Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Global Trends and Research Progress of Photodynamic Therapy in Skin Cancer: A Bibliometric Analysis and Literature Review
Authors Sun J , Zhao H, Fu L, Cui J , Yang Y
Received 12 December 2022
Accepted for publication 16 February 2023
Published 21 February 2023 Volume 2023:16 Pages 479—498
DOI https://doi.org/10.2147/CCID.S401206
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Jiachen Sun,1,* Hongqing Zhao,1,* Lin Fu,2,* Jing Cui,3,* Yuguang Yang1
1Department of Dermatology, Fourth Medical Center of Chinese PLA General Hospital, Beijing, People’s Republic of China; 2Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 3Navy Clinical College, the Fifth School of Clinical Medicine, Anhui Medical University, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuguang Yang, Email [email protected]
Background: Based on photochemical reactions through the combined use of light and photosensitizers, photodynamic therapy (PDT) is gaining popularity for the treatment of skin cancer. Various photosensitizers and treatment regimens are continuously being developed for enhancing the efficacy of PDT on skin cancer. Reviewing the development history of PDT on skin cancer, and summarizing its development direction and research status, is conducive to the further research.
Methods: To evaluate the research trends and map knowledge structure, all publications covering PDT on skin cancer were retrieved and extracted from Web of Science database. We applied VOSviewer and CiteSpace softwares to evaluate and visualize the countries, institutes, authors, keywords and research trends. Literature review was performed for the analysis of the research status of PDT on skin cancer.
Results: A total of 2662 publications were identified. The elements, mechanism, pros and cons, representative molecular photosensitizers, current challenges and research progress of PDT on skin cancer were reviewed and summarized.
Conclusion: This study provides a comprehensive display of the field of PDT on skin cancer, which will help researchers further explore the mechanism and application of PDT more effectively and intuitively.
Keywords: photodynamic therapy, photosensitizers, skin cancer, bibliometric
Plain Language Summary
- Reviewing the development history, and summarizing its development direction and research status to map knowledge structure, is conducive to the further research of PDT on skin cancer.
- In total, 2662 publications covering PDT on skin cancer were retrieved and extracted from Web of Science database. VOSviewer and CiteSpace softwares were employed to evaluate and visualize the countries, institutes, authors, keywords and research trends. Then, literature review was performed for the analysis of the research status of PDT on skin cancer. The elements, mechanism, pros and cons, representative molecular photosensitizers, current challenges and research progress of PDT on skin cancer were reviewed and summarized.
- PDT is a relatively new treatment option and usually performed as an outpatient procedure with high effective rate and minimal side effects. PDT is most commonly used to treat precancerous skin conditions such as actinic keratosis, as well as basal cell and squamous cell carcinomas. Recently, studies have focused on the efficacy comparison between PDT and different treatment methods or the improvement of efficacy brought by the combined application. Many studies have also focused on the continuous upgrading of photosensitizers. More research is urgently needed to determine PDT’s effectiveness in treating various types of skin cancer and its long-term outcomes.
Background
Photodynamic therapy (PDT) is a treatment method based on photochemical reactions through the combined use of light and light-induced activation chemicals, namely photosensitizers (PSs).1,2 With the rapid and excessive formation of reactive oxygen species (ROS), cell killing, microvascular damage effects and local immune responses were triggered through the lipid peroxidation and DNA damage, manifested as apoptosis, necrosis, or immunogenic cell death.3–8 Over the past 30 years, PDT has been increasingly applied to treat various solid tumors, including brain, lung, gastrointestine, bone, bladder, prostate, breast, cervix, ovary, etc.9,10
For the skin is the outermost layer of the body which is convenient for the irradiation of the light, Dermatology is a department with plenty of opportunities of PDT application, for the treatment of diseases from acne, naevus flammeus, to skin cancer.11,12 Skin cancer can be simply divided into melanoma skin cancer (MSC) and nonmelanoma skin cancer (NMSC).13 MSCs have a high degree of malignancy and are prone to metastasis but were not an indication of PDT. NMSC is the most common cancer worldwide, of which approximately 75–80% are Basal cell carcinomas (BCCs), 20–25% are squamous cell carcinomas (SCCs), and others include Actinic Keratosis (AK), Bowen’s disease, and Merkel cell carcinoma (MCC).14
In view of the rapid development of PDT in skin cancer in recent years, reviewing its development history, and summarizing the development direction and research status, is conducive and urgently needed to the further research of PDT on skin cancer. Thus, we reviewed the global trends of PDT on skin cancer based on the bibliometrics analysis, with the research progress of PDT on NMSC summarized, to help researchers quickly grasp the development trends in the field and lay the foundation for future research directions.
Materials and Methods
Data Sources and Search Strategies
The data of PDT on skin cancer were obtained from the Web of Science Core Collection from 1988 to 2022. Literature retrieval was conducted within 1 day (September 5, 2022) to avoid fluctuations in citations caused by rapid updates of publications. The search formula was set to TS=(Photodynamic Therapy OR PDT) AND TS=(skin) AND TS=(carcinoma OR cancer). A total of 3083 papers were identified. Next, 418 studies were excluded, including meeting abstracts (n=55), proceeding papers (n=277), editorial materials (n=32), letters (n=27), early access (n=17), and others (n=10). Further, only articles and reviews written in English were kept (n=2662), and enrolled for bibliometrics analysis, including the following information: the number of publications and citations, titles, publication year, countries/regions, affiliations, authors, journals, key words and references. This procedure was conducted by three researchers (J.S., J.C., and H.Z.) independently with any potential differences discussed. The detailed flowchart is shown in Figure 1.
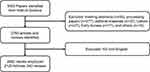 |
Figure 1 Flow diagram of literature selection and screening in this study. |
Statistical Analysis
Microsoft Office Excel 2021 was employed for the data integration. Further, VOSviewer (version 1.6.18) and CiteSpace (version 6.1.R3) were applied for the data visualization, including the cooperation among countries and institutions, cluster analysis, bursts of references and keywords, and timeline views. The nodes colors represented various clusters or times, and the sizes for the number of publications, the thickness of the line for the strength of the relation.
Results
The Initiation and Development of PDT in Skin Cancer
The combinations of light and chemical agents for the treatment of diseases took its shape approximately 3000 years ago, when the ancient inhabitants of Egypt and Indian used psoralen to treat the depigmentation of vitiligo under sunlight.15,16 In 1903, Nobel Prize in Physiology or Medicine was awarded to Niels Finsen for his contribution to this field. Also in 1903, Von Tappeiner used a combination of light and organic dye eosin to treat skin cancer, and named it photodynamic action, which was the beginning of modern PDT.15
From 1988 to 2022, totally 2120 articles and 542 reviews were associated with PDT in skin cancer (Figure 2A). The first clinical trial of PDT on skin cancer was reported in 1988, which was scrapped halfway although.17 In 1990, Kennedy et al first successfully used 5-aminolaevulinic acid (ALA)-PDT for the treatment of AK, BCC and SCC.18 In 1999, ALA-PDT was firstly approved by the USA Food and Drug Administration (FDA) for the treatment of AK.19 In 2001, the European Union, Australia and New Zealand approved methyl aminolevulinate (MAL) for the PDT of AK and BCC.20,21 The curve fitting analysis revealed an overall increasing trend of the annual number of publications, which highlighted the rapidly progression of interests in the field of PDT on skin cancer. So far, PDT has developed into an increasingly mature technology, and eight PSs have been approved for clinical use. New PSs such as Pc4 (developed by Case Western Reserve University) and PHOTOCYANINE (developed by Fuzhou University) are already under clinical trials.22,23
The Bibliometrics of PDT in Skin Cancer
Totally, 79 countries around the world participated in the study of PDT in skin cancer (Figure 3). The top 10 countries with the highest number of outputs are displayed in Table 1. Especially, the USA contributed the largest number of studies with a total of 627 publications accounting for 23.55%. It was followed by China (289, 10.86%), Germany (263, 9.88%), England (126, 8.87%), and Italy (157, 5.90%) (Figure 2B). With publications greater than or equal to 10 as the filter condition, a further cooperation network of countries was constructed, showing the relatively close cooperation between countries. Among them, the USA, England and China are in the core area with most of the cooperation and exchanges with other countries (Figure 3).
