Back to Journals » Open Access Emergency Medicine » Volume 11
Global Ultrasound Check for the Critically lll (GUCCI)—a new systematized protocol unifying point-of-care ultrasound in critically ill patients based on clinical presentation
Authors Tavares J , Ivo R, Gonzalez F , Lamas T , Mendes JJ
Received 22 December 2018
Accepted for publication 2 May 2019
Published 10 July 2019 Volume 2019:11 Pages 133—145
DOI https://doi.org/10.2147/OAEM.S199137
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Hans-Christoph Pape
This paper has been retracted
João Tavares,1 Rita Ivo,2 Filipe Gonzalez,3 Tomás Lamas,4 João João Mendes4
1Internal Medicine Department, Hospital da Luz, Lisbon, Portugal; 2Internal Medicine Department, Hospital Egas Moniz, Lisbon, Portugal; 3Intensive Care Unit, Hospital Garcia de Orta, Almada, Portugal; 4Intensive Care Unit, Hospital CUF Infante Santo, Lisbon, Portugal
Abstract: Ultrasound technology is an essential tool in the management of critically ill patients. Point-of-care ultrasonography (POCUS) enables data collection from different anatomic areas to achieve the most probable diagnosis and administer the right therapy at the right time. Despite the increasing utilization of POCUS, there is still a lack of standards to establish how to use different bedside ultrasound protocols, and it is imperative to develop a unifying protocol. Thus, the aim of this paper is to establish a new systematized approach that can be adopted by all physicians to implement POCUS for critically ill patient management. To achieve this, we propose a new systematized approach—Global Ultrasound Check for the Critically Ill (GUCCI)—that integrates multiple protocols. This protocol is organized based on three syndromes (acute respiratory failure, shock, and cardiac arrest) and includes ultrasound-guided procedures.
Keywords: ultrasonography, interventional ultrasonography, respiratory failure, shock, cardiac arrest, echocardiography, intensive care
Introduction
Point-of-care ultrasound (POCUS) is a technique that employs ultrasound imaging to answer objective clinical questions. Clinicians perform POCUS as an extension of the physical examination in a problem-oriented approach. In critical care, POCUS should be objective, quick, and repeated as often as necessary to monitor the rapid evolution of the patient’s critical condition.1
While using POCUS, one has to keep in mind the sensitivity, specificity, and pretest and posttest condition probability to wisely guide diagnosis and treatment. It should be noted that clinical evaluation is necessary to define the pretest probability of the condition, whereas the specific sensitivity and specificity of a given ultrasound finding will help determine the posttest probability of a given condition.2 For example, the presence of B-lines has been reported to have 94% sensitivity and 92% specificity with respect to the diagnosis of cardiogenic pulmonary edema.3 If B-lines are used as a screening method in a healthy 30-year-old man (1% pretest probability for heart failure), the posttest probability will just be 10%. However, if it is used as a screening method in patients with acute dyspnea in the emergency department (pretest probability of around 43%), the posttest probability will be 90%.4
Since 2001, several protocols have been published to standardize the specific use of POCUS to examine critically ill patients (Table 1). For the purpose of integrating POCUS protocols, we propose a new systematized approach—Global Ultrasound Check for the Critically Ill (GUCCI). This is organized based on three syndromes (acute respiratory failure, shock, and cardiac arrest) and includes ultrasound-guided procedures.
 |
Table 1 Chronology of point-of-care ultrasound protocols |
Acute respiratory failure
Acute respiratory failure represents loss of the ability of the respiratory system to ventilate adequately or to provide adequate oxygen delivery to meet metabolic demands. The diagnosis of acute respiratory failure is based on clinical data and blood gas analysis, but POCUS can be extremely useful in terms of differential diagnosis.11
Studies have shown that, in these patients, lung ultrasound has high diagnostic accuracy in identifying pneumothorax, consolidation/atelectasis, interstitial syndromes (eg, pulmonary edema of cardiogenic or noncardiogenic origin), pleural effusion, and pneumonia.25–27 As a result, lung ultrasound is likely to have a significant impact on clinical decision-making and therapeutic management of these patients.28
GUCCI proposes a two-step approach using a quick algorithm to integrate lung ultrasound with complementary cardiac and vascular ultrasound in a stepwise approach to exclude the most severe diagnoses and those with possible immediate intervention (Figure 1).
 |
Figure 1 Acute respiratory failure algorithm. ARDS: Acute Respiratory Distress Syndrome. |
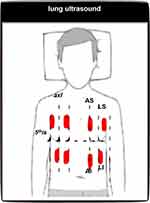
With respect to lung ultrasound, different probes such as low-frequency probes (3.5–5 MHz) to examine deeper structures (eg, heart, pleural effusion) and high-frequency probes (>5 MHz) to examine superficial structures (eg, pleural sliding) can be used.11 However, an organized approach with multiple points of examination is recommended.29 Initially, with the patient in a dorsal decubitus position, the chest is scanned bilaterally in four different areas, which are defined by the anterior axillary line and fifth intercostal space line (Figure 2). The diaphragm should be carefully identified. In some cases, to allow better pleural effusion and consolidation pattern recognition, the patient is placed in the lateral decubitus position.
