leading metabolic disorder is the Warburg Effect, indicating that cancer cells prefer aerobic glycolysis even when there is enough oxygen to maintain mitochondrial oxidative phosphorylation.7 During the procedure, the uptake of glucose and lactate generation increased. This effect not only accelerates the pace of synthesizing adenosine triphosphate (ATP)5 and anabolic processes8 but also remodels the tumor microenvironment through H+ ions.9 Targeting glucose metabolism may gain major therapeutic benefits since glucose is the most abundant nutrient in circulation and the most frequent metabolic substrate utilized by malignant cells.10 The restriction of key metabolic steps or the deprivation of intracellular glucose probably avoid the downregulation of mitochondrial aerobic respiration, block NADPH generation, and interrupt pentose phosphate synthesis, inhibiting tumor development.11 Several medicines that target glycolytic enzymes and transporters are being investigated in preclinical investigations and clinical trials.12,13 The brief glucose metabolism pathway and agents referred to in this review are shown in Figure 1.
Although ST has distinct advantages, several obstacles limit further clinical application. Different agents targeting glucose metabolism have their own limitations. For example, most small molecular drugs display poor solubility and short half-life, which limit their therapeutic efficacy.14 Glucose oxidase (GOx) would be degraded by proteinase during circulation.15 Meanwhile, despite malignant cells being profiled with a higher rate of metabolism, normal tissues share similar metabolic pathways. The off-target effect probably causes systemic adverse reactions. Immune cell differentiation would be impeded as well.16 Besides, the compensation of other metabolites would lead to drug resistance to targeting a single nutrient and restrict the efficacy.6,17 To address these concerns, nanomaterials have been constructed to facilitate ST.
Nanotechnology-assisted treatment techniques have received a great deal of interest in recent years. Employing the tumor-specific antigens18 or specific cell membranes,19 nanomedicines are able to accumulate in malignant regions to reduce systemic toxicity. Various material systems have been applied to optimize delivery efficiency,20 improve targeting efficacy,21 overcome biological barriers,22 and increase the half-life of therapeutics.23 More intriguingly, nanotechnology enables the simultaneous administration of multiple therapeutic agents for synergistic therapy. The pharmacodynamics and pharmacokinetics of diverse drugs can be coordinated in a nanodrug, thus increasing the therapeutic impact. Novel therapeutic alternatives based on the development of nanotechnologies, such as photothermal therapy (PTT),24 photodynamic therapy (PDT),25 chemodynamic therapy (CDT),26 sonodynamic therapy (SDT),27 and immunotherapy,28 have popped up like mushrooms, offering a ray of hope in the fight against cancer. These benefits make up for the drawbacks of conventional ST intervention.
Here, we looked at studies that included methods consuming glucose29 or limiting glucose uptake30 and anabolic processes (Figure 2).31 Interventions on glucose metabolism without the use of GOx that are rarely mentioned before are also involved. The design concepts of these formulations, as well as their anticancer effects, are highlighted.
Mechanisms of Agents Targeting Glucose Metabolism
Malignant cells tend to increase the import and usage of glucose to synthesize ATP rapidly. Moreover, the metabolic products from aerobic glycolysis can further flue the tricarboxylic acid cycle and the pentose phosphate pathway. Glucose metabolism is involved in the synthesis of hexosamine, amino acids, as well as lipids.6 As the most prominent and well-studied metabolic disorder, glucose metabolism has been targeted by lots of agents. Enzymes, small molecule drugs, and even siRNAs have been applied in glucose metabolism blockage.
The most widely utilized molecule to block glucose metabolism is GOx. It serves as an efficient biocatalyst for the oxidation of glucose to gluconic acid and hydrogen peroxide (H2O2) with the assistance of oxygen (O2),32 as shown in the following reaction:
By depleting glucose in the tumor area, tumor cells would thus be devoid of nutrients, grow slowly, or even die. Aside from starving tumor cells, GOx has the potential of increasing acidity, hypoxia, and H2O2 level in the tumor microenvironment (TME). Several nanodrugs made up of components responding to specific environments have been constructed using this property.
Besides GOx, there are plenty of drugs interfering with glycolysis under investigation,6 which target glucose transporter 1 (GLUT1), pyruvate kinase isozyme M2 (PKM2), hexokinase (HK), 6-phosphofructo 2-kinase-fructose-2,6-biphosphatase 3 (PFKFB3) and so on.13,33 As the initial stage in cellular glucose metabolism, GLUT1 possesses the ability to transfer glucose into cells for subsequent reactions.34 It has also been considered an ideal biomarker for solid tumor prognosis and survival.35 Therefore, blocking GLUT1 by diclofenac or BAY-876 can greatly reduce the amount of glucose available in tumor cells. In addition to GLUT1, HK has been targeted by several nanomaterials as well. Catalyzing the conversion of glucose to glucose-6-phosphate, the rate-limiting process in glycolysis, HK is crucial in sustaining the high glucose catabolic rate required for tumor cells to flourish.36 Pharmacological suppression of HK2 inhibits tumor development and restores tumor cell sensitivity to therapies.37 Some pharmaceuticals have been included in nanomaterials to interrupt the function of HK, such as 2-deoxy-d-glucose (2DG)38 and bromopyruvate.39 Even small interfering ribonucleic acid (siRNA) targeting PKM2 has been utilized to sabotage glycolysis by knocking down PKM2, which is overexpressed in rapidly proliferating cancer cells.40 PKM2 is regarded to be crucial not only in producing ATP and pyruvates but also in regulating the expression of genes involved in multiple steps of cell survival.41 Inhibition of this enzyme potentially brings about novel glycolysis inhibition concepts by altering metabolic flux. To sum up, the applications of distinctive medications disrupting glucose metabolism have made the field bloom (Table 1).
 |  |  |
Table 1 Summary of Nanodrugs Targeting Glucose Metabolism |
Monotherapy Targeting Glucose Metabolism
A great many nanodrugs have been developed for glucose-based starvation monotherapy. A number of nanosystems carrying GOx have been synthetized to enhance ST by avoiding hazardous side effects and preventing degradation by proteinases.42–44 To ensure long-lasting catalytic activity, Yang et al devised a nanovehicle to load GOx coordinated with folic acid and Zn2+ termed GOx@PDA.45 The nanodrug was further modified with a polydopamine (PDA) shell. Because of the protection of the PDA shell, GOx in the core was prevented from passing through the capsule membrane, while free glucose substrates could be transported. Therefore, even under harsh conditions, GOx activity was maintained. Furthermore, the authors incorporated GOx@PDA with microneedles (MNs) made of hyaluronic acid (HA) and polyvinylpyrrolidone, which allowed GOx@PDA to penetrate the skin for precise delivery to melanoma. Interestingly, the in vivo inhibition ratio of mice bearing B16F10 tumors was up to 91%.
Unfortunately, ST alone may induce medication resistance for elevated hypoxia or the supplementary of other metabolic pathways.46 Autophagy triggered by metabolizing pressure is another factor weakening starvation therapy efficacy.47 To inhibit that, several autophagy inhibitors have been applied in nanomedicine.48,49 A nanoparticle co-delivering GOx and the autophagy inhibitor 3-methyladenine (3-MA) has been reported.50 Aside from GOx, a variety of medicines that interfere with glycolysis are being investigated. Yang et al synthesized black phosphorus (BP)-based 2D nanosheets modified with polyethylene glycol (PEG)-NH2 (Figure 3).38 Regrettably, instead of loading 2DGs on BPs, the two substances were administered separately. The extracellular lactate produced by glycolysis was dramatically reduced as a result of competitively restricting glucose absorption by GLUT1 and noncompetitively decreasing glucose phosphorylation by HK.51 The levels of lactate dehydrogenase A (LDHA), HK2, and MYC were lowered, and the ATP level was cut in half. The antineoplastic impact on tumor-bearing mice was considerable, owing to the restriction of lysosomal degradation and autophagic flux driven by BP nanosheets and ST. If BP nanosheets were to be employed as carriers as in other articles,52 follow-up research to adjust the ratio of 2DGs to BPs and load 2DGs onto BP nanosheets would likely boost the treatment efficiency even more.
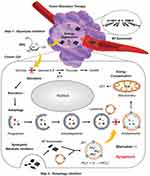 |
Figure 3 Schematic illustration for the mechanism of autophagy inhibition-augmented tumor-ST. Step 1: 2DG is applied to restrain glycolysis and initiate severe starvation of cancer cells. Step 2: BP nanosheet inhibits downstream protective autophagy, cuts off the compensatory nutrition supply and finally promotes apoptosis. Reproduced with permission from: Yang B, Ding L, Chen Y, Shi J. Augmenting Tumor-Starvation Therapy by Cancer Cell Autophagy Inhibition. Adv Sci (Weinh). 2020;7(6):1902847. doi:10.1002/advs.201902847.38 Copyright 2020 The Authors. Creative Commons Attribution License. |
Combined Therapy Targeting Glucose Metabolism
Although several nanomedicines conducting ST alone achieved curative effect, it is a pity that ST alone is difficult to eliminate the tumor. The abundant capillary supplying glucose and other nutrients compensating for the lack of glucose metabolism would result in poor efficacy and drug resistance.46 Besides, combined therapy is known to minimize medicine dosage, reduce drug toxicity, and boost effectiveness.53 Therefore, it is reasonable to combine ST with other treatment modalities.
Chemotherapy Based on Glucose Metabolism Intervention
Chemotherapy, as a traditional therapeutic approach, has proven to be beneficial in healing most human malignancies. Chemotherapy significantly extended the survival time of patients with specific chemosensitive tumors. Serving an essential role as adjuvant treatment combined with radical local treatments, the characteristics of chemotherapy are expanding and widely applied.54,55 Nevertheless, it is challenging to cure tumors using chemotherapy individually due to a variety of factors, including the multidrug resistance (MDR) effect.56 Additionally, chemotherapeutic side effects arising from nonselective cytotoxicity on normal cells are a barrier.57 Hence, synergistic therapies are desired to improve chemotherapy therapeutic efficacy while lowering toxic side effects. Starvation could sensitize certain malignant cells to chemotherapy, probably because of attenuated chemoresistance, while switching normal cells to a defensive mode.58,59 Although fasting has been used in conjunction with chemotherapy in clinical studies, intolerability and nonspecificity might impede its further applications.60 To cope with this, platforms incorporating starvation agents and chemotherapeutic medicines have been devised.61–64 Lately, Yang et al designed Lip-(2DG+DOX) to co-encapsulate 2DG and doxorubicin (DOX) hydrochloride (Figure 4).65 Cancer cells, such as HeLa, 4T1, and B16 cells, were considerably injured by the pharmacological action of 2DG and DOX, resulting in mitochondrial depolarization and an increase in reactive oxygen species (ROS), but normal cells were less impacted. The differential metabolism-regulated pathways increased tumor mortality while sparing other tissues. What is more intriguing is that limiting glucose metabolism potentially helped with MDR by reducing medication efflux via P-glycoprotein (P-gp), an ATP-binding cassette transporter.66 The concrete mechanism of conquering MDR was reported by Chen et al, engineering a nanovesicle containing ferrocene, GOx, and Pt (GOx&Pt@FcNV).67 The therapeutic technique reduced the expression of P-gp by about 41%, which led to improved therapeutic efficacy. It illustrated that combining a chemotherapeutic medication with suppression of the MDR mechanism could result in considerable cytotoxicity. In these nanosystems, the role of drugs regulating glucose metabolism was eye-catching and irreplaceable.