 |
Table 1 Top 10 Countries in the Field of PDT on Skin Cancer |
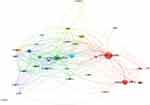 |
Figure 3 The contributions and cooperation of countries/regions in the field of PDT on skin cancer. Circles with larger diameters represent countries with more publications. |
Further, we profiled institutions which are influential at the forefront of PDT in skin cancer. A total of 181 institutions published more than or equal to 10 publications. The top 10 institutions with the most publications are listed in Table 2. Harvard University was the leading institutions with the largest number of publications (n=92), followed by Universidade de Sao Paulo (n=83) and University of London (n=83). The contribution and cooperation network indicated the strong cooperation between the organizations within the same country (Figure 4). International cooperation still needs to be strengthened.
 |
Table 2 Top 10 Institutions in the Field of PDT on Skin Cancer |
 |
Figure 4 The contributions and cooperation of different institutions focusing on PDT on skin cancer. Circles with larger diameters represent institutions with more publications. |
Focusing on individuals, we find that the continued progress of PDT in skin cancer depends partly on the efforts of up to 342 researchers with publications greater than or equal to 5. The 10 authors with the most outputs are listed in Table 3, who may probably be the most representative Principal Investigators in the field. Among them, Szeimies RM from Germany was the most productive author with 59 publications, followed by Moan J from Norway (44 publications) and Haedersdal M from Denmark (38 publications). Morton CA from Scotland was the author with the highest average citation index (ACI). Further, collaboration between authors with five or more publications was visualized by VOSviewer as shown in Figure 5.
 |
Table 3 Top 10 Authors in the Field of PDT on Skin Cancer |
 |
Figure 5 Visual cluster analysis of top authors in the field of PDT on skin cancer. Circles with larger diameters represent authors with more publications. |
As a platform for the display and exchange of academic achievements, 180 journals have 3 or more publications on PDT and skin cancer (Figure 6). The top 10 journals are listed in Table 4 with 817 publications. Photodiagnosis and Photodynamic Therapy unsurprisingly won the crown with 187 publications and the ACI of 16.9. But the journal with the highest 71.07 ACI was the Journal of the American Academy of Dermatology, with 44 publications. The top 10 cited publications were presented in Table 5. The publication with the most citation was written by Brown SB in 2004 with 1358 citations, who revealed the present and future role of photodynamics.24 Then, the second and third most-cited articles both focus on the discussion of PSs, proving its core position in the development of PDT.25,26
 |
Table 4 Top 10 Journals in the Field of PDT on Skin Cancer |
 |
Table 5 Top 10 Most Cited Publications in the Field of PDT on Skin Cancer |
 |
Figure 6 Journal analysis in the field of PDT on skin cancer. Circles with larger diameters represent journals with more publications. |
To explore the development direction and research hotspots of the field, the timeline changes of the top 20 keywords were further analyzed and shown in Figure 7. Immune modulation and fluorescence imaging have successively become the research focus since the beginning of the 1990s. The research emphasis of PDT on AK and SCC started in the 1990s. BCC began to take the spotlight in the 1995, accompanied by the exploration on contact hypersensitivity of PDT. Since the 2000s, topical agents and topical delivery have gradually become the focus of PDT research.
 |
Figure 7 Timeline distribution of the top 20 keywords in the field of PDT on skin cancer. |
Elements of PDT in Skin Cancer
Photosensitizers
As the key of PDT, PSs have been in continuous iterative development over the past 30 years. An ideal PS should have a relatively single and stable component, with a good absorption peak for the 600–900 nm light source, and has excellent solubility under physiological pH conditions. In addition, qualified PSs should be outstanding in the rapid and massive ROS generation under light irradiation, while maintaining its stable baseline state in the absence of light irradiation. PSs should be selectively and rapidly absorbed by tumor cells, and keep non-absorbed or less absorbed by healthy tissues. Also, PSs should have good pharmacokinetic characteristics, that is, the excess drug should be quickly excreted from the body after the completion of treatment.
Light Sources
The light sources currently used for PDT are mainly blue light (wavelength around 410 nm) and red light (wavelength 630–635 nm).27 For lesions in the cavity, it is recommended to use a semiconductor laser with a fiber, a helium–neon laser, or a special LED light source. For multiple or large skin lesions, LED light source with a larger spot was helpful. The efficacy of PDT is positively correlated with the time and power of illumination within a moderate range. Given that conventional illumination of red light may cause unbearable pain in patients, novel protocols, including the Phosistos protocol (P-PDT) or the Flexitheralight protocol have emerged in recent years, which emit illumination with fabric-based biophotonic devices.28 A multicenter intraindividual randomized controlled trial (RCT) has proved that P-PDT was noninferior to traditional PDT in terms of efficacy for treating AK with much lower pain scores and fewer adverse effects.27 In addition, sunlight has also been tried as a light source of PDT for pain relief.29
Oxygen and Photochemical Reactions
Oxygen molecules participate in the photochemical reaction of PDT.30 In type I PDT, oxygen molecules can participate in PSs-induced electron transfer from substrates to generate superoxide anion (O2·-). In type II PDT, oxygen molecules can generate singlet oxygen (1O2) with PSs activated by light, which could promote denaturation of protein, inactivation of enzymatic activities, DNA damage and destruction of lipid membrane structure in tumor cells. After being endocytosed by cells, the PS 5-ALA was mainly in mitochondria, and can be converted into protoporphyrin IX (PpIX) via oxygen-based enzymatic reaction, which can inhibit the biosynthetic activity of ATP by disturbing the electron transport chain and promoting ROS generation. Various approaches have been developed to address hypoxia in the tumor microenvironment, such as the introduction of second near-infrared photocatalytic O generation.30
The Mechanism of PDT in Skin Cancer
The conventional treatment of skin cancer is mainly traumatic surgical resection, including both conventional surgical resection and morphic microsurgical resection. In addition, chemotherapy or radiotherapy is often applied for large or non-resectable skin cancer to improve the prognosis of patients. In recent years, PDT has been gradually used alone or as a supplement to treat skin cancer, and its anti-cancer effect mainly depends on the following three aspects.
Cytotoxicity
As a process with massive production of ROS, PDT can trigger cancer regression by inducing cell apoptosis, necrosis, and ferroptosis. Excessive apoptosis is the main way for PDT to exert its anti-cancer effect, which depends mainly on the mitochondrial apoptosis pathway.31 The deposition of PSs in mitochondria can stimulate the irreversible over-opening of mitochondrial permeability transition pore (MPTP), leading to the release of cytochrome C from the intermembrane space into the cytoplasm, which then binds to the apoptosis protease-activating factor-1 (Apaf-1), contributing to the recruitment and self-cleavage of Caspase-9. Cleaved Caspase-9 can activate the downstream Caspase-3 to initiate a cascade reaction of Caspase protein family, and finally induce nuclear pyknosis and cell apoptosis. In addition, PDT can also damage cancer tissues via the activation of the matrix metalloprotease family (MMP).32 Besides, PDT can induce tumor cell necrosis through the activation of receptor-interacting protein kinase 1 (RIPK1), lysosome damage, and intracellular calcium overload.33,34 Cell death could also be triggered by PDT via immunogenic way by the emission of damage-associated molecular patterns (DAMP), including HSP70, HSP90, HMGB1 and IL-1β.35,36
Microvascular Killing
Through the application of intravascular PSs, PDT can directly damage vascular endothelial cells, triggering the contraction and necrosis of micro-blood vessels of the cancer tissues in the light-irradiated area. Besides, PDT can also trigger platelet aggregation in some larger blood vessels, leading to blockage and obstruction of blood flow, then further inducing hypoxia and ischemia.