With the probe placed between two rib spaces in the craniocaudal direction, the typical lung pattern (Figure 3A) consists of two echogenic interfaces: the acoustic shadows (produced by the two adjacent ribs), and a hyperechoic horizontal line (produced by the visceral and parietal pleural surfaces) that represents the interface between the chest wall and aerated lung. The reverberation of ultrasound waves between the pleura and the probe produces horizontal artifact lines that are equidistant from each other; they are referred to as A-lines.30 Respiratory movements cause the lung to expand and contract, generating the lung sliding sign30 that represents the sliding of the visceral pleura against the parietal pleura. This sign, which is dynamic on B-mode, can be recorded as a static sign on M-mode, generating the characteristic seashore sign30 (Figure 3B) (the pleural surface is the boundary between a wave-like pattern, representing the motionless chest wall, and a sandy beach-like pattern, representing the air-filled lung). The pattern of the predominant A-lines along with lung sliding represents the normal lung pattern—A-profile.30
 |
Figure 3 Ultrasound images of normal lung pattern (A-profile): A) B-mode and B) M-mode (seashore sign). |
The absence of the lung sliding sign, which generates the characteristic barcode sign30 on M-mode (the normal sandy beach-like pattern below the pleural line is replaced by horizontal lines), signifies no lung movement (Figure 4). Two conditions, lung atelectasis and pneumothorax, may generate these findings, which can be differentiated by two specific signs. The presence of a lung pulse (heart activity perception at the pleural line) aids in identifying lung atelectasis, whereas the presence of a lung point (alternating seashore sign, indicating lung sliding, and barcode sign, indicating absent lung sliding in the same intercostal space) aids in identifying pneumothorax.30
 |
Figure 4 Ultrasound image of abnormal lung presentation with the absence of lung sliding (M-mode): barcode sign. |
Pleural effusion is characterized by the presence of an anechoic space between the visceral and parietal pleura. However, quantifying the volume of pleural effusion still remains a challenge although there are multiple methods to do so.31 We generally estimate its volume (in milliliters) in the supine patient with the probe positioned transversally in the posterior axillary line at the pulmonary base. Following this, we measure the maximum distance (in millimeters) between the lung and the thoracic wall and multiply it by twenty.32 Pleural effusions can exhibit one of the following sonographic patterns:33 1) anechoic, which is typical of transudates; 2) complex nonseptated (echogenic material strewn in a nonhomogeneous pattern without septations), which is typical of exudates; 3) complex septated (evidence of strands or septae in a lattice-like pattern), which is typical of various types of exudates; and 4) homogeneously echogenic (echogenic material strewn homogeneously), which is typical of hemorrhagic effusion and empyema. In the presence of moderate to large pleural effusions, the adjacent lung may become atelectatic and appear as a tissue-like pattern flapping in the pleural effusion (flapping lung sign). Clinically, if the pleural effusion is identified as the cause (or a major contributor) of acute respiratory failure, ultrasound-guided therapeutic thoracentesis or chest drain insertion should be considered.
In the presence or absence of pleural effusion, the tissue-like pattern may be associated with either pneumonia (Figure 5) or atelectasis.30 If the presence of a dynamic air bronchogram (punctiform or linear hyperechoic artifacts within the tissue-like pattern with centrifugal inspiratory movement >1 mm) is detected, this indicates patent bronchi. Furthermore, the presence of a dynamic air bronchogram has a high positive predictive value with respect to diagnosing pneumonia,34 which is further augmented by the presence of a shred sign29 (subpleural hypoechoic area with ragged margins).