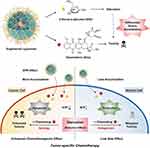 |
Figure 4 Schematic illustration of engineered liposome nanomedicines and their interaction with cancer cells and normal cells enabling differential stress sensitization of chemotherapy. Reproduced with permission from: Yang B, Chen Y, Shi J. Tumor-Specific Chemotherapy by Nanomedicine-Enabled Differential Stress Sensitization. Angew Chem Int Ed Engl. 2020;59(24):9693–9701. doi:10.1002/anie.202002306.65 Copyright 2020, Wiley-VCH. |
Apart from MDR, starvation therapy is capable to induce prodrug-to-drug conversion for spatiotemporal precision. Several prodrugs have been identified, such as hypoxia-activated tirapazamine (TPZ)68–70 and banoxantrone dihydrochloride (AQ4N),71,72 pDOXs responding to acid,73 and BDOX responding to H2O2.74 In addition to generating products because of the distinctive environmental characteristics aggravated by GOx, prodrugs reacting with the original tumor microenvironment for precise release have been advanced.75 By analogy, DOX-Duplex formed by loading DOX in deoxyribonucleic acid (DNA) segments enabled DOX to release in the presence of external ATP. Fortunately, ATP was overexpressed in tumor tissues for a high degree of anabolic processes. On account of the properties of DOX-Duplex, Jiang et al established a nanomedicine named BAY-876@(mPEG-SS-PEI-DSPE-DOX-Duplex) (short for P-B-D), delivering DOX-Duplex and BAY876 rationally (Figure 5).30 The carrier, polyethylene glycol-disulfide bond-polyethylenimine-1,2-Distearoyl-sn-glycero-3-phosphoethanolamine, was manufactured with disulfide bonds reacting with glutathione (GSH), leading to GSH deprivation and TME-triggered discharge of cargos. The double guarantee of ATP and GSH activation ensured precious release and minimal DOX side effects. As a chemical that inhibits GLUT1, BAY876 substantially reduced glucose absorption. The intracellular ATP level was reduced for at least 72 hours after one dose of DOX-Duplex therapy by depleting previously created ATP and preventing de novo production. Therefore, 4T1 cells underwent ferroptosis while normal tissues suffered little.
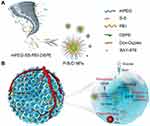 |
Figure 5 (A) Schematic illustration of the structure of P-B-D NPs. (B) Schematic illustration of the anti-tumor mechanism for synergistic ST/chemotherapy. Reproduced with permission from: Jiang W, Luo X, Wei L, et al. The Sustainability of Energy Conversion Inhibition for Tumor Ferroptosis Therapy and Chemotherapy. Small. 2021;17(38):2102695. doi:10.1002/smll.202102695.30 Copyright 2021, Wiley-VCH. |
Immunotherapy Based on Glucose Metabolism Intervention
Immunotherapy, defined as harnessing antitumor immune responses to recognize and attack cancer cells, has been a fundamental strategy in a variety of solid and hematologic malignancies. Various types of immunotherapies, including cytokine therapy, adoptive cell therapy, checkpoint inhibitors (ICIs), oncolytic viruses, and cancer vaccines, have been developed.76,77 While manipulating the immune system to reactivate the antitumor immune response, immunotherapy has its own set of side effects.78,79 Moreover, the limited population benefited from checkpoint inhibitor-based immunotherapy restrictions its wider applications.80 ST has brought about a novel method to enhance the efficiency of immunotherapy with other agents.
Another impediment to effective immunotherapy is the intrinsic immunosuppressive TME, including anti-inflammatory and protumor M2 macrophages secreting immunosuppressive cytokines.81,82 Fortunately, ROS have been proven to repolarize tumor-associated macrophages (TAMs) from the M2 phenotype to the tumoricidal M1 phenotype.83 As an agent to induce ROS, GOx has been included in nanodrugs to remodel the TME and promote immune reactivity.84,85 A upconversion nanosystem (UCNP)-based nanocatalyst activated by TME was designed by Wang et al (Figure 6).86 UCNPs with peroxidase-like catalytic activity were grafted by cysteine (Cys) complexed by Cu. They were further covalently linked with GOx to form UCNPs@Cu-Cys-GOx (abbreviated as UCCG). Intratumoral GSH was exhausted by a reaction involving disulfide bonds and Cu2+. Subsequently, Cu+ deriving from Cu2+ elevated ROS concentrations by Fenton-like reactions. M2 phenotype TAMs were satisfactorily repolarized to the M1 phenotype, attributable to the formation of ROS by cyclic reactions of CDT and ST. In addition, certain factors conducive to the immune response, such as the ratio of dendritic cells (DC) maturation, the number of cytotoxic T cells (CTLs), and the secretion of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), rose considerably. The population of regulatory T cells (Tregs), previously thought to be a cell population associated with a poor prognosis, was suppressed. In general, ROS transformed the immunosuppressive TME into a “hot” state. UCCGs also teamed up with α-PD-L1, a checkpoint inhibitor licensed by the US Food and Drug Administration (FDA). Not only was the growth of tumors impaired in situ, but distal tumors were also remarkably attenuated, indicating that the introduction of immunotherapy was a promising treatment modality.
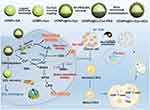 |
Figure 6 Synthetic route of UCCG and TME-activated enzymatic cascade catalytic reaction for synergistic cancer ST/CDT/immunotherapy process. Reproduced with permission from: Wang M, Chang M, Li C, et al. Tumor-Microenvironment-Activated Reactive Oxygen Species Amplifier for Enzymatic Cascade Cancer Starvation/Chemodynamic /Immunotherapy. Adv Mater. 2021;34(4):e2106010. doi:10.1002/adma.202106010.86 Copyright 2021, Wiley-VCH. |
Moreover, immunogenic cell death (ICD) might play an important role in M1 phenotype TAM repolarization. During the period, dying tumor cells release damage-associated molecular patterns (DAMPs) and tumor-associated antigens (TAAs), such as calreticulin and high mobility group box 1, for antigen presentation and CTL infiltration.87–89 It was discovered that ROS generated when GOx oxidizes glucose induces ICD. Sun et al found a method that utilized tadpole-ovoid manganese-doped hollow mesoporous silica to administer gold nanoparticles (AuNPs), DOX, and aspirin (ASA) to strengthen ICD (Figure 7).90 When the structure collapsed in an acidic and GSH-sufficient environment, AuNPs were exposed to glucose for GOx-like catalysis, and Mn2+ was liberated for a Fenton-like reaction. ROS was then produced by the dual effects. DOX was put into nanoparticles and coupled with ROS to trigger ICD. Furthermore, ASA inhibited the cyclooxygenase-2 expression and prostaglandin E2 secretion, which are barriers between tumor cells and T cells due to the anti-inflammatory ability. As a result of the enormous release of TAAs, DCs matured, increased antitumor cytokines were released, and more CTLs infiltrated. Above all, the combination of GOx-strengthened ROS production and chemotherapy is an ideal method to promote ICD for oncology immunotherapy.
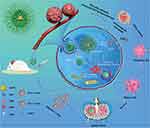 |
Figure 7 Schematic diagram of PEGylated Au@HMnMSNs ICD nanoinducers for eliciting potent antitumor immunotherapeutic efficacy. Reproduced with permission from: Sun K, Hu J, Meng X, et al. Reinforcing the Induction of Immunogenic Cell Death Via Artificial Engineered Cascade Bioreactor-Enhanced Chemo-Immunotherapy for Optimizing Cancer Immunotherapy. Small. 2021;17(37):e2101897. doi:10.1002/smll.202101897.90 Copyright 2021, Wiley-VCH. |
PDT Based on Glucose Metabolism Intervention
Photodynamic therapy is a novel treatment approach that has excellent selectivity, noninvasiveness, and minimal adverse effects.25 Under irradiation, photosensitizers generate ROS such as singlet oxygen (1O2), hydroxyl radical (•OH), superoxide radical (O2•−), or H2O2 to eliminate tumors.91 However, hypoxia, which is one of the challenges of O2-dependent PDT,92 is a characteristic of the tumor microenvironment due to increased oxygen consumption, limited oxygen diffusion, and inadequate blood supply.93 Limited H2O2 concentration in TME is another obstruction. The participation of GOx offers more abundant H2O2 through the oxidation of glucose, which could be converted to •OH by irradiation or O2 with the assistance of other molecules. PDT would be consequently enhanced by the oxidation of glucose.
Plenty of devices that utilize MnO294–97 or catalase98–101 to raise local oxygen concentration have been reported. Recently, a cascade bioreactor Ce6/GOx@ZIF-8/PDA@MnO2 (abbreviated as CGZPM) permitted synergizing PDT, CDT, and ST, which was aided by self-oxygen production and GSH consumption.102 CGZPM was synthesized by a one-pot method as the scheme, co-loading GOx and chlorene6 (Ce6) with zeolitic imidazolate framework-8 (ZIF-8). The nanoparticle was also wrapped with PDA and MnO2. After the disintegration of ZIF-8 in acidy TME, the cargos underwent the following reactions:
The decomposition of glucose by GOx produced H2O2. It was further catalyzed by MnO2 to O2, which served as a substrate for PDT and continued glucose oxidation. Moreover, the PDA shell and MnO2 interacted with GSH, a cellular antioxidant defense system obstructing ROS-based therapy, and produced Mn2+ as a Fenton-like agent for CDT. The nanosystem made full use of each component. The volume of 4T1 tumors in mice was significantly suppressed when only GOx was transported, which reflected the therapeutic effect of ST. After adding Ce6 and light irradiation, the tumors were almost eradicated thanks to the combined therapy.
Although PDT is a potential anticancer treatment, limited penetration ability impedes its practical applicability.103 Instead of photoexcitation, scientists have looked at employing chemical energy produced by bis[2,4,5-trichloro-6-(pentoxycarbonyl)phenyl]oxalate to overcome this issue.104,105 For deeper penetration, a biomimetic UCNP was reported by Wang et al.106 The UCNPs took advantage of near-infrared (NIR) light that penetrates deeper to emit blue light, which has the capacity to directly photolyze H2O2 into •OH but has a low penetrating ability. After embedding GOx and UCNPs in polyacrylic acid-η-octylamine micelles, the cancer cell membrane was coated on that for homotypic targeting. Following cellular absorption, oxidation mediated by GOx released abundant H2O2 and cut off the essential energy sources of tumor cells. Upon 980 nm laser stimulation, UCNPs emitted 470 nm light, which photolyzed excessive H2O2 into hydroxyl radicals directly, damaging tumor cells. The in vivo intervention was found to effectively target subcutaneous 4T1 tumors and lung metastatic lesions. The subcutaneous 4T1 tumors were effectively controlled even without 980 nm light irradiation, whose volumes were about 30% compared to the controls. Their volumes were lowered much more once PDT was added. Regrettably, the authors failed to evaluate the therapeutic effect of lung metastatic lesions as an indicator of deep irradiation capability.
CDT Based on Glucose Metabolism Intervention
CDT is described as the production of oxidative •OH in tumors by disproportionation of H2O2 through a Fenton or Fenton-like reaction catalyzed by Fe2+ or other chemical compositions.26 As one of the most harmful ROS, •OH can effectively kill cancer cells by disrupting biomolecules.107 However, inadequate acidity and insufficient H2O2 render therapy ineffective.108
Combining Fenton reagents with GOx to fight cancer makes sense. GOx-based ST improves CDT by supplying gluconic acid and H2O2, whereas H2O2-deficient Fenton reactions produce O2 to provide raw ingredients for glucose oxidation.29 Thus, CDT could be enhanced through the mutual promotion of the two treatment modalities. Introducing Fenton reagents and GOx into one nanomedicine is a conventional practice.110−113 Zhang et al developed a liposomal nanosystem named lipoCaO2/Fe(OH)3-GOx to synergize the complementarity of CDT and ST by co-loading Fe(OH)3-doped CaO2 nanocomposites and GOx.29 In an acidy environment, the antitumor reaction of lipoCaO2/Fe(OH)3-GOx could be promoted greatly by H2O2 and O2 produced from CaO2. They serve as substrates for CDT and ST separately in this system. As designed, CaO2/Fe(OH)3 nanocomposites decomposed in acidic TME, H2O2 and Fe3+ were generated for the following Fenton reactions as well. Besides producing •OH, GOx-expedited Fenton reactions generated O2 to enhance the catalytic efficiency of GOx in turn. LipoCaO2/Fe(OH)3-GOx could relieve the hypoxic TME and inhibit MDA-MB-231 cells both in vivo and in vitro, owing to its self-supplied oxygen function and cycle-like catalytic process. In addition to synergizing CDT agents and GOx into a unified system, efforts have been undertaken to broaden the therapeutic direction. A nanomedicine was created that exploits broking endoperoxide bridges in artemisinin to boost ROS levels.85 Combining CDT and ST with PTT,113–116 SDT,117 chemotherapy,62,63,118,119 immunotherapy,84 and metal ion therapy120 are also gaining traction.