Immunity Response
Numerous studies have shown that PDT is closely related to systemic antitumor immunity. Compared with immunocompromised mice, immunocompetent mice exhibited more ablation of mammary sarcoma after receiving PDT.37 For vulvar intraepithelial neoplasia (VIN), patients with deficiency in major histocompatibility complex class I (MHC-1) molecules showed no response to ALA-PDT. while VIN patients with better response to ALA-PDT showed increased CD8+ T cells infiltration in the cancer.38 AK and Bowen’s disease in immunosuppressed patients show low response to PDT, with longer treatment cycles and possible new lesions.
While killing cancer tissues, PDT can also induce edema in the light-irradiated area, which is actually a local acute inflammatory response. As a protective effect of the body, acute inflammatory response is an innate response to injury, exerting its anti-cancer effect by inducing the chemotaxis, activation and phagocytosis of macrophages. Besides, a large number of neutrophils, hypertrophic cells, and dendritic cells also infiltrate rapidly to the lesion tissues in response to various inflammatory factors released by the damaged cells of the PDT site. Further, phagocytes that have engulfed tumor cells could migrate to regional lymph nodes and then differentiate into specific antigen-presenting cells. In response to tumor antigens, lymphocytes can proliferate rapidly, and target to the residual cancer tissues which were then eliminated by the antigen–antibody reaction and interleukin release of the lymphocytes. The reactivity of leukocytes in the blood to the BCC-related tumor antigen Hip-1 could be enhanced after BCC patients being treated with PDT.39 The immunoreactivity following PDT was negatively correlated with its treatment area and light dose.39 Therefore, the blockade of anti-inflammatory factors such as IL-10 and TGF-β after PDT may improve the efficacy of PDT by maintaining or strengthening the local inflammatory response. It should be noted that the anti-cancer immunity mediated by PDT to activate humoral immunity and cellular immunity is still unclear, which may be related to the maturation and activation of dendritic cells and the enhancement of T cells. In addition to the direct apoptosis and cell death induced by PSs, studies have demonstrated that apoptotic cells induced by ALA-PDT can strengthen the production of dendritic cells vaccines, which exhibit growth-inhibitory effects on SCC in mice, indicating possible directions of dendritic cells vaccine-based cancer immunotherapy in PDT.40
Representative Photosensitizers
Thus far, hundreds of PSs have been applied in preclinical research, preclinical experiments, or clinical therapy. Most PSs, including porphyrin, chlorin and phthalocyanine derivatives, have tetrapyrrole structures. BODIPY, cyanine dyes, semiconductors, fullerenes, and aggregation-induced emission (AIE) fluorogens have been recently employed as novel PSs owing to their unique physical and chemical properties, which enable improvements in PDT efficiency.
As the most widely studied PSs families, porphyrins and phthalocyanines are widely used for the fabrication of supramolecular PSs. Porphyrins contain a cyclic array of four pyrrole rings, electronically π-conjugated through four methyl bridges. Since the core framework of porphyrins is composed of a macrocyclic conjugated system containing 18 π electrons, it has unique electron absorption properties, including a Soret band around 400 nm and four Q bands in the 500–700 nm region. Chemical modification enables porphyrins to be rationally transformed into various derivatives or porphyrin-like compounds with more excellent photoactivity. For example, chloride, a porphyrin derivative obtained by reducing one of the peripheral cross-conjugated double bonds, has a red-shifted absorption maximum and a larger extinction coefficient than porphyrin.
Phthalocyanines are porphyrazine derivatives that contain four Schiff base nitrogens in place of the methane bridge connecting the four pyrrole rings. Due to the extended conjugated structures, phthalocyanines absorb light in the near-infrared region compared to most other porphyrins. Owing to the extended conjugation provided by the two fused phenyl groups on each pyrrole ring, naphthyridine derivatives generally absorb light with longer wavelengths than porphyrins, with higher efficacy of singlet oxygen generation, showing great potential for being applied in PDT. So far, three main strategies have been used to develop structurally modified phthalocyanines, including tuning the peripheral aromatic rings, changing the metal ions in the center, and changing the axial ligands. Specifically, the complexation of closed shell and diamagnetic metal ions (eg, Al3+, Si4+, and Zn2+) enables phthalocyanine complexes with higher intersystem crossover rates and longer triplet lifetimes, whose complexes generally show higher ROS generation efficiency, and thus, become the main choice for PDT in the clinic.
Current Challenges in the Development of Photosensitizers
Although the PSs currently in clinic application already have their inherent advantages, there still have some inherent disadvantages, which pose a challenge to further improvement in efficacy. First, most of the current PSs are highly conjugated compounds, which leads to their low solubility ability or strong aggregation in aqueous solutions, resulting in a decrease in ROS generation after being activated by light. In addition, although PDT has good selectivity in both time and space manner, the selectivity of PSs to diseased cells is still unsatisfactory enough, and some PSs could still been taken up by adjacent healthy cells. The slow clearance of PSs from the body may also lead to potential therapeutic risks associated with eye damage and hyperpigmentation of healthy skin. For PSs with higher absorption peaks in the visible light region of 400–600 nm like photofrin, it is of great necessity to avoid light after PDT to minimize the side effects caused by the residual PSs. These problems in part contribute to people’s concerns about the application of PDT for skin cancer, which in turn hinders the further research and development of PSs.