 |
Figure 5 Tissue-like pattern characteristic of pneumonia. |
The alveolar-interstitial syndrome35 includes several heterogeneous conditions and is characterized by a B-profile (Figure 6). In contrast to the normal (A-profile) pattern, the B-profile is present when three or more B-lines30 (hyperechoic comet-tail-like artifacts perpendicular to the pleural line that erase A-lines) are identified at the same intercostal space.11 A focal or multifocal heterogeneous B-profile is suggestive (but not diagnostic) of pneumonia,35 whereas a homogeneous bilateral B-profile is suggestive of diffuse pulmonary edema35 of cardiogenic (acute cardiogenic pulmonary edema) or noncardiogenic etiology (acute respiratory distress syndrome), which can be distinguished both clinically and by evaluating the cardiac function (see “Shock”). Isolated B-lines (<3 per intercostal space) or B-lines that are confined to the last intercostal space above the diaphragm can be observed in healthy subjects and are of little clinical significance.30
 |
Figure 6 B-profile with more than three B-lines in the same intercostal space. |
If respiratory failure is detected along with a normal A-profile, then two conditions must be considered: obstructive pulmonary disease (asthma or chronic obstructive pulmonary disease) and pulmonary thromboembolism.11 Although clinical evaluation will differentiate them in most cases, searching for deep venous thrombosis with two-point compression ultrasound36 will help to corroborate pulmonary thromboembolism (Figure 7). To achieve this, a linear high-frequency probe is placed axially in two points (common femoral and popliteal vessels), and the vein is compressed. If a thrombus is visualized or a vein is not compressible, then deep vein thrombosis is likely.
 |
Figure 7 Two-point compression ultrasound for the diagnosis of deep venous thrombosis: (A) Left femoral vein-non-compressible thrombus; (B) Normal, compressible popliteal vein. |
Therapeutic thoracentesis and chest drain insertion
With the patient in a semi-recumbent position, a low-frequency (3.5–5 MHz) probe is used to visualize the pleural fluid distribution and select the best access site (the point at which the maximum width of the pleural effusion is detected). Qualitative information about the nature of the fluid and the clinical presentation should be used to select the drain (eg, thoracentesis catheters for anechoic pleural effusions, large-bore chest tubes for the homogeneously echogenic suspect of hemothorax or empyema). To guide needle/trocar insertion and confirm the pleural space needle tip position, an in-plane technique can be used. Following this, the classic thoracentesis or chest drain insertion technique37 is used. However, one major pitfall is the confusion regarding distinguishing ascitic and pleural fluid; thus, it is mandatory to identify the diaphragm and liver on the right side and the spleen on the left side.
Shock
Shock refers to the failure of the cardiocirculatory system to provide adequate oxygen to meet metabolic demands, which are clinically manifested by tissue hypoperfusion.38 Classically, shock can be classified into four broad etiological categories, which have been listed as follows: hypovolemic, cardiogenic, obstructive, and distributive. Even though this classification provides a useful way of determining the main underlying mechanism of shock, it is somewhat of an oversimplification. Moreover, it should be noted that multiple mechanisms may coexist, as is often the case in sepsis. Although the type and etiology of shock may be apparent from the medical history, physical examination, or clinical investigations, the diagnosis can be refined by conducting a POCUS evaluation.
Irrespective of whether the cause of shock is unknown or has been suspected/established, ultrasound may prove very useful in its diagnosis and management, and in monitoring ongoing treatments and clinical progression. It is recommended as a first-choice examination in consensus guidelines,39 as no other bedside tool possesses similar diagnostic capability.
GUCCI proposes a stepwise holistic approach for diagnosing shock, integrating cardiac, lung, vascular, and abdominal ultrasound, and guiding directed immediate therapeutic management (Figure 8).
 |
Figure 8 Shock algorithm.Abbreviations: RV, right ventricle; LV, left ventricle; IVS, interventricular septum. |
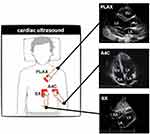
For cardiac ultrasound, low-frequency sectorial probes (3.5–5 MHz) are used, and an organized approach is recommended (Figure 9). Ideally, the heart is scanned in the left lateral decubitus position, but more frequently in the dorsal decubitus position, and three different views (parasternal long axis, apical four-chamber, and subxiphoid window) are obtained. This approach permits the evaluation of the crucial elements of the cardiac ultrasound examination (chamber size and shape, left ventricular systolic function, inferior vena cava (IVC) size, and collapsibility and pericardial effusion) and other gross morphological abnormalities (eg, mass in the heart chambers).40–42 Subsequent evaluation depends on the type of shock, combining clinical evaluation and cardiac ultrasound as follows.
If a tension pneumothorax is suspected either clinically or through cardiac ultrasound (mediastinal shift associated with pressure overload and/or dilated IVC in the right heart chambers), a lung ultrasound (limited to the anterior–superior area) can be conducted to confirm diagnosis (see “Acute respiratory failure”) while waiting for the drainage material.