SDT Based on Glucose Metabolism Intervention
SDT utilizes ultrasound to generate ROS for tumor ablation. It possesses the advantage of penetrating deeper than light for visceral tumor treatment and opens up new therapeutic avenues.27,121 As a popular therapeutic, the combination of SDT and ST has been researched.122–125 Similar to PDT, the efficacy of SDT was also boosted by the involvement of starvation therapy. PCN-224@Pt@GOx@EM nanocarriers (short for PPGE NCs) made most of the nanosized porphyrin-based MOF called PCN-224.126 PPGE co-loaded platinum nanoparticles (Pt NPs) and GOx molecules and camouflaged by erythrocyte membranes. Under the catalysis of Pt, a natural catalase-like enzyme with steady catalytic characteristics, oxygen generated from H2O2 aided the SDT and ST process. Therefore, even under a hypoxia environment, the viability of BxPC-3 cells decreased to less than 30% due to the function of O2-promoted glucose oxidation. The efficacy can be further enhanced under ultrasound irradiation. Overall, the reaction process can be mutually moderated by one another’s byproducts, bringing about a cycle-like process.
PTT Based on Glucose Metabolism Intervention
With NIR light irradiation, PTT mediated by photothermal agents can raise the temperature of the tumor area over 42 °C, causing cancer cells to undergo apoptosis by cell membrane disintegration, cytoskeleton damage, and DNA synthesis inhibition.127,128 With the advantages of noninvasive qualities and remarkable therapeutic accuracy, PTT has attracted increasing attention.129 However, upregulation of intracellular heat shock proteins (HSPs) expression might correct misfolded proteins, maintain intracellular homeostasis, and protect cells against hyperthermia-induced cell damage.130 As a consequence, restricting HSP production appears to be the most likely remedy to this problem, and several molecules have been adopted.131,132 The biosynthesis of HSPs is highly reliant on the level of intratumor ATP.130 From this perspective, the suppression of glucose metabolism, which prevented the generation of ATP, is critical in the nanodrugs combining ST and PTT. It offered a novel approach to lowering HSPs without the usage of HSP inhibitors.
GOx has been incorporated into multiple nanomedicines for PTT/ST synergistic treatment as a star molecule.133–137 Furthermore, multimodal treatments combining PTT/ST with PDT,98,138–140 CDT,113–116,141 chemotherapy,142–144 gas therapy145 and immunotherapy146,147 have been developed. Since nanomaterials utilizing GOx were discussed in detail in previous reviews,46,148 we will focus on other compounds altering glucose metabolism introduced in PTT/ST bimodal therapy.
Dai et al provided novel therapeutic concepts by encapsulating 2DG and a newly synthesized semiconducting polymer termed DPQ inside FA-modified liposomes.31 The synthetic nanodrug was named Lip(DPQ+2DG). FA awarded the system merit for its capacity of targeting malignant cells overexpressing folate receptors. As the cornerstone of PTT, DPQ had outstanding NIR-II fluorescence imaging performance and PTT effectiveness. Meanwhile, as a glucose analog acting as an antiglycolytic agent in clinical trials (Phase I/II),149,150 2DG not only starved tumor cells by cutting off glycolysis but also inhibited the production and function of HSPs, allowing for a superimposed impact of PTT. More crucially, 2DG outperformed GOx as 2DG does not denature or deactivate under high-temperature conditions. Beneficial from the specific targeting ability on glycolysis, Lip(DPQ+2DG) demonstrated extraordinary sensitivity to tumor cells compared to normal cells preferring to synthesize ATP through oxidative phosphorylation (OXPHOS) rather than glycolysis. On the other hand, such an effect allowed normal cells to defend themselves and lessened the negative effects. As a result of the outstanding photothermal conversion efficiency of DPQ and glucose starvation ability of 2DG, cell viability in vitro decreased to 8.41% and tumor weight in vivo declined by 90.4%. Similarly, Chen et al reported a PTT/ST bimodal nanomedicine by decreasing glucose uptake with diclofenac, a small drug targeting GLUT1.151
Apart from HK targeted by 2DG, PKM2 has also been considered a potential cancer-fighting target. Certain noncoding ribonucleic acids (RNAs), such as siRNAs and micro ribonucleic acids (microRNAs), have revealed the tremendous potential for cancer therapy by reducing the expression of target proteins.152–154 Interestingly, Dang et al developed an intelligent system named D-I/P@HSA NCs co-loading the photothermal agent indocyanine green (ICG) and siRNA against PKM2 (siPKM2) for PTT/ST synergistic therapy.155 Spherical helical polypeptides (DPPs) carrying ICG and siRNAs were prepared using amine-terminating polyamidoamine and N-carboxy anhydride. Guanidines and human serum albumin (HSA) were utilized as well for serum stability. Well-designed nanoparticles facilitated cell membrane penetration and endolysosomal escape for siRNA distribution. ATP levels fell because of the high effectiveness of PKM2 silencing at the messenger ribonucleic acid (mRNA) and protein levels. Therefore, HSPs, the bridge between ST and PTT, were repressed. MCF-7 cell apoptotic levels in vitro were 94.34%, and tumors in vivo were fully suppressed or even slightly ablated when exposed to comparable interactions as those described above.
Gas Therapy Based on Glucose Metabolism Intervention
Gas therapy appears to be an emerging and promising “green” therapeutic option due to its high efficacy, biosafety, and biocompatibility.156 It utilizes specific gases, such as nitric oxide (NO),157 carbon monoxide (CO),158,159 hydrogen sulfide (H2S),160 and sulfur dioxide (SO2),161 to complement other treatments. However, it is a challenge to transport gases to defined areas for poor solubility, diffusivity, and inaccurate release. Therefore, nanocarriers encapsulating gas releasing molecules (GRMs) for tumor targeting and stimuli-responsive release are desired.156 Gas release induced by starvation therapy products provides superb inspiration.
NO is able to react with ROS to produce extremely toxic peroxynitrites (ONOO−),162 inhibit P-gp expression,163 and maintain vascular homeostasis for oxygen supply.164 Certain nanosystems have been manufactured with the combination of GOx and L-arginine (L-Arg) serving as the GRM.165–167 A typical tumor targeting and TME-activatable nanomedicine known as GCAH was constructed by Fu et al (Figure 8).168 With spherical calcium phosphate (CaP) biomineralized on the foundation of GOx, GCAH carried L-Arg for ST-facilitated gas therapy. Due to the unique interactions between HA on the nanoparticles and glycoprotein CD44, GCAH could concentrate in tumor tissues overexpressing CD44. GOx and L-Arg were released as primary therapeutic agents and underwent the following two-step cascade reactions:
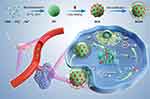 |
Figure 8 Schematic illustration of the preparation process of GCAH and its application for synergistic cancer ST/gas therapy. Reproduced with permission from: Fu LH, Li C, Yin W, et al. A Versatile Calcium Phosphate Nanogenerator for Tumor Microenvironment-activated Cancer Synergistic Therapy. Adv Healthc Mater. 2021;10(23):e2101563. doi:10.1002/adhm.202101563.198 Copyright 2021, Wiley-VCH. |
As a result, glucose was depleted, and NO was discharged for gas therapy. In vitro experiments showed that when the drug concentration reached 1.5μg/mL, the effect of starvation treatment alone could reduce the cell viability by about 50%. Additionally, NO was systematically produced for optimal response productivity. It converted the proangiogenic phenotype into vascular stabilization signals in addition to directly eradicating tumor cells. Thus, organically combining starvation therapy with GOx and NO-gas therapy has been illustrated to be a promising therapeutic strategy.
In addition to NO, CO has been proven to coordinate momentous biological effects as a homeostatic and cytoprotective molecule.168 Since stimuli-responsive CO-releasing molecules can be initiated by a high level of H2O2, it is promising to unite CO-based gas treatment with GOx-based ST. Manganese carbonyl (Mn2(CO)10, simplified as MnCO), a CO prodrug, was coupled with GOx in the platform of hollow mesoporous organosilica nanoparticles (HMONs).169 It was further decorated with arginine-glycine-aspartic acid (RGD) on the exterior for selectively recognizing tumor cells overexpressing αvβ3 integrin, allowing tumor-targeted delivery. The disulfide bonds in the spherical framework and the benzoic-imine covalent bonds between HMONs and GOx enabled the release of MnCO in the TME. These subtle designs guaranteed the spatiotemporal delivery of MnCO regardless of negative effects. Furthermore, the inherent effects of the nanocatalyst could reinforce therapeutic functions each other by chemical reactions listed below:
GOx was able to oxidize glucose to gluconic acid and H2O2, accelerating the production of CO from MnCO concurrently. Conversely, MnOx originating from MnCO supplied raw materials for further redox reactions. The ATP level was thence further reduced by the addition of MnCO. With the interaction between ST and ROS derived from damaged mitochondria impaired by CO, the apoptotic signaling pathway was notably upregulated. More interestingly, CO might block the respiratory chain as a complement to starvation therapy.170 Above all, these explorations exhibited a viable technique for optimizing the nanotherapeutic efficacy by targeting the microenvironment dedicatedly with a high concentration of H+, GSH, and glucose.
Other Treatments Based on Glucose Metabolism Intervention
Several metallic materials, such as silver nanoparticles (AgNPs), zinc oxide nanoparticles, and gold nanoparticles, have been proven to induce mitochondrial damage, oxidative stress, and apoptosis through their inherent cytotoxicity.171,172 Because of the accompaniment of the generation of gluconic acid and H2O2, GOx has been incorporated in nanosystems as an enhancer with AgNPs that generate Ag+ at high concentrations of H2O2 and low pH.173,174 Sun et al developed ZIF-8@GOx-AgNPs@MBN based on ZIF-8 MOF, co-delivering GOx and AgNPs.175 Owing to its acidic breakdown property, ZIF-8 crumbled to release cargos while generating Zn2+, realizing spatial tumor treatment. Zn2+, along with Ag+ generated from AgNPs, served as metal-ion therapy agents. In the absence of glucose, Ag+ and Zn2+ were able to kill cancer cells at a lower concentration, indicating that ST could induce increased sensitivity of tumor cells to metal-ion therapy. Notably, Zn2+ has been reported to inhibit glycolysis by inactivating GAPDH and decreasing NAD+.176 Zn2+-activating DNAzymes cleaving GLUT1 mRNA were loaded in zinc-rich ZIF-8 for synergistic treatment as well.