Recently, great efforts have been made to develop PSs for overcoming the above-mentioned problems. Several studies have focused on developing highly water-soluble and non-aggregating phthalocyanine derivatives to generate higher levels of ROS with higher stability.41 In addition, some studies have improved the selectivity of PSs by attaching cell-targeting groups to the PSs, mitigating damage to healthy cells.42 Nano drug delivery system is a new method that has been in full swing recently.43 While greatly improving biocompatibility, the better targeting of nano drug delivery system to cancer cells is also conducive to the improvement of curative effect.44 Besides, combining PSs with oxygen-carrying groups can solve the problem of hypoxic microenvironment during PDT.45 Research in the area has led to a new “supramolecular” strategy for developing new smart PSs. Supramolecular PSs utilize non-covalent intermolecular interactions to construct PDT-active assemblies, including self-assembly of the same PSs molecules, co-assembly of different PSs molecules, co-assembly of PSs molecules with other non-PSs small molecules, and co-assembly of PSs molecules with other materials. The noncovalent interactions endow these types of supramolecular PSs with controllable photoactivity. Also, transition metal complexes have received increasing attention as PSs in PDT.46 Ru (II) polypyridine complexes have emerged as promising PSs for PDT, with rich photochemical and photophysical properties, derived from the various excited-state electron configurations accessible to visible and near-infrared light, and can be exploited for energy and electron transfer process.47 These lead to much more efficient oxygen-dependent and/or oxygen-independent photobiological activity.48
The Research Progress of PDT on Skin Cancer
Seborrheic Keratosis
Commonly seen on the head and face of the middle-aged and elderly, seborrheic keratosis (SK) is a benign lesion with little chance of transformation into malignancy like SCC. There are only two cases about the treatment of SK with PDT. The first was an 81-year-old man presented with a reddish-brown plaque on the scalp and was diagnosed as SK with SCC. The patient underwent carbon dioxide laser for 3 times, followed by ALA-PDT, and was followed up for 1 year without recurrence.49 The second was a 61-year-old woman presented with a 4.0 cm × 4.5 cm SK on her vertex, which was effectively reduced and thinned by ALA-PDT.50
Actinic Keratosis
As the most frequent premalignant skin disease in the Caucasian race, AK is commonly seen in the sun-exposed site of the elderly such as the face, ears, palmar region and forearm, and can sometimes develop into SCC.51–53
PDT is currently approved for the treatment of AK in the USA, Canada, and the European Union, etc.54 However, which PSs work best in the treatment of AK is still inconclusive. MAL-PDT has been shown to ameliorate lesions till the dermis underlying and surrounding the AK sites, which is more effective than ingenol mebutate and diclofenac plus hyaluronate gel (DHA).55 In a single-blind RCT of four Dutch hospitals, totally 624 AK patients with five or more lesions on the head were recruited, involving one continuous area of 25 to 100 cm2.51 3 months after the treatment, the cure rate of the MAL-PDT was 76.0% (117/154), similar to the 75.8% (113/149) of the Imiquimod, and significantly higher than the 0.015% Ingenol mebutate (67.3%, 101/150). No unexpected toxic effects were documented in the MAL-PDT group.51 Including 15 independent RCTs with 4252 patients, a network meta-analysis (NMA) focused on the long-term efficacy of AK after at least 12 months.56 They found that, as for the complete clearance of AK, ALA-PDT showed the most favorable risk ratio (RR) compared with placebo (RR, 8.06), followed by 5% Imiquimod (RR, 5.98), MAL-PDT (RR, 5.95), and cryosurgery (RR, 4.67).56 ALA-PDT also showed the highest RR in the NMA for lesion-specific clearance (RR, 5.08).56 Based on the traditional ALA-PDT, the emerging daylight PDT is thought to have higher efficacy for mild-to-moderate AK lesions, with better patient compliance and relatively low variability in outcomes.53,57–61 An intra-individual design with 50 patients in 6 Germany centers has proved that with daylight as the light source, BF-200 ALA-PDT was superior to the vehicle with respect to total lesion clearance rates (86.0% vs 32.9%, P<0.01) to complete clearance (67.3% vs 12.2%, P<0.01).62 Meanwhile, BF-200 ALA-PDT had a lower one-year overall lesion recurrence rate (14.1% vs 27.4%, P<0.01), and more satisfactory cosmetic outcome.62 A multicenter RCT has also found that BF-200 ALA-PDT is more effective and cost-effective than MAL-PDT for grade I–II AK, consistent with another study including 52 patients carried out in Germany and Spain.63,64
In addition to the application in AK that has already occurred, PDT has also been shown to be useful in the prevention of AK after solid organ transplantation. The clinical trial of 25 patients with kidney transplantation showed that AK occurred in 63% of the patients, but PDT treatment every 6 months for 5 years was effective in reducing the onset of AK to 28%.65
The combined use of PDT with cryotherapy, laser therapy, topical interventions or microneedling can improve their therapeutic effect with similar tolerability compared to the respective monotherapy.66–68 A randomized split-scalp study confirmed that topical application of calcipotriol could enhance the efficacy of MAL-PDT on type II AK from 63% to 90% which may be owing to the elevated PpIX levels.69 A bilaterally controlled trial of 17 patients found that the pretreatment of 5-FU for 6 days could enhance the PpIX level to two- to three-fold immediately after MAL-PDT, with altered expression of heme-synthetic enzymes and induction of p5352. With the assistance of 5-FU pretreatment, the relative clearance rates of MAL-PDT increased from 45% to 75% at 3 months (P<0.05), and from 39% to 67% at 6 months (P<0.05), respectively.52 A study of 58 patients found that VD3 deficiency resulted in the PDT clearance rate of AK to drop from 62.6% to 40.9%.70 Meanwhile, high-dose VD3 supplementation (10,000 IU daily for 5 or 14 days) significantly improved the response rate of AK from 54.4% to 72.5%.70 This regimen has good prospects and good tolerance, and can be considered for further promotion in clinical practice of PDT.
Basal Cell Carcinoma
Originating from basal cells or the outer root sheath cells of the hair follicle, BCC is a common skin malignancy with increasing incidence worldwide. European Dermatology Forum guidelines on topical PDT 2019 confirmed that nodular BCC should be treated with surgery first, but for other types of BCC, the lesion clearance rates of surgery were not always superior, and topical non-surgical treatments, such as PDT or creams were recommended.71 In Netherlands, a RCT of 601 patients from seven hospitals found that, after 1 year of treatment, the proportion of tumor residue or recurrence in the MAL-PDT group was 52 of 196 patients, comparable with 31 of 189 and 39 of 198 in the Imiquimod and 5-FU groups, respectively.72 A follow-up of BCC treatment showed that the 3-year tumor-free survival rate was 58.0% of MAL-PDT, 79.7% of Imiquimod, and 68.2% of 5-FU.73 PDT may be associated with higher pain, but its duration is shorter than other topical treatments, with superiority in cosmetic results.74,75 Also, serious adverse reactions of PDT are less common than Imiquimod or 5-FU, indicating its relative safety.75 ALA-PDT was proved to have equal effective rate as MAL-PDT in treating BCC.76 A Phase III RCT of non-invasive BCC in Germany and the England showed a complete response rate of 93.4% in the BF-200 ALA group and 91.8% in the MAL-PDT group.77 Furthermore, a study in 2020 pointed out that hexyl aminolevulinate (HAL) is an interesting alternative PS for BCC, which can achieve lesion clearance similar to the previous PSs at lower concentrations.78
Combination of PDT with other treatment modalities showed lower recurrence rates and better cosmetic results for BCC treatment. The CO2 ablative fractional laser (AFL)-assisted PDT for superficial BCC was significantly less painful with the same efficacy as well as comparable side-effects as traditional PDT.79 The pretreatment of skin with CO2 AFL prior to MAL-PDT could also enhance drug penetration with the incubation time minimized from 3 hours to 1 hour with the same efficacy for BCC.80 Er: YAG AFL-primed MAL-PDT was also proved to have an overall complete response rate of 84.5%, much higher than the 50.0% of the MAL-PDT, with the cosmetic outcomes or safety untouched.81 Li et al explored the efficacy of hematoporphyrine injection (HpD)-based PDT on BCC which was activated by laser irradiation, and found that it could be significantly enriched in the tumor site at 48 and 72 hours after injection, and significantly reduced after laser irradiation, with good therapeutic effect and metabolic pattern.82
Squamous Cell Carcinoma