If cardiac tamponade is clinically suspected, a cardiac ultrasound demonstrating pericardial effusion and collapse of the right heart chambers along with dilated IVC can be conducted to confirm the diagnosis.40 The pericardial effusion appears as an anechoic image surrounding the heart (there may be echogenicity within the pericardial sac if the effusion is exudative or hemorrhagic), best seen in the parasternal long axis and subxiphoid views (Figure 10). In the parasternal long axis, pericardial effusion can be differentiated from pleural effusion, as pericardial effusion is located anterior to the descending aorta. The effusion can be quantified according to its maximum thickness, which is measured during diastole: small, <1 cm not circumferential; moderate, <1 cm circumferential around the heart; large, 1–2 cm circumferential; and very large, >2 cm. It should be noted that recognizing the features of the cardiac tamponade ultrasound is extremely important. The observable features have been listed as follows: right atria collapse (right atria inversion during ventricular end-diastole), right ventricular diastolic collapse (absence of right ventricular free wall expansion during early diastole), and dilated IVC. After the diagnosis of cardiac tamponade is established, ultrasound-guided pericardiocentesis should be considered as the standard of care.
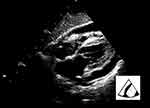 |
Figure 10 Pericardial effusion with tamponade. |
Massive pulmonary thromboembolism should be suspected in the adequate clinical context if right heart chamber dilatation (right/left ventricular ratio >0.6 in the apical four-chamber view (Figure 11)) is detected. Rarely, an intracardiac free-flowing echogenic thrombus or, more frequently, a deep venous thrombosis can be seen with two-point compression ultrasound (see “Acute respiratory failure”).40
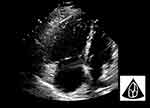 |
Figure 11 Massive pulmonary thromboembolism. |
Cardiogenic shock is most commonly caused due to left ventricular systolic dysfunction (as evaluated by ejection fraction) in the presence of elevated filling pressure, which results in hydrostatic pulmonary edema (as evaluated by diffuse B-lines (see “Acute respiratory failure”)). Visually, left ventricular ejection fraction estimation (“eyeball”) is a feasible and accurate method to evaluate left ventricular systolic function and is well correlated with other quantitative methods43 (eg, Simpson biplane ejection fraction). The normal left ventricular ejection fraction is usually >55%; however, when it is <30%, this indicates severe left ventricular systolic dysfunction.44 With focused training on eyeball cardiac function evaluation, even nonexperienced physicians can achieve good agreement with cardiologists.45
In patients who experience hypovolemic shock, the left ventricle becomes small (the lumen may even become obliterated with “kissing” ventricular46 walls), and the IVC collapses (Figure 12). In this setting, it is mandatory to conduct an abdominal ultrasound to check for hemorrhage, aortic aneurysm rupture, or other organ lesions. A global abdominal ultrasound, employing the three focused assessment with sonography for trauma views (right flank, left flank, and pelvis), should be performed when no obvious sources of bleeding can be identified in the context of hypovolemic shock40 to allow the detection of other arterial catastrophes (eg, rupture of splenic artery aneurysm47). The proximal section of the abdominal aorta lies along the mid-line of the abdomen on the left side of the IVC and should be screened to detect aortic aneurysm (aortic diameter >3 cm) (Figure 13) which, in the adequate clinical context, makes aneurysmal rupture probable.48
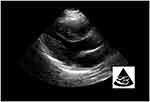 |
Figure 12 "Kissing" ventricular walls in hypovolemic shock. |
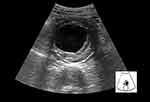 |
Figure 13 Aortic aneurysm using FAST views. |
Pericardiocentesis
With the patient in the dorsal decubitus position, a low-frequency cardiac probe (3.5–5 MHz) is used to visualize the distribution of the pericardial fluid and select the best approach (apical, parasternal, or subxiphoid). An in-plane technique is used to guide needle insertion, whereas the tip position of the pericardial space needle is confirmed through a saline bubble injection. Following this, a classic Seldinger technique is used to insert the pericardial catheter.49
Shock treatment
The first step in the shock treatment algorithm includes treating shock-reversible etiologies by following the shock diagnosis protocol (eg, thoracic drainage in tension pneumothorax, pericardiocentesis in cardiac tamponade, fibrinolysis in massive pulmonary thromboembolism).