Yue et al developed a nanomedicine for synergistic GOx-based glucose starvation and gene therapy by integrating a hypoxia-activated plasmid (RTP801::p53) holding the post of a wild type p53 gene.177 In the nanodrug, GOx consumed oxygen to form a hypoxia environment and functioned as an inducer of gene therapy. To realize H2O2 and hypoxia dual activation, GOx was loaded in ferrocene (Fc)-capped Au nanoparticles and was released via the oxidation of Fc to ferrocenium (Fc+) in an H2O2-sufficient environment. Additionally, GOx-mediated glucose oxidation would speed up the release of GOx by H2O2 and further activate the gene integrated with the hypoxia-activatable promoter RTP801 by oxygen consumption. With the delicate architecture, the nanomedicine above upregulated p53 mRNA and protein. Due to H2O2-triggered drug release, GOx exhibited cytotoxicity to tumor cells, while did little on normal cells. Tumor cells underwent apoptosis and necrosis under ST and gene therapy.
Another nanoplatform generating ROS by Pt NPs under an alternating electric field termed electrodynamic therapy (EDT) was established by Lu et al.178 By combining with GOx, the efficiency of EDT could be further improved. Porous platinum nanospheres functioned as GOx carriers, catalysts for converting H2O2 to O2, and EDT agents. Significant therapeutic benefits could be detected with the combination of EDT and GOx-based ST augmented by the O2 generated through Pt catalysis. Overall, this research explored the wide range of applications of EDT as an emerging method to produce ROS without in situ O2 or H2O2.
Conclusion and Outlook
The finite pathways of tumor cells remodeling their metabolism make it possible to adopt a unified and simplified tumor treatment scheme. Besides, there are conclusive clues to prove that metabolic disorders play a significant role in resistance to conventional therapies, including chemotherapy and immunotherapy.179 Whether the resistance to carboplatin, 5-fluorouracil, or paclitaxel are all found to be related to glucose metabolism.180–182 As for immunotherapy, PKM2 enhances the expression of PD-L1 as well as the recruitment of myeloid-derived suppressor cells, which leads to the failure of immunotherapy.183,184 The metabolic intervention probably enhances the efficacy of traditional treatments or reinvigorates treatments that have been deemed ineffective. Therefore, starvation therapy has swiftly evolved into a potential complementary therapeutic approach for other therapies. Treatments targeting tumor-favored metabolites, such as the ketogenic diet, have grown in popularity in recent years.185 Plenty of drugs are being explored in clinical trials, not only alone but also in combination with certain therapies. For example, 2-DG has been combined with docetaxel in a Phase I clinical trial with promising results.186 What is more interesting, targeting the stronger glycolytic metabolism level and treatment-induced increased oxidative metabolism of cancer stem cells make it possible to alleviate cancer stem cell resistance.179
Regrettably, systemic starvation therapy itself remains defective and needs further exploration. The interconnected metabolic pathways also hinder the efficacy of starvation therapy.179 Furthermore, long-term systemic starvation therapy would lead to side effects like weight loss and cardiovascular diseases, which impedes its clinical application.187 Thence, spatiotemporal drug delivery with more precise therapeutic effects and combination with other therapeutic methods conceivably be the way forward.
Nanomaterial-assisted starvation therapy targeting glucose metabolism partly resolves the drawbacks of standard systemic drug delivery by improving targeting efficacy and precise drug release, avoiding harmful side effects, and maximizing the benefits of ST. It is even more valuable that targeting tumor-specific glucose metabolic pathways is more accurate and has less impact on other organs. Therefore, glucose metabolism has been targeted by biological enzymes, small compound molecules, or inorganic substances with enzyme-like effects. PDT, SDT, PTT, chemotherapy, and immunotherapy were combined with ST for interactive therapeutic effects and enhanced therapeutic outcomes. In this review, we reviewed the significant progress made, which covers monotherapy and multimodal therapeutic methods combined with ST. The design of nanomedicines and exquisite connections between these therapy options were focused on.
Nevertheless, nanomaterial-assisted starvation treatment is still in its infancy. There are still some potential challenges before entering clinical application. First, systematic investigations on the long-term biosafety of nanomedicines should be conducted. Although every study listed biosafety data, most of these studies only observed biosafety for a very short period,112 and the differences between rats and humans probably count a lot.188 Therefore, long-term toxicity, pharmacokinetics, and pharmacodynamics should be validated. Second, although several nanomedicines have demonstrated impressive therapeutic effects, the majority of them were based on complex structures synthesized through complicated multistep reactions, resulting in heterogeneity. And it is difficult to reproduce them for batch production. Fewer components, on the other hand, would diminish refinement and therapeutic efficacy. Introducing substances with diverse functions may be a solution.148 In general, the balance between the complexity and efficacy of nanodrugs is an urgent problem to be solved. Finally, the interaction between ST and other therapy approaches involves hypoxia, increasing acidity, and oxidative stress, all of which have been linked to a negative prognosis.189,190 Therefore, whether the factors attributed to tumorigenesis and development or the therapeutic effects of drugs are dominant needs to be evaluated.
There is still much to investigate when it comes to starvation therapy. Apart from glucose metabolism referred to in this review, cancer cells also enhance the metabolism of amino acids,191 fatty acids,192 and other necessary nutrients. However, molecules interrupting the metabolism of these nutrients are like virgin land in the field of nanomedicine possibly due to a lack of understanding of the mechanisms. Although certain nanoparticles have been reported to target nutrients other than glucose,193–195 more research is needed to fill the gap. Additionally, a combination of multiple drugs is the trend in starvation therapy. Thence, dual metabolic blockage and metabolic inhibition combined with other treatment methods are being investigated. It’s also promising to compensate for the lack of fasting by using nanomedicines with starvation therapy properties. The combination of nanodrugs targeting specific nutrients and milder dietary interventions possibly enhance therapeutic efficacy while reducing systemic toxicity. Moreover, metabolic reprogramming influences the function of immune cells in addition to tumor cells. Tumor endothelial cells also exhibit a high glucose metabolism phenotype to promote blood vessel growth.196 Tumor cells even obtain nutrients through crosstalk with cells in TME to ease metabolic stress.197 Regulation of cellular metabolism within the TME and modulation of the relationship between tumor cells and infiltrating immune cells are additional inspiring directions of exploration. Developing nanoparticles specifically targeting cells in TME might be a feasible method. With the increasingly clear role of nutrients in tumors, it can be predicted that more nanodrugs conducting ST will be developed and boost cancer treatment in the future.
Abbreviations
ST, starvation therapy; ATP, adenosine triphosphate; GOx, glucose oxidase; PTT, photothermal therapy; PDT, photodynamic therapy; CDT, chemodynamic therapy; SDT, sonodynamic therapy; H2O2, hydrogen peroxide; O2, oxygen; TME, tumor microenvironment; GLUT1, glucose transporter 1; PKM2, pyruvate kinase isozyme M2; HK, hexokinase; PFKFB3, 6-phosphofructo 2-kinase-fructose-2,6-biphosphatase 3; 2DG, 2-deoxy-d-glucose; siRNA, small interfering ribonucleic acid; PDA, polydopamine; MNs, microneedles; HA, hyaluronic acid; 3-MA, 3-methyladenine; BP, black phosphorus; PEG, polyethylene glycol; LDHA, lactate dehydrogenase A; MDR, multidrug resistance; DOX, doxorubicin; ROS, reactive oxygen species; P-gp, P-glycoprotein; TPZ, tirapazamine; AQ4N, banoxantrone dihydrochloride; DNA, deoxyribonucleic acid; GSH, glutathione; ICI, checkpoint inhibitor; TAM, tumor-associated macrophage; UCNP, upconversion nanosystem; Cys, cysteine; DC, dendritic cell; CTL, cytotoxic T cell; IL, interleukin; TNF-α, tumor necrosis factor-α; Treg, regulatory T cell; FDA, the US Food and Drug Administration; ICD, immunogenic cell death; DAMP, damage-associated molecular pattern; TAA, tumor-associated antigen; AuNP, gold nanoparticle; ASA, aspirin; 1O2, singlet oxygen; •OH, hydroxyl radical; O2•−, superoxide radical; Ce6, chlorene6; ZIF-8, zeolitic imidazolate framework-8; NIR, near-infrared; Pt NP, platinum nanoparticle; HSP, heat shock protein; OXPHOS, oxidative phosphorylation; RNA, ribonucleic acid; microRNA, micro ribonucleic acid; ICG, indocyanine green; siPKM2, siRNA against PKM2; DPP, spherical helical polypeptide; HSA, human serum albumin; mRNA, messenger ribonucleic acid; NO, nitric oxide; CO, carbon monoxide; H2S, hydrogen sulfide; SO2, sulfur dioxide; GRM, gas releasing molecule; ONOO−, peroxynitrites; L-Arg, L-arginine; CaP, calcium phosphate; HMON, hollow mesoporous organosilica nanoparticle; RGD, arginine-glycine-aspartic acid; AgNP, silver nanoparticle; Fc, ferrocene; EDT, electrodynamic therapy.
Funding
This work was supported by the National Natural Science Foundation of China (81602103 and 82172645), the Natural Science Foundation of Jiangsu Province (BK20200052), and Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (2021-LCYJ-MS-09 and 2021-LCYJ-PY-17).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA
3. Gotwals P, Cameron S, Cipolletta D, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17(5):286–301. doi:10.1038/nrc.2017.17
4. Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer. 2021;21(3):162–180. doi:10.1038/s41568-020-00320-2
5. Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211–218. doi:10.1016/j.tibs.2015.12.001
6. Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14(1):11–31. doi:10.1038/nrclinonc.2016.60
7. Warburg O. The metabolism of carcinoma cells. J Cancer Res. 1925;9(1):148–163. doi:10.1158/jcr.1925.148
8. Heiden MGV, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi:10.1126/science.1160809
9. Estrella V, Chen T, Lloyd M, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013;73(5):1524–1535. doi:10.1158/0008-5472.CAN-12-2796
10. Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17(4):351–359. doi:10.1038/ncb3124
11. Flaveny CA, Griffett K, El-Gendy Bel D, et al. Broad anti-tumor activity of a small molecule that selectively targets the Warburg effect and lipogenesis. Cancer Cell. 2015;28(1):42–56. doi:10.1016/j.ccell.2015.05.007
12. Marchiq I, Pouysségur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med. 2016;94(2):155–171. doi:10.1007/s00109-015-1307-x
13. Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12(11):829–846. doi:10.1038/nrd4145
14. Yang Z, Gao D, Cao Z, et al. Drug and gene co-delivery systems for cancer treatment. Biomater Sci. 2015;3(7):1035–1049. doi:10.1039/C4BM00369A
15. Kotov NA. Inorganic nanoparticles as protein mimics. Science. 2010;330(6001):188–189. doi:10.1126/science.1190094
16. Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. doi:10.1016/j.cell.2015.08.016
17. Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168(4):657–669. doi:10.1016/j.cell.2016.12.039
18. Wickens JM, Alsaab HO, Kesharwani P, et al. Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov Today. 2017;22(4):665–680. doi:10.1016/j.drudis.2016.12.009
19. Oroojalian F, Beygi M, Baradaran B, Mokhtarzadeh A, Shahbazi M-A. Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small. 2021;17(12):e2006484–e2006484. doi:10.1002/smll.202006484
20. Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi:10.1038/nrc.2016.108
21. Tay ZW, Chandrasekharan P, Chiu-Lam A, et al. Magnetic particle imaging-guided heating in vivo using gradient fields for arbitrary localization of magnetic hyperthermia therapy. ACS Nano. 2018;12(4):3699–3713. doi:10.1021/acsnano.8b00893
22. Huo D, Jiang X, Hu Y. Recent advances in nanostrategies capable of overcoming biological barriers for tumor management. Adv Mater. 2020;32:27.
23. Zhang L, Wang Z, Zhang Y, et al. Erythrocyte membrane cloaked metal-organic framework nanoparticle as biomimetic nanoreactor for starvation-activated colon cancer therapy. ACS Nano. 2018;12(10):10201–10211. doi:10.1021/acsnano.8b05200
24. Chang M, Hou Z, Wang M, Li C, Lin J. Recent advances in hyperthermia therapy-based synergistic immunotherapy. Adv Mater. 2020;33:2004788.