SCC is a malignant tumor originating from epidermal or adnexal keratinocytes in sun-exposed areas, like the head and neck. In addition to surgical resection, PDT is increasingly applied in the clinical treatment of SCC, with the complete response rate as high as 82%.83–85 But due to the poor tissue penetration of light and PSs, PDT is more suitable for single-shot, superficial, small-area SCCs rather than larger or thicker SCCs. To solve this problem, there are studies developed visible light-induced PS nanoparticles, based on the conjugation of Ce6, cleavable Caspase-3 peptide and monomethyl auristatin E (MMAE), which requires lower energy irradiation for the activation of Caspase-3 to cleave the anticancer drug MMAE from the nanoparticles.86 The subsequent strong cytotoxic effects could further amplify cell death in the absence of visible light irradiation, thus leading to better treatment outcomes of SCC.86 Furthermore, brusatol (Bru) was loaded on the surface of ultraviolet A (UVA)-responsive zinc oxide (ZnO)-coated magnetic nanoparticles (FeO@ZnO-Bru), which has been shown to have a strong inhibitory effect on SCC, and can inhibit the scavenging effect of SCC on ROS by significantly inhibiting the Nrf2 signaling pathway.87 ALA-loaded polylactic-co-glycolic acid (PLGA) nanoparticle (NP)-assisted PDT demonstrated stronger PpIX production and more efficient cell killing in SCCs.88
Combination use with other treatment modalities can also improve the efficacy of PDT for SCC. Enhanced glycolysis and reduced oxidative phosphorylation, namely the Warburg effect, play a key role in the resistance of SCCs to PDT. Studies have shown that pretreatment with metformin can effectively prevent the metabolic reprogramming of SCC during PDT treatment and enhance the efficacy of PDT.89 The pretreatment of 5-FU for 3 days could help ALA-PDT trigger more PpIX enrichment in SCCs and thus result in more cell death, whose mechanism may be related to changes in the expression of key enzymes, including upregulated coproporphyrinogen oxidase and downregulated ferrochelatase.90 A three- to six-fold induction of p53 in 5-FU-pretreated tumors was also noted, which may play a certain role in the apoptosis induction and cell death.90 In a RCT of 45 patients with micro-invasive SCC, the overall complete response rates were higher in AFL-primed MAL-PDT than the MAL-PDT group, both in 3 months and 24 months after treatment, which indicated that AFL can be used as an auxiliary means for SCC patients receiving PDT treatment.91
Extramammary Paget’s Disease
As an intraepithelial cancer, extramammary Paget’s Disease (EMPD) is prone to recurrence and sometimes refractory to therapy. PDT had a good therapeutic effect with no obvious side effects in EMPD. Compared with the traditional wide local excision (WLE) of EMPD, PDT had shorter operation time, lower rate of recurrence and functional sequelae.92 MAL-PDT can exert EMPD killing effect by promoting the expression of Toll-like receptor-7 and the infiltration of Langerhans cells and T cells in the lesion.93 ALA-PDT showed a higher complete remission rate than MAL-PDT, meanwhile systemic PDT with intravenous sodium porfimer had much higher response rates but can be associated with more adverse reactions.94 A study of 7 patients with EMPD was exposed to 635 nm laser to irradiate the lesion area with a power of 177 J/cm for 15 minutes. After 2–4 cycles of ALA-PDT, no local recurrence occurred during the follow-up period in 2.9 years.95 In an open-label, single arm, pilot study of 11 patients, the thickness of the EMPD lesions was 0.8–6.7 mm (mean, 2.9 mm), and the complete remission rate of HpD-PDT after the first month was 90.1% with good cosmetic outcomes.96
PDT can also be used in combination with holmium laser, Imiquimod, etc, to improve the efficacy on EMPD.93,97–99 In a case study of 13 patients, compared with the WLE-only group, 4 times of PDT preprocessing reduced the size of the lesion by more than 58%, and the recurrence rate after further surgical resection was reduced from 25% to 9.1%.100 For the treatment of EMPD in the scrotum, ALA-PDT combined with WLE showed good clinical efficacy, with a low complication rate (6.25%) and a recurrence rate as low as 12.5%.101
Vulvar Paget disease (VPD) accounts for 1% of all vulvar cancer. The standard treatment of VPD is surgical resection, whose recurrence rates were rather high, with unavoidable anatomical, functional and sexual complications.102 PDT could solve these problems well, but at the same time, 6 out of the 10 patients report moderate-to-severe pain, which was an unignorable problem to be solved urgently.103 The light emitting fabric has been well developed for the treatment of AK and has also been shown to be adjunctive to MAL-PDT in the treatment of VPD with marked improvement in tolerability.104
Pros and Cons of PDT on Skin Cancer
The Pros of PDT on Skin Cancer
Located on the body surface, skin cancer has unique advantages in the application of PSs and light source irradiation, compared with tumors of the internal organs. Also, compared with traditional treatment options, PDT has obvious advantages listed below.
First, the light dependence of PSs activation makes PDT more selective and specific in time and space manner, thus greatly reduces unnecessary cytotoxicity and damage to other parts of the body which may be unavoidable in radiotherapy and chemotherapy.105
Second, in view of the fact that PDT kills cancer cells through instantaneously generated excess ROS, it can quickly and effectively kill all lesions of the light-irradiated site without missing potential minor lesions, which can greatly reduce the possibility of recurrence from invisible minimal lesions.106 In addition, the hyperplastic tissue in skin cancer can accumulate more PSs, which can emit a unique brick-red fluorescence under the irradiation of ultraviolet light, making the boundary of cancer tissues visualized, facilitating the determination of treatment plan.
Third, compared with traditional surgical treatment, bleeding is almost non-existent during PDT, which can preserve normal tissues as much as possible with less trauma.107 Meanwhile, the nursing work after PDT does not require frequent dressing change or other special precautions except for the protection from light. This greatly guarantees patients’ convenience and compliance. For skin cancer that require multiple treatments, PDT has almost no initiating resistance or drug resistance issues.
Fourth, PDT can be used alone or in combination with other treatment modalities, including radiotherapy, chemotherapy, surgery, gene therapy, sonodynamic therapy or immunotherapy.108
The Cons of PDT on Skin Cancer
Despite these advantages, what cannot be neglected is that PDT also has some obvious shortcomings, which limit its further promotion in clinical treatment. First of all, the skin of different parts, types, or colors may have different absorption, transmission, scattering and reflection characteristics of light, with different PpIX generation, so that different patients or different parts of the same patient may differ in responses to the same PDT process.109 This undoubtedly puts forward higher requirements for dermatologist’s skills and experience.
Second, the penetration depth of light in the skin is limited by the wave-particle duality of light. The wavelength of light is proportional to its penetration depth. Namely, the longer the wavelength of light, the deeper the penetration, but meanwhile the lower the energy it carries. Light with a wavelength greater than 850 nm cannot effectively activate the PSs to generate enough ROS due to its low energy, thus cannot be effectively used in clinical treatment. Therefore, light in the wavelength range of 650–850 nm is generally considered to be a more suitable wavelength range for PDT. However, light in this wavelength range can only penetrate a few millimeters of skin. With the deepening of the light penetration distance, the energy carried by light decreases exponentially. That is, the PSs in the subdermal skin may not be effectively activated by the attenuated light. So, PDT may be ineffective for the deep invasion of skin cancer.
Second, the generation of ROS during PDT is dependent on the presence of oxygen. When the cancer microenvironment is in a hypoxic state, or when the cancer is deeply infiltrated, the treatment efficiency of PDT will be limited. This aspect also limits the efficacy of PDT in the treatment of skin cancer with deep infiltration depths.
Third, patients may experience tingling, burning sensation, or itching during PDT, and local erythema, edema after treatment.110 Some patients may also experience temporary hyperpigmentation. The response of residual PSs in the body to sunlight after treatment may cause unexpected side effects, which requires patients to remain in a dark environment for a period of time after treatment, relying heavily on the compliance of patients.
Fourth, the relatively high price of PDT may be another key factor limiting its large-scale application. In the Netherlands. a trial-based analysis was performed to determine which commonly prescribed field-directed treatment was the most cost-effective, when comparing 5% 5-fluorouracil (5-FU), 5% imiquimod (IMQ), 0.015% ingenol mebutate (IM) and MAL-PDT for AK in the head and neck region.111 At 12 months post-treatment, the total mean cost of MAL-PDT was €1621, significantly higher than 5-FU, IMQ and IM, respectively, €433, €728, and €775111.
The Current Status and Prospects of PDT on Skin Cancer in China
Nowadays, PDT on skin cancer has been widely promoted in the department of Dermatology in China. The large population and relatively weaker awareness of UV protection have resulted in a correspondingly large number of skin cancer patients. This has given PDT great prospects for application, but also poses many challenges.