The second step includes assessing preload and fluid responsiveness using IVC dynamics (Figure 14). The evaluation of the IVC can begin at the subcostal classical view, moving slightly off the midline to the right of the abdominal aorta on the transverse view.40 The IVC size should be measured in the longitudinal view—2 cm caudal to the point where the IVC joins the right atrium. In patients with spontaneous breathing effort, due to a change in intrathoracic pressure, the IVC collapses on inspiration and distends on expiration, whereas the reverse occurs in patients on mechanical ventilation. A totally collapsed IVC implies low preload and fluid responsiveness; on the other hand, a plethoric IVC (dilated with no collapse) implies high preload and no fluid responsiveness. For patients with IVC dynamics that stand between these opposite scenarios, the collapsibility index should be used [(maximum IVC diameter—minimum IVC diameter)/maximum IVC diameter] if spontaneously breathing, and the distensibility index should be used [(maximum IVC diameter—minimum IVC diameter)/minimum IVC diameter] if mechanically ventilated. A collapsibility index50 superior to 0.40 or a distensibility index51 superior to 0.18 translates into potential fluid responsiveness. The endpoint of fluid administration entails the appearance of anterior B-lines, indicating iatrogenic interstitial edema (which is often clinically silent but precedes alveolar edema and worsens respiratory failure). Thus, striking a balance between fluid responsiveness and interstitial edema is key to administering adequate fluids.52
 |
Figure 14 Shock treatment algorithm. *Tidal volume 8-10 mL/Kg, volume-controlled ventilation, positive end-expiratory pressure (PEEP) 4-6 cm H2O and plateau pressure <30 cm H2O. |
The third and final step includes evaluating the left ventricular systolic function (see “Shock”). In patients with high preload, fluid responsiveness, or fluid responsiveness with interstitial edema, a depressed left ventricular systolic function signifies that inotropic drug support should be considered. On the other hand, in the case of normal systolic left ventricle function (or hyperdynamic heart), vasopressors should be considered. The treatment protocol should be repeated after each intervention or if clinical changes are noted.
Cardiopulmonary resuscitation
Patients in cardiac arrest must be treated through algorithm-based management, such as basic life support and advanced life support. However, the resuscitation guidelines of the American Heart Association, the European Resuscitation Council, and the International Liaison Committee on Resuscitation21,53 recommend identifying and treating the correctable causes of cardiac arrest.
POCUS included in the advanced life support algorithm7,53 can help to diagnose/exclude some of the potentially treatable causes of cardiac arrest, such as cardiac tamponade, massive pulmonary embolism, severe ventricular dysfunction, and hypovolemia. Moreover, it can help distinguish “pseudo-pulseless electric activity” (PEA) (coordinated electrical activity with no palpable pulse, but with coordinated cardiac activity) from “true-PEA” (coordinated electrical activity with no palpable pulse or detectable cardiac motion). Breitkreutz et al17 demonstrated that 35% of patients with an electrocardiographic diagnosis of asystole experienced ongoing coordinated cardiac motion. This was associated with a better prognosis with 55% surviving to hospital admission, in contrast to “true-PEA”, which conferred a poor prognosis with only 8% surviving to hospital admission. This survival benefit further improved when a potentially treatable cause was detected through echocardiography.54,55 Namely, 59% were detected with reduced left ventricular function, whereas 8% had a dilated right ventricle and 4% were hypovolemic. Furthermore, patient management was directly altered as a result of echocardiographic findings in 51% of cases.
GUCCI proposes a stepwise holistic approach for cardiopulmonary resuscitation and integrating cardiac, lung, vascular, and abdominal ultrasound (Figure 15). A member of the ultrasound check should be a part of the cardiopulmonary resuscitation team and, to obtain the best echocardiographic view, must be positioned on the right side caudal to the compressor member (Figure 16). GUCCI proposes a three-step approach using an ultrasound cardiac low-frequency (3.5–5 MHz) probe in a subcostal view in nonshockable rhythms (and selected cases of shockable rhythms), which are eventually complemented by thoracic, abdominal, and vascular ultrasound. A unique probe type and a single window are used to minimize the time spent acquiring the appropriate cardiac window (maximum 10-s interval). It should be noted that previous studies have shown that it is possible to acquire echocardiographic images during a cardiac arrest on a timely basis.10
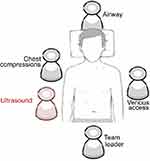 |
Figure 16 Ultrasound check member position in CPR team. |
The first step includes seeking one out of four patterns (subcostal window during pulse check)—myopathic pattern, pericardial effusion, right heart chamber dilatation, or hyperdynamic heart—and acting quickly accordingly. The myopathic pattern includes ineffective myocardial contraction (intrinsic movement of the myocardium coordinated with cardiac valve movement), disorganized myocardial contraction (which implies probable ventricular fibrillation), and standstill. In the case of ineffective myocardial contraction, adrenaline should be withheld and mechanical support (eg, veno-arterial extracorporeal membrane oxygenation) considered,56 whereas in the case of disorganized myocardial contraction, delivery of a shock should be considered (after optimization of myocardial perfusion). Standstill refers to a situation where a patient is in “true-PEA”/asystole and, besides a bad prognosis, the cardiac arrest etiology is inconclusive. Thus, in such cases, one must think about other nonmechanical reversible causes (eg, metabolic, hypoxia, and hypothermia). Pericardial effusion refers to a situation where a cardiac arrest indicates tamponade until proven otherwise, and for which immediate pericardiocentesis should be performed. Pericardial effusion size can be misleading, as severity depends on the rate of pericardial fluid accumulation. Furthermore, dilatation of right heart chambers during cardiac arrest can be difficult to define according to the usual guidelines (right/left ventricular ratio >0.6). Generally, when the right ventricle is bigger than the left ventricle, there is a likelihood of a massive pulmonary embolism or hypertensive pneumothorax. A hyperdynamic heart is characterized by a small hyperkinetic left ventricle and an obliterated cavity in some cases—“kissing ventricle” sign—associated with a collapsed IVC, which prompts rapid fluid therapy.