25. Chen D, Xu Q, Wang W, Shao J, Huang W, Dong X. Type I photosensitizers revitalizing photodynamic oncotherapy. Small. 2021;17:2006742.
26. Tang Z, Liu Y, He M, Bu W. Chemodynamic therapy: tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew Chem Int Ed Engl. 2019;58(4):946–956. doi:10.1002/anie.201805664
27. Liang S, Deng X, Ma P, Cheng Z, Lin J. Recent advances in nanomaterial-assisted combinational sonodynamic cancer therapy. Adv Mater. 2020;32(47):2003214. doi:10.1002/adma.202003214
28. Liang J-L, Luo G-F, Chen W-H, Zhang X-Z. Recent advances in engineered materials for immunotherapy-involved combination cancer therapy. Adv Mater. 2021;33(31):2007630. doi:10.1002/adma.202007630
29. Zhang X, He C, Chen Y, et al. Cyclic reactions-mediated self-supply of H2O2 and O2 for cooperative chemodynamic/starvation cancer therapy. Biomaterials. 2021;275:120987. doi:10.1016/j.biomaterials.2021.120987
30. Jiang W, Luo X, Wei L, et al. The sustainability of energy conversion inhibition for tumor ferroptosis therapy and chemotherapy. Small. 2021;17(38):2102695. doi:10.1002/smll.202102695
31. Dai Y, Sun Z, Zhao H, et al. NIR-II fluorescence imaging guided tumor-specific NIR-II photothermal therapy enhanced by starvation mediated thermal sensitization strategy. Biomaterials. 2021;275:120935. doi:10.1016/j.biomaterials.2021.120935
32. Bankar SB, Bule MV, Singhal RS, Ananthanarayan L. Glucose oxidase–an overview. Biotechnol Adv. 2009;27(4):489–501. doi:10.1016/j.biotechadv.2009.04.003
33. Ooi AT, Gomperts BN. Molecular pathways: targeting cellular energy metabolism in cancer via inhibition of SLC2A1 and LDHA. Clin Cancer Res. 2015;21(11):2440–2444. doi:10.1158/1078-0432.CCR-14-1209
34. Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34(2–3):121–138. doi:10.1016/j.mam.2012.07.001
35. Wang J, Ye C, Chen C, et al. Glucose transporter GLUT1 expression and clinical outcome in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8(10):16875–16886. doi:10.18632/oncotarget.15171
36. Akins NS, Nielson TC, Le HV. Inhibition of glycolysis and glutaminolysis: an emerging drug discovery approach to combat cancer. Curr Top Med Chem. 2018;18(6):494–504. doi:10.2174/1568026618666180523111351
37. Ciscato F, Ferrone L, Masgras I, Laquatra C, Rasola A. Hexokinase 2 in cancer: a prima donna playing multiple characters. Int J Mol Sci. 2021;22(9):4716. doi:10.3390/ijms22094716
38. Yang B, Ding L, Chen Y, Shi J. Augmenting tumor-starvation therapy by cancer cell autophagy inhibition. Adv Sci. 2020;7(6):1902847. doi:10.1002/advs.201902847
39. Hanafy NA, Dini L, Citti C, Cannazza G, Leporatti S. Inhibition of glycolysis by using a micro/nano-lipid bromopyruvic chitosan carrier as a promising tool to improve treatment of hepatocellular carcinoma. Nanomaterials. 2018;8(1):34. doi:10.3390/nano8010034
40. Shen J, Kim HC, Su H, et al. Cyclodextrin and polyethylenimine functionalized mesoporous silica nanoparticles for delivery of siRNA cancer therapeutics. Theranostics. 2014;4(5):487–497. doi:10.7150/thno.8263
41. Li YH, Li XF, Liu JT, et al. PKM2, a potential target for regulating cancer. Gene. 2018;668:48–53. doi:10.1016/j.gene.2018.05.038
42. Huang C, Zhu C, Chen J, et al. Nano-platelets as an oxygen regulator for augmenting starvation therapy against hypoxic tumor. Front Bioeng Biotechnol. 2020;8:571993. doi:10.3389/fbioe.2020.571993
43. Li J, Anraku Y, Kataoka K. Self-boosting catalytic nanoreactors integrated with triggerable crosslinking membrane networks for initiation of immunogenic cell death by pyroptosis. Angew Chem Int Ed Engl. 2020;59(32):13526–13530. doi:10.1002/anie.202004180
44. Dinda S, Sarkar S, Das PK. Glucose oxidase mediated targeted cancer-starving therapy by biotinylated self-assembled vesicles. Chem Commun. 2018;54(71):9929–9932. doi:10.1039/C8CC03599G
45. Zeng Y, Zhou H, Ding J, Zhou W. Cell membrane inspired nano-shell enabling long-acting Glucose Oxidase for Melanoma starvation therapy via microneedles-based percutaneous delivery. Theranostics. 2021;11(17):8270–8282. doi:10.7150/thno.60758
46. Fu LH, Qi C, Hu YR, Lin J, Huang P. Glucose oxidase-instructed multimodal synergistic cancer therapy. Adv Mater. 2019;31(21):e1808325. doi:10.1002/adma.201808325
47. Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol. 2018;20(9):1013–1022. doi:10.1038/s41556-018-0176-2
48. Wang X, Li Y, Deng X, et al. Colloidally stabilized DSPE-PEG-glucose/calcium phosphate hybrid nanocomposites for enhanced photodynamic cancer therapy via complementary mitochondrial Ca(2+) overload and autophagy inhibition. ACS Appl Mater Interfaces. 2021;13(33):39112–39125. doi:10.1021/acsami.1c11583
49. Jing M, Li Y, Wang M, et al. Photoresponsive PAMAM-assembled nanocarrier loaded with autophagy inhibitor for synergistic cancer therapy. Small. 2021;17(38):e2102295–e2102295. doi:10.1002/smll.202102295
50. Wu F, Liu Y, Cheng H, et al. Enhanced cancer starvation therapy based on glucose oxidase/3-methyladenine-loaded dendritic mesoporous organosilicon nanoparticles. Biomolecules. 2021;11(9):1363. doi:10.3390/biom11091363
51. Ralser M, Wamelink MM, Struys EA, et al. A catabolic block does not sufficiently explain how 2-deoxy-D-glucose inhibits cell growth. Proc Natl Acad Sci U S A. 2008;105(46):17807–17811. doi:10.1073/pnas.0803090105
52. Chan L, Chen X, Gao P, et al. Coordination-driven enhancement of radiosensitization by black phosphorus via regulating tumor metabolism. ACS Nano. 2021;15(2):3047–3060. doi:10.1021/acsnano.0c09454
53. Zhongqi F, Xiaodong S, Yuguo C, Guoyue L. Can combined therapy benefit immune checkpoint blockade response in hepatocellular carcinoma? Anticancer Agents Med Chem. 2019;19(2):222–228. doi:10.2174/1871520618666181114112431
54. Wei G., Wang Y, Yang G, Wang Y, Ju R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics. 2021; 11(13):6370-6392.
55. Pinedo HM, Giaccone G. Chemotherapy. Lancet 1997;349(Suppl 2):Sii7–Sii9. doi:10.1016/S0140-6736(97)90012-X
56. Pauwels EK, Erba P, Mariani G, Gomes CM. Multidrug resistance in cancer: its mechanism and its modulation. Drug News Perspect. 2007;20(6):371–377. doi:10.1358/dnp.2007.20.6.1141496
57. Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi:10.1016/j.ejpb.2015.03.018
58. Lee C, Raffaghello L, Brandhorst S, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4(124):124ra127–124ra127. doi:10.1126/scitranslmed.3003293
59. Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Nat Acad Sci. 2008;105(24):8215–8220. doi:10.1073/pnas.0708100105
60. Nencioni A, Caffa I, Cortellino S, Longo VD. Fasting and cancer: molecular mechanisms and clinical application. Nat Rev Cancer. 2018;18(11):707–719. doi:10.1038/s41568-018-0061-0
61. Lu R, Zhou L, Liu Q, et al. Skillfully collaborating chemosynthesis with GOx-enabled tumor survival microenvironment deteriorating strategy for amplified chemotherapy and enhanced tumor ablation. Biomater Sci. 2021;9(5):1855–1871. doi:10.1039/D0BM01950J
62. Fu LH, Wan Y, Qi C, et al. Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer therapy. Adv Mater. 2021;33(7):e2006892. doi:10.1002/adma.202006892
63. Fu LH, Hu YR, Qi C, et al. Biodegradable manganese-doped calcium phosphate nanotheranostics for traceable cascade reaction-enhanced anti-tumor therapy. ACS Nano. 2019;13(12):13985–13994. doi:10.1021/acsnano.9b05836
64. Yang C, Gao M, Zhao H, et al. A dual-functional biomimetic-mineralized nanoplatform for glucose detection and therapy with cancer cells in vitro. J Mater Chem B. 2021;9(18):3885–3891. doi:10.1039/D1TB00324K
65. Yang B, Chen Y, Shi J. Tumor-specific chemotherapy by nanomedicine-enabled differential stress sensitization. Angew Chem Int Ed Engl. 2020;59(24):9693–9701. doi:10.1002/anie.202002306
66. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13(10):714–726. doi:10.1038/nrc3599
67. Chen Y, Yao Y, Zhou X, et al. Cascade-reaction-based nanodrug for combined chemo/starvation/chemodynamic therapy against multidrug-resistant tumors. ACS Appl Mater Interfaces. 2019;11(49):46112–46123. doi:10.1021/acsami.9b15848
68. Tang Y, Ji Y, Yi C, et al. Self-accelerating H(2)O(2)-responsive plasmonic nanovesicles for synergistic chemo/starving therapy of tumors. Theranostics. 2020;10(19):8691–8704. doi:10.7150/thno.45392
69. Ma Y, Zhao Y, Bejjanki NK, et al. Nanoclustered cascaded enzymes for targeted tumor starvation and deoxygenation-activated chemotherapy without systemic toxicity. ACS Nano. 2019;13(8):8890–8902. doi:10.1021/acsnano.9b02466
70. Shan L, Fan W, Wang W, et al. Organosilica-based hollow mesoporous bilirubin nanoparticles for antioxidation-activated self-protection and tumor-specific deoxygenation-driven synergistic therapy. ACS Nano. 2019;13(8):8903–8916. doi:10.1021/acsnano.9b02477
71. Li J, Wei Z, Lin X, et al. Programmable therapeutic nanodevices with circular amplification of H(2) O(2) in the tumor microenvironment for synergistic cancer therapy. Adv Healthc Mater. 2019;8(10):e1801627. doi:10.1002/adhm.201801627
72. Zhang R, Feng L, Dong Z, et al. Glucose & oxygen exhausting liposomes for combined cancer starvation and hypoxia-activated therapy. Biomaterials. 2018;162:123–131. doi:10.1016/j.biomaterials.2018.02.004
73. Ren C, Liu H, Lv F, et al. Prodrug-based nanoreactors with tumor-specific in situ activation for multisynergistic cancer therapy. ACS Appl Mater Interfaces. 2020;12(31):34667–34677. doi:10.1021/acsami.0c09489
74. Cheng H, Jiang XY, Zheng RR, et al. A biomimetic cascade nanoreactor for tumor targeted starvation therapy-amplified chemotherapy. Biomaterials. 2019;195:75–85. doi:10.1016/j.biomaterials.2019.01.003
75. Liu X, Liu J, Meng C, et al. Pillar[6]arene-based supramolecular nanocatalysts for synergistically enhanced chemodynamic therapy by the intracellular cascade reaction. ACS Appl Mater Interfaces. 2021;13(45):53574–53585. doi:10.1021/acsami.1c15203
76. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175(2):313–326. doi:10.1016/j.cell.2018.09.035
77. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104. doi:10.3322/caac.21596
78. Thompson JA. New NCCN guidelines: recognition and management of immunotherapy-related toxicity. J Natl Compr Canc Netw. 2018;16(5s):594–596. doi:10.6004/jnccn.2018.0047
79. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi:10.1038/nrclinonc.2017.148
80. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542–e551. doi:10.1016/S1470-2045(16)30406-5
81. Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30(1):36–50. doi:10.1016/j.cmet.2019.06.001
82. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–382. doi:10.1038/s41577-019-0127-6
83. Chen L, Zhou L, Wang C, et al. Tumor-targeted drug and CpG delivery system for phototherapy and docetaxel-enhanced immunotherapy with polarization toward M1-type macrophages on triple negative breast cancers. Adv Mater. 2019;31(52):e1904997. doi:10.1002/adma.201904997
84. Zhang Y, Yang Y, Shi J, Wang L. A multimodal strategy of Fe(3)O(4)@ZIF-8/GOx@MnO(2) hybrid nanozyme via TME modulation for tumor therapy. Nanoscale. 2021;13(39):16571–16588. doi:10.1039/D1NR04196G
85. Shao Y, Wang Z, Hao Y, et al. Cascade catalytic nanoplatform based on “butterfly effect” for enhanced immunotherapy. Adv Healthc Mater. 2021;10(8):e2002171. doi:10.1002/adhm.202002171
86. Wang M, Chang M, Li C, et al. Tumor-microenvironment-activated reactive oxygen species amplifier for enzymatic cascade cancer starvation/chemodynamic /immunotherapy. Adv Mater. 2021;34(4):e2106010. doi:10.1002/adma.202106010
87. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi:10.1038/nri.2016.107
88. Garg AD, Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280(1):126–148. doi:10.1111/imr.12574
89. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31(1):51–72. doi:10.1146/annurev-immunol-032712-100008
90. Sun K, Hu J, Meng X, et al. Reinforcing the induction of immunogenic cell death via artificial engineered cascade bioreactor-enhanced chemo-immunotherapy for optimizing cancer immunotherapy. Small. 2021;17(37):e2101897–e2101897. doi:10.1002/smll.202101897
91. Yang B, Chen Y, Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem Rev. 2019;119(8):4881–4985. doi:10.1021/acs.chemrev.8b00626
92. Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9(12):674–687. doi:10.1038/nrclinonc.2012.171
93. Wang Z, Gong X, Li J, et al. Oxygen-delivering polyfluorocarbon nanovehicles improve tumor oxygenation and potentiate photodynamic-mediated antitumor immunity. ACS Nano. 2021;15:5405–5419.