Firstly, there may be large differences in the application and quality of PDT in the treatment of skin cancer in hospitals of different levels. This may lead to inconsistent reputations for PDT, affecting its further acceptance by patients. Secondly, doctors have different criteria for judging whether skin cancer is suitable for PDT treatment, and there may be some patients who undergo useless PDT treatment or unnecessary trauma from surgery. In addition, the standard operating procedures and post-operative effectiveness evaluations for PDT in treating different types of skin cancer may vary, and there is no widely recognized consensus till now. Furthermore, although many new PSs are already being explored in clinical trials, the majority of PSs used in clinical treatment for skin cancer are still 5-ALA, leaving doctors with limited options.
To solve the above problems, a series of measures should be implemented for the development of PDT on skin cancer. First, considering the skin characteristics of the Chinese and the epidemiological features of skin cancer in China, the formulation of a detailed inclusion criteria for skin cancer to PDT will be the first step in standardizing PDT for skin cancer treatment. Second, standardized protocols for the treatment process and curative effect evaluation of various types of skin cancer need to be established urgently. Considering the diversity of patients’ conditions, the development of artificial intelligence to assist doctors in the treatment and evaluation of skin cancer may help to improve the efficacy of PDT. Doctors and scientists in China urgently need to promote more preclinical experiments and clinical trials to accelerate the process of new PSs from laboratory to clinical application. In November 2022, the State Drug Administration of China issued the Technical Guidelines for the Clinical Development of Anti-tumor Photodynamic Therapy Drugs. This marked China’s progress in the standardized research and development of PDT.
Conclusions
The development of PDT is the crystallization of the cross-coordinated fields of physics, chemistry, biology and medicine. Owing to the non-invasive characteristic, PDT is not only suitable for the treatment of patients in good physical condition but also especially suitable for those who cannot tolerate surgical treatment, such as elderly patients, solid organ transplant recipients, and other infirm patients, who are prone to skin cancer but may not be able to withstand radiation or chemotherapy.84,112 Besides, PDT has a good targeting effect on skin cancer, which is especially suitable for the assistance of difficult-to-resect tumors of the periorbital, ocular, paranasal, etc.113–115 PDT has the advantages of aesthetics under the premise of ensuring safety and effectiveness. For skin cancer with deep invasion or large area, the use of PDT and other treatment modalities can be combined to improve the cure rate. For skin cancer with recurrence after prior treatment, supplementation with PDT may be considered for the elimination of potentially tiny or invisible lesions.75,116
Recently, the modification of PSs has been continuously improved, such as the addition of molecules that help target tumor cells or improve the hypoxic environment, and the coupling of pro-apoptotic proteins. Besides developing novel PSs, improving the skin penetration of PSs by physical means is also a promising direction for drug delivery enhancements, like elongated microparticles, microneedles or dermaroller.117 Also, considering the large individual differences in the efficacy of PDT on skin cancer, further research on the characteristics of individuals and types of skin cancer with poor efficacy may help to further find targets for improving PSs.
At present, there is no standardized process for patient enrollment, treatment operation, and effect evaluation in the treatment of skin cancer in PDT, which needs to be further improved to ensure the curative effect of patients. It should be noted that not all patients could receive PDT. PDT is not suitable for people suffering from porphyria, allergic to porphyrins or other types of PSs. Patients were generally advised against PDT with a recent history of PSs taking, suffering systemic lupus erythematosus or chronic photosensitivity dermatitis, etc. The long-term effect of PDT on skin cancer should also be continuously observed to avoid cancer recurrence or metastasis.
Data Sharing Statement
Further information and requests for data may be directed to and will be fulfilled by the Lead Contact: Prof. Yuguang Yang ([email protected]).
Consent for Publication
The details of the manuscript can be published and all the authors providing consent have been shown the article contents to be published.
Acknowledgments
I am grateful to Miss Yi Ding for the selfless help and support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Qu Z, Wang Z, Qiu S, Cui G, Li C. Efficacy of photodynamic therapy with 5-aminolevulinic acid for the treatment of cervical high-grade squamous intraepithelial lesions with high-risk HPV infection: a retrospective study. Photodiagnosis Photodyn Ther. 2022;40:103068. doi:10.1016/j.pdpdt.2022.103068
2. Pham TC, Nguyen VN, Choi Y, Lee S, Yoon J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem Rev. 2021;121:13454–13619. doi:10.1021/acs.chemrev.1c00381
3. Tang D, Yu Y, Zhang J, et al. Self-sacrificially degradable pseudo-semiconducting polymer nanoparticles that integrate NIR-II fluorescence bioimaging, photodynamic immunotherapy, and photo-activated chemotherapy. Adv Mater. 2022;34(34):e2203820. doi:10.1002/adma.202203820
4. Stockwell BR. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401–2421. doi:10.1016/j.cell.2022.06.003
5. Zhang S, Sun X, Wang Z, et al. Molecularly self-engineered nanoamplifier for boosting photodynamic therapy via cascade oxygen elevation and Lipid ROS accumulation. ACS Appl Mater Interfaces. 2022;14(34):38497–38505. doi:10.1021/acsami.2c09209
6. Huang K, Yan M, Zhang H, Xue J, Chen J. A phthalocyanine-based photosensitizer for effectively combating triple negative breast cancer with enhanced photodynamic anticancer activity and immune response. Eur J Med Chem. 2022;241:114644. doi:10.1016/j.ejmech.2022.114644
7. Wei X, Song M, Jiang G, et al. Progress in advanced nanotherapeutics for enhanced photodynamic immunotherapy of tumor. Theranostics. 2022;12(12):5272–5298. doi:10.7150/thno.73566
8. Zhang X, Wan J, Mo F, et al. Targeting bone tumor and subcellular endoplasmic reticulum via near infrared II fluorescent polymer for photodynamic-immunotherapy to break the step-reduction delivery dilemma. Adv Sci. 2022;9(24):e2201819. doi:10.1002/advs.202201819
9. Fan W, Huang P, Chen X. Overcoming the Achilles’ heel of photodynamic therapy. Chem Soc Rev. 2016;45:6488–6519. doi:10.1039/c6cs00616g
10. Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi:10.3322/caac.20114
11. Morton CA, Szeimies R-M, Basset‐Séguin N, et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 2: emerging indications - field cancerization, photorejuvenation and inflammatory/infective dermatoses. J Eur Acad Dermatol Venereol. 2020;34:17–29. doi:10.1111/jdv.16044
12. Barbaric J, Abbott R, Posadzki P, et al. Light therapies for acne: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol. 2018;178:61–75. doi:10.1111/bjd.15495
13. Fitzmaurice C, Abate D, Abbasi N; Global Burden of Disease Cancer, C. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5:1749–1768. doi:10.1001/jamaoncol.2019.2996
14. Porceddu SV, Veness MJ, Guminski A. Nonmelanoma cutaneous head and neck cancer and Merkel cell carcinoma: current concepts, advances, and controversies. J Clin Oncol. 2015;33:3338–3345. doi:10.1200/JCO.2014.60.7333
15. Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi:10.1038/nrc1071
16. Celli JP, Spring BQ, Rizvi I, et al. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010;110:2795–2838. doi:10.1021/cr900300p
17. Pennington DG, Waner M, Knox A. Photodynamic therapy for multiple skin cancers. Plast Reconstr Surg. 1988;82:1067–1071. doi:10.1097/00006534-198812000-00021
18. Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B. 1990;6:143–148. doi:10.1016/1011-1344(90)85083-9
19. Lang K, Schulte KW, Ruzicka T, Fritsch C. Aminolevulinic acid (Levulan) in photodynamic therapy of actinic keratoses. Skin Therapy Lett. 2001;6(1–2):5.