The second step includes conducting a noncardiac ultrasound evaluation to complement the pattern found in the first step. This can be accomplished during chest compressions to avoid further delay in the diagnosis. In the case of right heart dilatation, hypertensive pneumothorax must be excluded with lung ultrasound (see “Acute respiratory failure” and “Shock”). To establish the absence of lung sliding, ventilation is mandatory. The absence of pneumothorax signs with right heart dilatation increases the possibility of massive pulmonary embolism. Further echocardiography and vascular ultrasound can reveal an intracavitary thrombus or deep vein thrombosis to corroborate the diagnosis.40 In the case of a hyperdynamic heart, a hemorrhagic focus should be sought (see “Shock”).
The third step embodies three main goals, which have been listed as follows: confirm the previous findings, conduct reevaluation after therapy (eg, thrombolysis, fluids), and determine prognosis (eg, persistent standstill after recovery of spontaneous circulations seems very unlikely after 10 min).57
Conclusions
We propose a new systematized protocol—GUCCI (Global Ultrasound Check for the Critically Ill)—that integrates all POCUS protocols in critical care. It is organized according to three syndromes—acute respiratory failure, shock, and cardiac arrest—and includes ultrasound-guided procedures. The GUCCI strategy will help intensivists and naive ultrasound doctors to adopt a global approach without a dead-end protocol. The primary aim of GUCCI is to provide the right therapy at the right moment to prevent missed emergent diagnosis.
Abbreviation list
GUCCI, Global Ultrasound Check for the Critically Ill; IVC, inferior vena cava; POCUS, point-of-care ultrasonography; PEA, pulseless electric activity.
Disclosure
The authors declare that they have no conflict of interest in this work.
References
1. Blanco P, Volpicelli G. Common pitfalls in point-of-care ultrasound: a practical guide for emergency and critical care physicians. Crit Ultrasound J. 2016;8(1):15. doi:10.1186/s13089-016-0052-x
2. Nicoll CD, Mark LC. Diagnostic testing & medical decision making. In: Papadakis MA, McPhee SJ, Rabow MW., editors. Current Medical Diagnosis & Treatment. New York, NY: McGraw-Hill Education; 2017. Available from: http://accessmedicine.mhmedical.com/content.aspx?aid=1145380368
3. Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014;21(8):843–852. doi:10.1111/acem.12435
4. Guttikonda SNR, Vadapalli K. Approach to undifferentiated dyspnea in emergency department: aids in rapid clinical decision-making. Int J Emerg Med. 2018;11(1). doi:10.1186/s12245-018-0181-z
5. Atkinson PRT, McAuley DJ, Kendall RJ, et al. Abdominal and Cardiac Evaluation with Sonography in Shock (ACES): an approach by emergency physicians for the use of ultrasound in patients with undifferentiated hypotension. Emerg Med J. 2009;26(2):87–91. doi:10.1136/emj.2007.056242
6. Bahner DP. Trinity: a hypotensive ultrasound protocol. J Diagnostic Med Sonogr. 2002;18:193–198. doi:10.1177/875647930201800402
7. Jones AE, Tayal VS, Sullivan DM, Kline JA. Randomized, controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med. 2004;32:1703–1708. doi:10.1097/01.CCM.0000133017.34137.82
8. Seif D, Perera P, Mailhot T, Riley D, Mandavia D. Bedside ultrasound in resuscitation and the rapid ultrasound in shock protocol. Crit Care Res Pract. 2012;2012:503254. doi:10.1155/2012/503254
9. Jensen MB, Sloth E, Larsen KM, Schmidt MB. Transthoracic echocardiography for cardiopulmonary monitoring in intensive care. Eur J Anaesthesiol. 2004. doi:10.1017/S0265021504009068
10. Breitkreutz R, Walcher F, Seeger FH. Focused echocardiographic evaluation in resuscitation management: concept of an advanced life support-conformed algorithm. Crit Care Med. 2007;35:S150–S161. doi:10.1097/01.CCM.0000260626.23848.FC
11. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure the BLUE protocol. Chest. 2008;134:117–125. doi:10.1378/chest.07-2800
12. Hernandez C, Shuler K, Hannan H, Sonyika C, Likourezos A, Marshall J. C.A.U.S.E.: cardiac arrest ultra-sound exam-A better approach to managing patients in primary non-arrhythmogenic cardiac arrest. Resuscitation. 2008;76:198–206. doi:10.1016/j.resuscitation.2007.06.033
13. Gunst M, Ghaemmaghami V, Sperry J, et al. Accuracy of cardiac function and volume status estimates using the bedside echocardiographic assessment in trauma/critical care. J Trauma - Inj Infect Crit Care. 2008;65:509–516. doi:10.1097/TA.0b013e3181825bc5
14. Boyd JH, Walley KR. The role of echocardiography in hemodynamic monitoring. Curr Opin Crit Care. 2009;15:239–243. doi:10.1097/MCC.0b013e32832b1fd0
15. Weingart SD, Duque DNB Rapid Ultrasound for Shock and Hypotension (RUSH-HIMAPP). Available from: http://emedhome.com/.
16. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: rapid ultrasound in shock in the evaluation of the critically lll. Emerg Med Clin North Am. 2010;28(1):29–56, vii. doi:10.1016/j.emc.2009.09.010
17. Breitkreutz R, Price S, Steiger HV, et al. Focused echocardiographic evaluation in life support and peri-resuscitation of emergency patients: a prospective trial. Resuscitation. 2010;81:1527–1533. doi:10.1016/j.resuscitation.2010.07.013
18. Elmer J, Noble VE. An evidence-based approach for integrating bedside ultrasound into routine practice in the assessment of undifferentiated shock. ICU Dir. 2010;1:163–174. doi:10.1177/1944451610369150
19. Lanctôt JF, Valois M, Beaulieu Y. EGLS: echo-guided life support. Crit Ultrasound J. 2011;3:123–129. doi:10.1007/s13089-011-0083-2
20. Ferrada P, Murthi S, Anand RJ, Bochicchio GV, Scalea T. Transthoracic focused rapid echocardiographic examination: real-time evaluation of fluid status in critically ill trauma patients. J Trauma - Inj Infect Crit Care. 2011;70:56–64. doi:10.1097/TA.0b013e318207e6ee
21. Lichtenstein D, Karakitsos D. Integrating lung ultrasound in the hemodynamic evaluation of acute circulatory failure (the fluid administration limited by lung sonography protocol). J Crit Care. 2012;27(5):
22. Liteplo A, Noble V, Atkinson P. My patient has no blood pressure: point-of-care ultrasound in the hypotensive patient: FAST and RELIABLE. Ultrasound. 2012;20:64–68. doi:10.1258/ult.2011.011044
23. Wu TS. The CORE scan: concentrated overview of resuscitative efforts. Crit Care Clin. 2014;30(1):151–75, vi. doi:10.1016/j.ccc.2013.08.001
24. Atkinson P, Bowra J, Milne J, et al. International Federation for Emergency Medicine Consensus Statement: sonography in hypotension and cardiac arrest (SHoC): an international consensus on the use of point of care ultrasound for undifferentiated hypotension and during cardiac arrest - CORRIG. Cjem. 2017;19(4):327. doi:10.1017/cem.2017.31
25. Xirouchaki N, Magkanas E, Vaporidi K, et al. Lung ultrasound in critically ill patients: comparison with bedside chest radiography. Intensive Care Med. 2011;37(9):1488–1493. doi:10.1007/s00134-011-2317-y
26. Volpicelli G. Sonographic diagnosis of pneumothorax. Intensive Care Med. 2011;37:224–232. doi:10.1007/s00134-010-2079-y
27. Bouhemad B, Liu ZH, Arbelot C, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med. 2010;38:84–92. doi:10.1097/CCM.0b013e3181b08cdb
28. Xirouchaki N, Kondili E, Prinianakis G, Malliotakis P, Georgopoulos D. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014. doi:10.1007/s00134-013-3133-3
29. Turner JP, Dankoff J. Thoracic Ultrasound. Emerg Med Clin North Am. 2012;30:451–473. doi:10.1016/j.emc.2011.12.003
30. Feldman MK, Katyal S, Blackwood MS. US Artifacts. RadioGraphics. 2009;29:1179–1189. doi:10.1148/rg.294085199
31. Ibitoye BO, Idowu BM, Ogunrombi AB, Afolabi BI. Ultrasonographic quantification of pleural effusion: comparison of four formulae. Ultrasonography. 2018;37:254–260. doi:10.14366/usg.17050
32. Balik M, Plasil P, Waldauf P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32:318–321. doi:10.1007/s00134-005-0024-2
33. Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. Am J Roentgenol. 1992;159:29–33. doi:10.2214/ajr.159.1.1609716
34. Lichtenstein D, Mezière G, Seitz J. The dynamic air bronchogram: a lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest. 2009;135:1421–1425. doi:10.1378/chest.08-2281
35. Lichtenstein DA, Lascols N, Mezière G, Gepner A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004;30:276–281. doi:10.1007/s00134-003-2075-6
36. Zitek T, Baydoun J, Yepez S, Forred W, Slattery D. Mistakes and pitfalls associated with two-point compression ultrasound for deep vein thrombosis. West J Emerg Med. 2016;17:201–208. doi:10.5811/westjem.2016.1.29335