94. Yang C, Liu Y, Su S, Gao N, Jing J, Zhang X. A multifunctional oxygen-producing MnO(2)-based nanoplatform for tumor microenvironment-activated imaging and combination therapy in vitro. J Mater Chem B. 2020;8(43):9943–9950. doi:10.1039/D0TB00529K
95. Liu P, Zhou Y, Shi X, et al. A cyclic nano-reactor achieving enhanced photodynamic tumor therapy by reversing multiple resistances. J Nanobiotechnol. 2021;19(1):149. doi:10.1186/s12951-021-00893-6
96. Pan W, Ge Y, Yu Z, et al. A cancer cell membrane-encapsulated MnO(2) nanoreactor for combined photodynamic-starvation therapy. Chem Commun. 2019;55(35):5115–5118. doi:10.1039/C9CC01386E
97. Yang X, Yang Y, Gao F, Wei JJ, Qian CG, Sun MJ. Biomimetic hybrid nanozymes with self-supplied H(+) and accelerated O(2) generation for enhanced starvation and photodynamic therapy against hypoxic tumors. Nano Lett. 2019;19(7):4334–4342. doi:10.1021/acs.nanolett.9b00934
98. Zhou Y, Niu B, Zhao Y, et al. Multifunctional nanoreactors-integrated microneedles for cascade reaction-enhanced cancer therapy. J Control Release. 2021;339:335–349. doi:10.1016/j.jconrel.2021.09.041
99. Wan X, Zhang H, Pan W, Li N, Tang B. An enzyme nanopocket based on covalent organic frameworks for long-term starvation therapy and enhanced photodynamic therapy of cancer. Chem Commun. 2021;57(44):5402–5405. doi:10.1039/D0CC07544B
100. Li SY, Cheng H, Xie BR, et al. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano. 2017;11(7):7006–7018. doi:10.1021/acsnano.7b02533
101. Liu S, Yan T, Sun J, et al. Biomimetic cascade polymer nanoreactors for starvation and photodynamic cancer therapy. Molecules. 2021;26(18):5609.
102. Zhang L, Yang Z, He W, Ren J, Wong CY. One-pot synthesis of a self-reinforcing cascade bioreactor for combined photodynamic/chemodynamic/starvation therapy. J Colloid Interface Sci. 2021;599:543–555. doi:10.1016/j.jcis.2021.03.173
103. Zhou Z, Song J, Nie L, Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem Soc Rev. 2016;45(23):6597–6626. doi:10.1039/c6cs00271d
104. Yu Z, Zhou P, Pan W, Li N, Tang B. A biomimetic nanoreactor for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat Commun. 2018;9(1):5044. doi:10.1038/s41467-018-07197-8
105. Yin SY, Liu W, Yang J, Li J. Synergistically enhanced multienzyme catalytic nanoconjugates for efficient cancer therapy. J Mater Chem B. 2021;9(29):5877–5886. doi:10.1039/D1TB00821H
106. Wang H, Wang Z, Tu Y, et al. Homotypic targeting upconversion nano-reactor for cascade cancer starvation and deep-tissue phototherapy. Biomaterials. 2020;235:119765. doi:10.1016/j.biomaterials.2020.119765
107. Lin LS, Song J, Song L, et al. Simultaneous Fenton-like ion delivery and glutathione depletion by MnO(2) -based nanoagent to enhance chemodynamic therapy. Angew Chem Int Ed Engl. 2018;57(18):4902–4906. doi:10.1002/smll.202001518
108. Augustine R, Kalva N, Kim HA, Zhang Y, Kim I. pH-responsive polypeptide-based smart nano-carriers for theranostic applications. Molecules. 2019;24(16):2961. doi:10.3390/molecules24162961
109. Zhang H, Lu F, Pan W, et al. A dual-catalytic nanoreactor for synergistic chemodynamic-starvation therapy toward tumor metastasis suppression. Biomater Sci. 2021;9(10):3814–3820. doi:10.1039/D1BM00240F
110. Wan X, Song L, Pan W, Zhong H, Li N, Tang B. Tumor-targeted cascade nanoreactor based on metal-organic frameworks for synergistic ferroptosis-starvation anticancer therapy. ACS Nano. 2020;14(9):11017–11028. doi:10.1021/acsnano.9b07789
111. Zhang L, Wan SS, Li CX, Xu L, Cheng H, Zhang XZ. An adenosine triphosphate-responsive autocatalytic Fenton nanoparticle for tumor ablation with self-supplied H(2)O(2) and acceleration of Fe(III)/Fe(II) conversion. Nano Lett. 2018;18(12):7609–7618. doi:10.1021/acs.nanolett.8b03178
112. Huo M, Wang L, Chen Y, Shi J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat Commun. 2017;8(1):357. doi:10.1038/s41467-017-00424-8
113. Yuan P, Dou G, Liu T, et al. On-demand manipulation of tumorigenic microenvironments by nano-modulator for synergistic tumor therapy. Biomaterials. 2021;275:120956. doi:10.1016/j.biomaterials.2021.120956
114. Singh P, Youden B, Yang Y, et al. Synergistic multimodal cancer therapy using glucose Oxidase@CuS nanocomposites. ACS Appl Mater Interfaces. 2021;13(35):41464–41472. doi:10.1021/acsami.1c12235
115. Ying W, Zhang Y, Gao W, et al. Hollow magnetic nanocatalysts drive starvation-chemodynamic-hyperthermia synergistic therapy for tumor. ACS Nano. 2020;14(8):9662–9674. doi:10.1021/acsnano.0c00910
116. Yang C, Younis MR, Zhang J, Qu J, Lin J, Huang P. Programmable NIR-II photothermal-enhanced starvation-primed chemodynamic therapy using glucose oxidase-functionalized ancient pigment nanosheets. Small. 2020;16(25):e2001518.
117. Hu C, Wang J, Liu S, et al. Urchin-shaped metal organic/hydrogen-bonded framework nanocomposite as a multifunctional nanoreactor for catalysis-enhanced synergetic therapy. ACS Appl Mater Interfaces. 2021;13(4):4825–4834. doi:10.1021/acsami.0c19584
118. Zhu P, Luo W, Qian J, et al. GSH/ROS dual-responsive supramolecular nanoparticles based on Pillar[6]arene and betulinic acid prodrug for chemo-chemodynamic combination therapy. Molecules. 2021;26(19):5900. doi:10.3390/molecules26195900
119. Peng H, Qin YT, Feng YS, He XW, Li WY, Zhang YK. Phosphate-degradable nanoparticles based on metal-organic frameworks for chemo-starvation-chemodynamic synergistic antitumor therapy. ACS Appl Mater Interfaces. 2021;13(31):37713–37723. doi:10.1021/acsami.1c10816
120. Guo Y, Jia HR, Zhang X, et al. A glucose/oxygen-exhausting nanoreactor for starvation- and hypoxia-activated sustainable and cascade chemo-chemodynamic therapy. Small. 2020;16(31):e2000897. doi:10.1002/smll.202000897
121. Di Ianni T, Bose RJC, Sukumar UK, et al. Ultrasound/microbubble-mediated targeted delivery of anticancer microRNA-loaded nanoparticles to deep tissues in pigs. J Control Release. 2019;309:1–10. doi:10.1016/j.jconrel.2019.07.024
122. Zhang R, Zhang L, Ran H, et al. A mitochondria-targeted anticancer nanoplatform with deep penetration for enhanced synergistic sonodynamic and starvation therapy. Biomater Sci. 2020;8(16):4581–4594. doi:10.1039/D0BM00408A
123. Wang J, Huang J, Zhou W, et al. Hypoxia modulation by dual-drug nanoparticles for enhanced synergistic sonodynamic and starvation therapy. J Nanobiotechnol. 2021;19(1):87. doi:10.1186/s12951-021-00837-0
124. Wen M, Shen J, Wang Z, et al. A cascaded enzyme-loaded Fe-hemoporfin framework for synergistic sonodynamic-starvation therapy of tumors. Nanoscale. 2021;13(11):5910–5920. doi:10.1039/D0NR08508A
125. Wu W, Pu Y, Lin H, Yao H, Shi J. Starvation-sensitized and oxygenation-promoted tumor sonodynamic therapy by a cascade enzymatic approach. Research. 2021;2021:9769867. doi:10.34133/2021/9769867
126. Bao Y, Chen J, Qiu H, et al. Erythrocyte membrane-camouflaged PCN-224 nanocarriers integrated with platinum nanoparticles and glucose oxidase for enhanced tumor sonodynamic therapy and synergistic starvation therapy. ACS Appl Mater Interfaces. 2021;13(21):24532–24542. doi:10.1021/acsami.1c05644
127. Huang X, Lu Y, Guo M, Du S, Han N. Recent strategies for nano-based PTT combined with immunotherapy: from a biomaterial point of view. Theranostics. 2021;11(15):7546–7569. doi:10.7150/thno.56482
128. Oei AL, Vriend LE, Crezee J, Franken NA, Krawczyk PM. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol. 2015;10:165. doi:10.1186/s13014-015-0462-0