20. Pariser DM, Lowe NJ, Stewart DM, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol. 2003;48:227–232. doi:10.1067/mjd.2003.49
21. Peng Q, Soler AM, Warloe T, Nesland JM, Giercksky KE. Selective distribution of porphyrins in skin thick basal cell carcinoma after topical application of methyl 5-aminolevulinate. J Photochem Photobiol B. 2001;62:140–145. doi:10.1016/s1011-1344(01)00173-7
22. Mangadlao JD, Wang X, McCleese C, et al. Prostate-specific membrane antigen targeted gold nanoparticles for theranostics of prostate cancer. ACS Nano. 2018;12(4):3714–3725. doi:10.1021/acsnano.8b00940
23. Chen J, Hou L, Zheng K, et al. Blood distribution and plasma protein binding of PHOTOCYANINE: a promising phthalocyanine photosensitizer inphase clinical trials. Eur J Pharm Sci. 2020;153:105491. doi:10.1016/j.ejps.2020.105491
24. Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi:10.1016/s1470-2045(04)01529-3
25. Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992;14:275–292. doi:10.1016/1011-1344(92)85108-7
26. O’Connor AE, Gallagher WM, Byrne AT. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol. 2009;85:1053–1074. doi:10.1111/j.1751-1097.2009.00585.x
27. Mordon S, Vignion-Dewalle AS, Abi-Rached H, et al. The conventional protocol vs. a protocol including illumination with a fabric-based biophotonic device (the Phosistos protocol) in photodynamic therapy for actinic keratosis: a randomized, controlled, noninferiority clinical study. Br J Dermatol. 2020;182:76–84. doi:10.1111/bjd.18048
28. Vicentini C, Vignion-Dewalle AS, Thecua E, et al. Photodynamic therapy for actinic keratosis of the forehead and scalp: a randomized, controlled, Phase II clinical study evaluating the noninferiority of a new protocol involving irradiation with a light-emitting, fabric-based device (the Flexitheralight protocol) compared with the conventional protocol involving irradiation with the Aktilite CL 128 lamp. Br J Dermatol. 2019;180:765–773. doi:10.1111/bjd.17350
29. Fernandez-Guarino M, Fonda Pascual P, Lizuain Gomez P, Harto Castano A, Jaen Olasolo P. Split-face study comparing conventional MAL photodynamic therapy in multiple actinic keratosis with complete time vs. half-time red light LED conventional illumination. J Eur Acad Dermatol Venereol. 2019;33:1529–1534. doi:10.1111/jdv.15566
30. Wang L, Kang K, Hou H, et al. NIR-II-driven intracellular photocatalytic oxygen-generation on Z-Scheme iron sulfide/cobalt sulfide nanosheets for hypoxic tumor therapy. J Colloid Interface Sci. 2022;625:145–157. doi:10.1016/j.jcis.2022.06.031
31. Wu Klingler W, Giger N, Schneider L, et al. Low-dose near-infrared light-activated mitochondria-targeting photosensitizers for PDT cancer therapy. Int J Mol Sci. 2022;23(17):9525. doi:10.3390/ijms23179525
32. Meng X, Song J, Lei Y, et al. A metformin-based nanoreactor alleviates hypoxia and reduces ATP for cancer synergistic therapy. Biomater Sci. 2021;9(22):7456–7470. doi:10.1039/d1bm01303c
33. Mohammadalipour Z, Rahmati M, Khataee A, Moosavi MA. Differential effects of N-TiO2 nanoparticle and its photo-activated form on autophagy and necroptosis in human melanoma A375 cells. J Cell Physiol. 2020;235:8246–8259. doi:10.1002/jcp.29479
34. Qian R, Wang K, Guo Y, et al. Minimizing adverse effects of Cerenkov radiation induced photodynamic therapy with transformable photosensitizer-loaded nanovesicles. J Nanobiotechnology. 2022;20(1):203. doi:10.1186/s12951-022-01401-0
35. Rodrigues MC, de Sousa Júnior WT, Mundim T, et al. Induction of immunogenic cell death by photodynamic therapy mediated by aluminum-phthalocyanine in nanoemulsion. Pharmaceutics. 2022;14(1):196. doi:10.3390/pharmaceutics14010196
36. Nguyen L, Christie C, Madsen SJ, et al. Inhibition of glioma development by doxorubicin-photochemical internalization generated macrophage vaccine: a survival study in rats. Photodiagnosis Photodyn Ther. 2022;38:102879. doi:10.1016/j.pdpdt.2022.102879
37. Korbelik M, Krosl G, Krosl J, Dougherty GJ. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 1996;56:5647–5652.
38. Daayana S, Winters U, Stern PL, Kitchener HC. Clinical and immunological response to photodynamic therapy in the treatment of vulval intraepithelial neoplasia. Photochem Photobiol Sci. 2011;10:802–809. doi:10.1039/c0pp00344a
39. Kabingu E, Oseroff AR, Wilding GE, Gollnick SO. Enhanced systemic immune reactivity to a Basal cell carcinoma associated antigen following photodynamic therapy. Clin Cancer Res. 2009;15:4460–4466. doi:10.1158/1078-0432.CCR-09-0400
40. Ji J, Zhang Y, Chen WR, Wang X. DC vaccine generated by ALA-PDT-induced immunogenic apoptotic cells for skin squamous cell carcinoma. Oncoimmunology. 2016;5:e1072674. doi:10.1080/2162402X.2015.1072674
41. Ayaz F, Yetkin D, Yuzer A, Demircioglu K, Ince M. Non-canonical anti-cancer, anti-metastatic, anti-angiogenic and immunomodulatory PDT potentials of water soluble phthalocyanine derivatives with imidazole groups and their intracellular mechanism of action. Photodiagnosis Photodyn Ther. 2022;39:103035. doi:10.1016/j.pdpdt.2022.103035
42. Li M, Xu Y, Pu Z, et al. Photoredox catalysis may be a general mechanism in photodynamic therapy. Proc Natl Acad Sci U S A. 2022;119(34):e2210504119. doi:10.1073/pnas.2210504119
43. Zhang H, Pan J, Wang T, et al. Sequentially activatable polypeptide nanoparticles for combinatory photodynamic chemotherapy of breast cancer. ACS Appl Mater Interfaces. 2022;14(35):39787–39798. doi:10.1021/acsami.2c09064
44. Khan NH, Mir M, Qian L, et al. Skin cancer biology and barriers to treatment: recent applications of polymeric micro/nanostructures. J Adv Res. 2022;36:223–247. doi:10.1016/j.jare.2021.06.014
45. Zhao J, Yang Y, Xu X, et al. Super light-sensitive photosensitizer nanoparticles for improved photodynamic therapy against solid tumors. Angew Chem Int Ed Engl. 2022;61(43):e202210920. doi:10.1002/anie.202210920
46. Alberto ME, Frances-Monerris A. A multiscale free energy method reveals an unprecedented photoactivation of a bimetallic Os(II)-Pt(II) dual anticancer agent. Phys Chem Chem Phys. 2022;24:19584–19594. doi:10.1039/d2cp02128e
47. Ponte F, Scopelliti DM, Sanna N, Sicilia E, Mazzone G. How computations can assist the rational design of drugs for photodynamic therapy: photosensitizing activity assessment of a Ru(II)-BODIPY assembly. Molecules. 2022;27(17):5635. doi:10.3390/molecules27175635
48. Monro S, Colón KL, Yin H, et al. Transition metal complexes and photodynamic therapy from a tumor-centered approach: challenges, opportunities, and highlights from the development of TLD1433. Chem Rev. 2019;119(2):797–828. doi:10.1021/acs.chemrev.8b00211