37. Peris A, Tutino L, Cianchi G, Gensini G. Ultrasound guidance for pleural-catheter placement. N Engl J Med. 2018;378:e19. doi:10.1056/NEJMvcm1102920
38. Vincent JL, De Backer D, Finfer SR, Vincent JL. Circulatory Shock. N Engl J Med. 2013;369:1726–1734. doi:10.1056/NEJMra1208943
39. Graham CA, Parke TRJ. Critical care in the emergency department: shock and circulatory support. Emerg Med J. 2005;22:17–21. doi:10.1136/emj.2003.012450
40. Mok KL. Make it SIMPLE: enhanced shock management by focused cardiac ultrasound. J Intensive Care. 2016. doi:10.1186/s40560-016-0176-x
41. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically Ill patients-part i: general ultrasonography. Crit Care Med. 2015;43(11):2479–2502. doi:10.1097/CCM.0000000000001216
42. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically Ill patients-part II: cardiac ultrasonography. Crit Care Med. 2016;44(6):1206–1227. doi:10.1097/CCM.0000000000001847
43. Gudmundsson P, Rydberg E, Winter R, Willenheimer R. Visually estimated left ventricular ejection fraction by echocardiography is closely correlated with formal quantitative methods. Int J Cardiol. 2005;101:209–212. doi:10.1016/j.ijcard.2004.03.027
44. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–271. doi:10.1093/ehjci/jev014
45. Bustam A, Azhar MN, Veriah RS, Arumugam K, Loch A. Performance of emergency physicians in point-of-care echocardiography following limited training. Emerg Med J. 2014;31:369–373. doi:10.1136/emermed-2012-201789
46. Leung JM, Levine EH. Left ventricular end-systolic cavity obliteration as an estimate of intraoperative hypovolemia. Anesthesiology. 1994;81:1102–1109. doi:10.1097/00000542-199411000-00003
47. Lo WL, Mok KL. Ruptured splenic artery aneurysm detected by emergency ultrasound—a case report. Crit Ultrasound J. 2015;7. doi:10.1186/s13089-015-0026-4
48. Tayal VS, Graf CD, Gibbs MA. Prospective study of accuracy and outcome of emergency ultrasound for abdominal aortic aneurysm over two years. Acad Emerg Med. 2003;10:867–871. doi:10.1197/aemj.10.8.867
49. Fitch MT, Nicks BA, Pariyadath M, McGinnis HD, Manthey DE. Videos in clinical medicine. Emergency pericardiocentesis. N Engl J Med. 2012. doi:10.1021/jp201018x
50. Muller L, Bobbia X, Toumi M, et al. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care. 2012;16:R188. doi:10.1186/cc11672
51. Barbier C, Loubières Y, Schmit C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30:1740–1746. doi:10.1007/s00134-004-2259-8
52. Lichtenstein D. Fluid administration limited by lung sonography: the place of lung ultrasound in assessment of acute circulatory failure (the FALLS-protocol). Expert Rev Respir Med. 2012;6:155–162. doi:10.1586/ers.12.13
53. Olasveengen TM, De Caen AR, Mancini ME, et al. International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations summary. Circulation. 2017. doi:10.1161/CIR.0000000000000541
54. Blaivas M, Fox JC. Outcome in cardiac arrest patients found to have cardiac standstill on the bedside emergency department echocardiogram. Acad Emerg Med. 2001;8:616–621. doi:10.1111/j.1553-2712.2001.tb00174.x
55. Bocka JJ, Overton DT, Hauser A. Electromechanical dissociation in human beings: an echocardiographic evaluation. Ann Emerg Med. 1988;17:450–452. doi:10.1016/S0196-0644(88)80234-8
56. Lamhaut L, Hutin A, Puymirat E, et al. A Pre-Hospital Extracorporeal Cardio Pulmonary Resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: an observational study and propensity analysis. Resuscitation. 2017;117:109–117. doi:10.1016/j.resuscitation.2017.04.014
57. Kim HB, Suh JY, Choi JH, Cho YS. Can serial focussed echocardiographic evaluation in life support (FEEL) predict resuscitation outcome or termination of resuscitation (TOR)? A pilot study. Resuscitation. 2016;101:21–26. doi:10.1016/j.resuscitation.2016.01.013
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.