129. Zhu X, Feng W, Chang J, et al. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat Commun. 2016;7:1.
130. Wang X, Chen M, Zhou J, Zhang X. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review). Int J Oncol. 2014;45(1):18–30. doi:10.3892/ijo.2014.2399
131. Talaei S, Mellatyar H, Asadi A, Akbarzadeh A, Sheervalilou R, Zarghami N. Spotlight on 17-AAG as an Hsp90 inhibitor for molecular targeted cancer treatment. Chem Biol Drug Des. 2019;93(5):760–786. doi:10.1111/cbdd.13486
132. Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–549. doi:10.1038/nrc2887
133. Zhu H, Li Y, Ming Z, Liu W. Glucose oxidase-mediated tumor starvation therapy combined with photothermal therapy for colon cancer. Biomater Sci. 2021;9(16):5577–5587. doi:10.1039/D1BM00869B
134. Shao L, Li Y, Huang F, et al. Complementary autophagy inhibition and glucose metabolism with rattle-structured polydopamine@mesoporous silica nanoparticles for augmented low-temperature photothermal therapy and in vivo photoacoustic imaging. Theranostics. 2020;10(16):7273–7286. doi:10.7150/thno.44668
135. Ren J, Zhang L, Zhang J, et al. Light-activated oxygen self-supplied starving therapy in near-infrared (NIR) window and adjuvant hyperthermia-induced tumor ablation with an augmented sensitivity. Biomaterials. 2020;234:119771. doi:10.1016/j.biomaterials.2020.119771
136. He T, Xu H, Zhang Y, et al. Glucose oxidase-instructed traceable self-oxygenation/hyperthermia dually enhanced cancer starvation therapy. Theranostics. 2020;10(4):1544–1554. doi:10.7150/thno.40439
137. Hu JJ, Liu MD, Gao F, et al. Photo-controlled liquid metal nanoparticle-enzyme for starvation/photothermal therapy of tumor by win-win cooperation. Biomaterials. 2019;217:119303. doi:10.1016/j.biomaterials.2019.119303
138. Li S, Lin K, Hu P, et al. A multifunctional nanoamplifier with self-enhanced acidity and hypoxia relief for combined photothermal/photodynamic/starvation therapy. Int J Pharm. 2021;611:121307. doi:10.1016/j.ijpharm.2021.121307
139. Wang Y, Wang B, Zhang L, et al. Mitochondria-targeted nanospheres with deep tumor penetration for photo/starvation therapy. J Mater Chem B. 2020;8(34):7740–7754. doi:10.1039/D0TB00001A
140. Cao J, Qiao B, Luo Y, et al. A multimodal imaging-guided nanoreactor for cooperative combination of tumor starvation and multiple mechanism-enhanced mild temperature phototherapy. Biomater Sci. 2020;8(23):6561–6578. doi:10.1039/D0BM01350A
141. Zhao L, Yang Q, Guo W, et al. Non-stoichiometric cobalt sulfide nanodots enhance photothermal and chemodynamic therapies against solid tumor. J Colloid Interface Sci. 2021;600:390–402. doi:10.1016/j.jcis.2021.05.058
142. Tian F, Zhong X, Zhao J, et al. Hybrid theranostic microbubbles for ultrasound/photoacoustic imaging guided starvation/low-temperature photothermal/hypoxia-activated synergistic cancer therapy. J Mater Chem B. 2021;9(45):9358–9369. doi:10.1039/D1TB01735G
143. Shubhra QTH, Guo K, Liu Y, Razzak M, Serajum Manir M, Moshiul Alam AKM. Dual targeting smart drug delivery system for multimodal synergistic combination cancer therapy with reduced cardiotoxicity. Acta Biomater. 2021;131:493–507. doi:10.1016/j.actbio.2021.06.016
144. Lu J, Liu F, Li H, Xu Y, Sun S. Width-consistent mesoporous silica nanorods with a precisely controlled aspect ratio for lysosome dysfunctional synergistic chemotherapy/photothermal therapy/starvation therapy/oxidative therapy. ACS Appl Mater Interfaces. 2020;12(22):24611–24622. doi:10.1021/acsami.0c06117
145. Su Y, Zhang X, Lei L, Liu B, Wu S, Shen J. Tumor microenvironment-activatable cyclic cascade reaction to reinforce multimodal combination therapy by destroying the extracellular matrix. ACS Appl Mater Interfaces. 2021;13(11):12960–12971. doi:10.1021/acsami.1c02011
146. Li Z, Rong L. A homotypic membrane-camouflaged biomimetic nanoplatform with gold nanocrystals for synergistic photothermal/starvation/immunotherapy. ACS Appl Mater Interfaces. 2021;13(20):23469–23480. doi:10.1021/acsami.1c04305
147. Cheng X, Hao Z, Chu S, et al. Plasmonic enhanced enzyme activity by catalytic cascade induced mutual benefit tumor starvation/immune/photothermal therapy. Biomater Sci. 2021;9(18):6116–6125. doi:10.1039/D1BM00551K
148. Wang M, Wang D, Chen Q, Li C, Li Z, Lin J. Recent advances in glucose-oxidase-based nanocomposites for tumor therapy. Small. 2019;15(51):e1903895. doi:10.1002/smll.201903895
149. Varghese E, Samuel SM, Líšková A, Samec M, Kubatka P, Büsselberg D. Targeting glucose metabolism to overcome resistance to anticancer chemotherapy in breast cancer. Cancers. 2020;12(8):2252. doi:10.3390/cancers12082252
150. Zhang D, Li J, Wang F, Hu J, Wang S, Sun Y. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014;355(2):176–183. doi:10.1016/j.canlet.2014.09.003
151. Chen WH, Luo GF, Lei Q, et al. Overcoming the heat endurance of tumor cells by interfering with the anaerobic glycolysis metabolism for improved photothermal therapy. ACS Nano. 2017;11(2):1419–1431. doi:10.1021/acsnano.6b06658
152. Lin YX, Wang Y, Blake S, et al. RNA nanotechnology-mediated cancer immunotherapy. Theranostics. 2020;10(1):281–299. doi:10.7150/thno.35568
153. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi:10.1016/j.cell.2009.01.002
154. Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15(2):188–200. doi:10.1101/gad.862301
155. Dang J, Ye H, Li Y, Liang Q, Li X, Yin L. Multivalency-assisted membrane-penetrating siRNA delivery sensitizes photothermal ablation via inhibition of tumor glycolysis metabolism. Biomaterials. 2019;223:119463. doi:10.1016/j.biomaterials.2019.119463
156. Jin D, Zhang J, Huang Y, et al. Recent advances in the development of metal-organic framework-based gas-releasing nanoplatforms for synergistic cancer therapy. Dalton Trans. 2021;50(4):1189–1196. doi:10.1039/D0DT03767B
157. Xu Y, Wang S, Chen Z, et al. Nitric oxide release activated near-infrared photothermal agent for synergistic tumor treatment. Biomaterials. 2021;276:121017. doi:10.1016/j.biomaterials.2021.121017
158. Zhu D, Liu Z, Li Y, Huang Q, Xia L, Li K. Delivery of manganese carbonyl to the tumor microenvironment using tumor-derived exosomes for cancer gas therapy and low dose radiotherapy. Biomaterials. 2021;274:120894. doi:10.1016/j.biomaterials.2021.120894
159. Wang M, Hou Z, Liu S, et al. A multifunctional nanovaccine based on l-arginine-loaded black mesoporous titania: ultrasound-triggered synergistic cancer sonodynamic therapy/gas therapy/immunotherapy with remarkably enhanced efficacy. Small. 2021;17(6):e2005728–e2005728. doi:10.1002/smll.202005728
160. Liu B, Liang S, Wang Z, et al. A Tumor-Microenvironment-Responsive Nanocomposite for Hydrogen Sulfide Gas and Trimodal-Enhanced Enzyme Dynamic Therapy. Adv Mater. 2021;33:2101223.
161. Yue L, Yang K, Li J, Cheng Q, Wang R. Self-propelled asymmetrical nanomotor for self-reported gas therapy. Small. 2021;17(34):e2102286–e2102286. doi:10.1002/smll.202102286
162. Xiang HJ, Deng Q, An L, Guo M, Yang SP, Liu JG. Tumor cell specific and lysosome-targeted delivery of nitric oxide for enhanced photodynamic therapy triggered by 808 nm near-infrared light. Chem Commun. 2016;52(1):148–151. doi:10.1039/C5CC07006F
163. Kim J, Yung BC, Kim WJ, Chen X. Combination of nitric oxide and drug delivery systems: tools for overcoming drug resistance in chemotherapy. J Control Release. 2017;263:223–230. doi:10.1016/j.jconrel.2016.12.026
164. Chen Y, Li Z-H, Pan P, Zeng R-Y, Zhang X-Z. Tumor-specific ONOO- nanogenerator for improved drug delivery and enhanced chemotherapy of tumor. ACS Nano. 2021;15:11514–11525.
165. Zhai M, Gong P, Li H, et al. Metastable interface biomimetic synthesis of a smart nanosystem for enhanced starvation/gas therapy. J Colloid Interface Sci. 2021;599:149–157. doi:10.1016/j.jcis.2021.04.042
166. Peng J, Gong P, Song S, et al. Biomineralized synthesis of a smart O(2)-regenerating nanoreactor for highly efficient starvation/gas therapy. Mater Sci Eng C Mater Biol Appl. 2021;126:112132. doi:10.1016/j.msec.2021.112132
167. Fan W, Lu N, Huang P, et al. Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving-like/gas therapy. Angew Chem Int Ed Engl. 2017;56(5):1229–1233. doi:10.1002/anie.201610682
168. Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9(9):728–743. doi:10.1038/nrd3228
169. Wu J, Meng Z, Exner AA, et al. Biodegradable cascade nanocatalysts enable tumor-microenvironment remodeling for controllable CO release and targeted/synergistic cancer nanotherapy. Biomaterials. 2021;276:121001. doi:10.1016/j.biomaterials.2021.121001
170. Guan Q, Zhou LL, Li YA, Dong YB. A nanoscale metal-organic framework for combined photodynamic and starvation therapy in treating breast tumors. Chem Commun. 2019;55(99):14898–14901. doi:10.1039/C9CC07510K
171. Soenen SJ, Parak WJ, Rejman J, Manshian B. (Intra)cellular stability of inorganic nanoparticles: effects on cytotoxicity, particle functionality, and biomedical applications. Chem Rev. 2015;115(5):2109–2135. doi:10.1021/cr400714j
172. Gurunathan S, Han JW, Eppakayala V, Jeyaraj M, Kim JH. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed Res Int. 2013;2013:535796. doi:10.1155/2013/535796
173. Zhang Y, Li Y, Gao Z, et al. Mesoporous silica-coated silver nanoframes as drug-delivery vehicles for chemo/starvation/metal ion multimodality therapy. Langmuir. 2020;36(23):6345–6351. doi:10.1021/acs.langmuir.0c00191
174. Zhang YF, Yang YC, Jiang SS, et al. Degradable silver-based nanoplatform for synergistic cancer starving-like/metal ion therapy. Mater Horizons. 2019;6(1):169–175. doi:10.1039/C8MH00908B
175. Sun D, Qi G, Ma K, et al. Tumor microenvironment-activated degradable multifunctional nanoreactor for synergistic cancer therapy and glucose SERS feedback. iScience. 2020;23(7):101274. doi:10.1016/j.isci.2020.101274
176. Wu S, Zhang K, Liang Y, et al. Nano-enabled tumor systematic energy exhaustion via zinc (II) interference mediated glycolysis inhibition and specific GLUT1 depletion. Adv Sci. 2021;9(7):e2103534. doi:10.1002/advs.202103534
177. Yue L, Sun T, Yang K, et al. Supramolecular nanovesicles for synergistic glucose starvation and hypoxia-activated gene therapy of cancer. Nanoscale. 2021;13(21):9570–9576. doi:10.1039/D1NR02159A
178. Lu Z, Gao J, Fang C, Zhou Y, Li X, Han G. Porous Pt nanospheres incorporated with GOx to enable synergistic oxygen-inductive starvation/electrodynamic tumor therapy. Adv Sci. 2020;7(17):2001223. doi:10.1002/advs.202001223
179. Li J, Eu JQ, Kong LR, et al. Targeting metabolism in cancer cells and the tumour microenvironment for cancer therapy. Molecules. 2020;25(20):4831. doi:10.3390/molecules25204831
180. Liu Y, He C, Huang X. Metformin partially reverses the carboplatin-resistance in NSCLC by inhibiting glucose metabolism. Oncotarget. 2017;8(43):75206–75216. doi:10.18632/oncotarget.20663
181. Sun H, Zhu A, Zhou X, Wang F. Suppression of pyruvate dehydrogenase kinase-2 re-sensitizes paclitaxel-resistant human lung cancer cells to paclitaxel. Oncotarget. 2017;8(32):52642–52650. doi:10.18632/oncotarget.16991
182. Shin YK, Yoo BC, Hong YS, et al. Upregulation of glycolytic enzymes in proteins secreted from human colon cancer cells with 5-fluorouracil resistance. Electrophoresis. 2009;30(12):2182–2192. doi:10.1002/elps.200800806
183. Palsson-McDermott EM, Dyck L, Zasłona Z, et al. Pyruvate kinase M2 is required for the expression of the immune checkpoint PD-L1 in immune cells and tumors. Front Immunol. 2017;8:1300. doi:10.3389/fimmu.2017.01300
184. Liu WR, Tian MX, Yang LX, et al. PKM2 promotes metastasis by recruiting myeloid-derived suppressor cells and indicates poor prognosis for hepatocellular carcinoma. Oncotarget. 2015;6(2):846–861. doi:10.18632/oncotarget.2749
185. Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer - where do we stand? Mol Metab. 2020;33:102–121. doi:10.1016/j.molmet.2019.06.026
186. Raez LE, Papadopoulos K, Ricart AD, et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71(2):523–530. doi:10.1007/s00280-012-2045-1
187. Lien EC, Westermark AM, Zhang Y, et al. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature. 2021;599(7884):302–307. doi:10.1038/s41586-021-04049-2
188. Cheon DJ, Orsulic S. Mouse models of cancer. Annu Rev Pathol. 2011;6(1):95–119. doi:10.1146/annurev.pathol.3.121806.154244
189. Jing X, Yang F, Shao C, et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18(1):157. doi:10.1186/s12943-019-1089-9
190. Lee JD, Cai Q, Shu XO, Nechuta SJ. The role of biomarkers of oxidative stress in breast cancer risk and prognosis: a systematic review of the epidemiologic literature. J Women's Health. 2017;26(5):467–482. doi:10.1089/jwh.2016.5973
191. Tsun Z-Y, Possemato R. Amino acid management in cancer. Semin Cell Dev Biol. 2015;43:22–32. doi:10.1016/j.semcdb.2015.08.002
192. Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. 2018;38(1):27. doi:10.1186/s40880-018-0301-4
193. Chen QW, Wang JW, Wang XN, et al. Inhibition of tumor progression through the coupling of bacterial respiration with tumor metabolism. Angew Chem Int Ed Engl. 2020;59(48):21562–21570. doi:10.1002/anie.202002649
194. Kou L, Jiang X, Tang Y, et al. Resetting amino acid metabolism of cancer cells by ATB(0,+)-targeted nanoparticles for enhanced anticancer therapy. Bioact Mater. 2022;9:15–28. doi:10.1016/j.bioactmat.2021.07.009
195. Cao S, Saw PE, Shen Q, Li R, Liu Y, Xu X. Reduction-responsive RNAi nanoplatform to reprogram tumor lipid metabolism and repolarize macrophage for combination pancreatic cancer therapy. Biomaterials. 2021;280:121264. doi:10.1016/j.biomaterials.2021.121264
196. De Bock K, Georgiadou M, Schoors S, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154(3):651–663. doi:10.1016/j.cell.2013.06.037
197. Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21(10):669–680. doi:10.1038/s41568-021-00378-6
198. Fu LH, Li C, Yin W, et al. A versatile calcium phosphate nanogenerator for tumor microenvironment-activated cancer synergistic therapy. Adv Healthc Mater. 2021;10(23):e2101563. doi:10.1002/adhm.202101563
199. Zhang YH, Qiu WX, Zhang M, Zhang L, Zhang XZ. MnO(2) motor: a prospective cancer-starving therapy promoter. ACS Appl Mater Interfaces. 2018;10(17):15030–15039. doi:10.1021/acsami.8b01818
200. Wen H, Fei Y, Cai R, et al. Tumor-activatable biomineralized nanotherapeutics for integrative glucose starvation and sensitized metformin therapy. Biomaterials. 2021;278:121165. doi:10.1016/j.biomaterials.2021.121165
201. Liu X, Liu J, Chen S, et al. Dual-path modulation of hydrogen peroxide to ameliorate hypoxia for enhancing photodynamic/starvation synergistic therapy. J Mater Chem B. 2020;8(43):9933–9942. doi:10.1039/D0TB01556C
202. Liu B, Wang Z, Li T, et al. Rapid decomposition and catalytic cascade nanoplatforms based on enzymes and Mn-etched dendritic mesoporous silicon for MRI-guided synergistic therapy. ACS Appl Mater Interfaces. 2020;12(41):45772–45788. doi:10.1021/acsami.0c12580
203. Zhu Y, Shi H, Li T, et al. A dual functional nanoreactor for synergistic starvation and photodynamic therapy. ACS Appl Mater Interfaces. 2020;12(16):18309–18318. doi:10.1021/acsami.0c01039
204. Ranji-Burachaloo H, Reyhani A, Gurr PA, Dunstan DE, Qiao GG. Combined Fenton and starvation therapies using hemoglobin and glucose oxidase. Nanoscale. 2019;11(12):5705–5716. doi:10.1039/C8NR09107B
205. Yao Z, Zhang B, Liang T, Ding J, Min Q, Zhu JJ. Promoting oxidative stress in cancer starvation therapy by site-specific startup of hyaluronic acid-enveloped dual-catalytic nanoreactors. ACS Appl Mater Interfaces. 2019;11(21):18995–19005. doi:10.1021/acsami.9b06034
206. Ming J, Zhu T, Yang W, et al. Pd@Pt-GOx/HA as a novel enzymatic cascade nanoreactor for high-efficiency starving-enhanced chemodynamic cancer therapy. ACS Appl Mater Interfaces. 2020;12(46):51249–51262. doi:10.1021/acsami.0c15211
207. Wang Y, Song M. pH-responsive cascaded nanocatalyst for synergistic like-starvation and chemodynamic therapy. Colloids Surf B Biointerfaces. 2020;192:111029. doi:10.1016/j.colsurfb.2020.111029
208. Wang Z, Liu B, Sun Q, et al. Fusiform-like copper(II)-based metal-organic framework through relief hypoxia and GSH-depletion co-enhanced starvation and chemodynamic synergetic cancer therapy. ACS Appl Mater Interfaces. 2020;12(15):17254–17267. doi:10.1021/acsami.0c01539
209. Hu Y, Wang K, Ye C. “Four-in-one” nanozyme and natural enzyme symbiotic system of Cu2-xSe-GOx for cervical cancer therapy. Chemistry. 2021;28:e202102885.
210. Kou Y, Dai Z, Cui P, et al. A flowerlike FePt/MnO(2)/GOx-based cascade nanoreactor with sustainable O(2) supply for synergistic starvation-chemodynamic anticancer therapy. J Mater Chem B. 2021;9(40):8480–8490. doi:10.1039/D1TB01539G
211. Meng X, Zhang F, Guo H, et al. One-pot approach to Fe(2+) /Fe(3+) -based MOFs with enhanced catalytic activity for Fenton reaction. Adv Healthc Mater. 2021;10(19):e2100780. doi:10.1002/adhm.202100780
212. Zhou J, Li M, Hou Y, et al. Engineering of a nanosized biocatalyst for combined tumor starvation and low-temperature photothermal therapy. ACS Nano. 2018;12(3):2858–2872. doi:10.1021/acsnano.8b00309
213. Gao P, Shi M, Wei R, et al. A biomimetic MOF nanoreactor enables synergistic suppression of intracellular defense systems for augmented tumor ablation. Chem Commun. 2020;56(6):924–927. doi:10.1039/C9CC08498C
214. Zhang MK, Li CX, Wang SB, et al. Tumor starvation induced spatiotemporal control over chemotherapy for synergistic therapy. Small. 2018;14(50):e1803602. doi:10.1002/smll.201803602
215. Hang L, Zhang T, Wen H, et al. Rational design of non-toxic GOx-based biocatalytic nanoreactor for multimodal synergistic therapy and tumor metastasis suppression. Theranostics. 2021;11(20):10001–10011. doi:10.7150/thno.65399
216. Du X, Zhang T, Ma G, Gu X, Wang G, Li J. Glucose-responsive mesoporous silica nanoparticles to generation of hydrogen peroxide for synergistic cancer starvation and chemistry therapy. Int J Nanomedicine. 2019;14:2233–2251. doi:10.2147/IJN.S195900
217. Song S, Peng J, Wu Y, et al. Biomimetic synthesis of a novel O(2)-regeneration nanosystem for enhanced starvation/chemo-therapy. Nanotechnology. 2021;33(2):025102.
218. Wang Y, Liu Z, Wang H, et al. Starvation-amplified CO generation for enhanced cancer therapy via an erythrocyte membrane-biomimetic gas nanofactory. Acta Biomater. 2019;92:241–253. doi:10.1016/j.actbio.2019.05.009
219. Yu J, He X, Wang Z, et al. Combination of starvation therapy and Pt-NP based chemotherapy for synergistic cancer treatment. J Mater Chem B. 2021;9(32):6406–6411. doi:10.1039/D1TB01222C
220. Gao W, Wei S, Li Z, et al. Nano magnetic liposomes-encapsulated parthenolide and glucose oxidase for ultra-efficient synergistic antitumor therapy. Nanotechnology. 2020;31(35):355104. doi:10.1088/1361-6528/ab92c8
221. Wu F, Zhang Q, Zhang M, et al. Hollow porous carbon coated FeS(2)-based nanocatalysts for multimodal imaging-guided photothermal, starvation, and triple-enhanced chemodynamic therapy of cancer. ACS Appl Mater Interfaces. 2020;12(9):10142–10155. doi:10.1021/acsami.0c00170
222. Xu K, Wu X, Cheng Y, et al. A biomimetic nanoenzyme for starvation therapy enhanced photothermal and chemodynamic tumor therapy. Nanoscale. 2020;12(45):23159–23165. doi:10.1039/D0NR05097K
223. Gu D, Liu Z, Wu H, et al. Dual catalytic cascaded nanoplatform for photo/chemodynamic/starvation synergistic therapy. Colloids Surf B Biointerfaces. 2021;199:111538. doi:10.1016/j.colsurfb.2020.111538
224. Zhao Y, Kong W, Wang P, et al. Tumor-specific multipath nucleic acid damages strategy by symbiosed Nanozyme@Enzyme with synergistic self-cyclic catalysis. Small. 2021;17(28):e2100766. doi:10.1002/smll.202100766
225. Wang Q, Niu D, Shi J, Wang L. A three-in-one ZIFs-derived CuCo(O)/GOx@PCNs hybrid cascade nanozyme for immunotherapy/enhanced starvation/photothermal therapy. ACS Appl Mater Interfaces. 2021;13(10):11683–11695. doi:10.1021/acsami.1c01006
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.