49. Fang S, Zhou Z, Wu Y, et al. Photodynamic therapy combined with carbon dioxide laser for successful treatment of cutaneous squamous cell carcinoma within a long-standing and huge seborrheic keratosis. Photodiagnosis Photodyn Ther. 2021;36:102536. doi:10.1016/j.pdpdt.2021.102536
50. Zhang H, Wei G, Zhang C, Xu Q, Zhang C. Aminolevulinate photodynamic therapy (ALA-PDT) for giant seborrheic keratosis of the head: a case report. Photodiagnosis Photodyn Ther. 2020;32:102015. doi:10.1016/j.pdpdt.2020.102015
51. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380(10):935–946. doi:10.1056/NEJMoa1811850
52. Maytin EV, Anand S, Riha M, et al. 5-fluorouracil enhances protoporphyrin IX accumulation and lesion clearance during photodynamic therapy of actinic keratoses: a mechanism-based clinical trial. Clin Cancer Res. 2018;24(13):3026–3035. doi:10.1158/1078-0432.CCR-17-2020
53. Karrer S, Aschoff RAG, Dominicus R, et al. Methyl aminolevulinate daylight photodynamic therapy applied at home for non-hyperkeratotic actinic keratosis of the face or scalp: an open, interventional study conducted in Germany. J Eur Acad Dermatol Venereol. 2019;33(4):661–666. doi:10.1111/jdv.15422
54. Jetter N, Chandan N, Wang S, Tsoukas M. Field cancerization therapies for management of actinic keratosis: a narrative review. Am J Clin Dermatol. 2018;19:543–557. doi:10.1007/s40257-018-0348-7
55. Arisi M, Zane C, Polonioli M, et al. Effects of MAL-PDT, ingenol mebutate and diclofenac plus hyaluronate gel monitored by high-frequency ultrasound and digital dermoscopy in actinic keratosis - A randomized trial. J Eur Acad Dermatol Venereol. 2020;34:1225–1232. doi:10.1111/jdv.16123
56. Steeb T, Wessely A, Petzold A, et al. Evaluation of long-term clearance rates of interventions for actinic keratosis: a systematic review and network meta-analysis. JAMA Dermatol. 2021;157(9):1066–1077. doi:10.1001/jamadermatol.2021.2779
57. Beiki D, Eggleston IM, Pourzand C. Daylight-PDT: everything under the sun. Biochem Soc Trans. 2022;50:975–985. doi:10.1042/BST20200822
58. Assikar S, Labrunie A, Kerob D, Couraud A, Bedane C. Daylight photodynamic therapy with methyl aminolevulinate cream is as effective as conventional photodynamic therapy with blue light in the treatment of actinic keratosis: a controlled randomized intra-individual study. J Eur Acad Dermatol Venereol. 2020;34:1730–1735. doi:10.1111/jdv.16208
59. Sotiriou E, Evangelou G, Papadavid E, et al. Conventional vs. daylight photodynamic therapy for patients with actinic keratosis on face and scalp: 12-month follow-up results of a randomized, intra-individual comparative analysis. J Eur Acad Dermatol Venereol. 2018;32(4):595–600. doi:10.1111/jdv.14613
60. Morton CA, Braathen LR. Daylight photodynamic therapy for actinic keratoses. Am J Clin Dermatol. 2018;19:647–656. doi:10.1007/s40257-018-0360-y
61. Lee CN, Hsu R, Chen H, Wong TW. Daylight photodynamic therapy: an update. Molecules. 2020;25:5195. doi:10.3390/molecules25215195
62. Ulrich M, Reinhold U, Dominicus R, et al. Red light photodynamic therapy with BF-200 ALA showed superior efficacy in the treatment of actinic keratosis on the extremities, trunk, and neck in a vehicle-controlled phase III study. J Am Acad Dermatol. 2021;85(6):1510–1519. doi:10.1016/j.jaad.2021.03.031
63. Rasanen JE, Neittaanmäki N, Ylitalo L, et al. 5-aminolaevulinic acid nanoemulsion is more effective than methyl-5-aminolaevulinate in daylight photodynamic therapy for actinic keratosis: a nonsponsored randomized double-blind multicentre trial. Br J Dermatol. 2019;181(2):265–274. doi:10.1111/bjd.17311
64. Dirschka T, Ekanayake-Bohlig S, Dominicus R, et al. A randomized, intraindividual, non-inferiority, Phase III study comparing daylight photodynamic therapy with BF-200 ALA gel and MAL cream for the treatment of actinic keratosis. J Eur Acad Dermatol Venereol. 2019;33(2):288–297. doi:10.1111/jdv.15185
65. Togsverd-Bo K, Omland SH, Wulf HC, Sorensen SS, Haedersdal M. Primary prevention of skin dysplasia in renal transplant recipients with photodynamic therapy: a randomized controlled trial. Am J Transplant. 2015;15:2986–2990. doi:10.1111/ajt.13358
66. Steeb T, Wessely A, Leiter U, et al. The more the better? An appraisal of combination therapies for actinic keratosis. J Eur Acad Dermatol Venereol. 2020;34(4):727–732. doi:10.1111/jdv.15998
67. Heppt MV, Steeb T, Leiter U, Berking C. Efficacy of photodynamic therapy combined with topical interventions for the treatment of actinic keratosis: a meta-analysis. J Eur Acad Dermatol Venereol. 2019;33:863–873. doi:10.1111/jdv.15459
68. Petukhova TA, Hassoun LA, Foolad N, Barath M, Sivamani RK. Effect of expedited microneedle-assisted photodynamic therapy for field treatment of actinic keratoses: a randomized clinical trial. JAMA Dermatol. 2017;153:637–643. doi:10.1001/jamadermatol.2017.0849
69. Torezan L, Grinblat B, Haedersdal M, et al. A randomized split-scalp study comparing calcipotriol-assisted methyl aminolaevulinate photodynamic therapy (MAL-PDT) with conventional MAL-PDT for the treatment of actinic keratosis. Br J Dermatol. 2018;179(4):829–835. doi:10.1111/bjd.16473
70. Bullock TA, Negrey J, Hu B, et al. Significant improvement of facial actinic keratoses after blue light photodynamic therapy with oral vitamin D pretreatment: an interventional cohort-controlled trial. J Am Acad Dermatol. 2022;87(1):80–86. doi:10.1016/j.jaad.2022.02.067
71. Morton CA, Szeimies R-M, Basset‐Seguin N, et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: treatment delivery and established indications - actinic keratoses, Bowen’s disease and basal cell carcinomas. J Eur Acad Dermatol Venereol. 2019;33:2225–2238. doi:10.1111/jdv.16017
72. Arits AH, Mosterd K, Essers BA, et al. Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal-cell carcinoma: a single blind, non-inferiority, randomised controlled trial. Lancet Oncol. 2013;14:647–654. doi:10.1016/S1470-2045(13)70143-8
73. Roozeboom MH, Arits AHMM, Mosterd K, et al. Three-year follow-up results of photodynamic therapy vs imiquimod vs fluorouracil for treatment of superficial basal cell carcinoma: a single-blind, noninferiority, randomized controlled trial. J Invest Dermatol. 2016;136:1568–1574. doi:10.1016/j.jid.2016.03.043
74. Thomson J, Hogan S, Leonardi-Bee J, Williams HC, Bath-Hextall FJ. Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev. 2020;11:CD003412. doi:10.1002/14651858.CD003412.pub3
75. Collier NJ, Haylett AK, Wong TH, et al. Conventional and combination topical photodynamic therapy for basal cell carcinoma: systematic review and meta-analysis. Br J Dermatol. 2018;179:1277–1296. doi:10.1111/bjd.16838
76. Tarstedt M, Gillstedt M, Wennberg Larko AM, Paoli J. Aminolevulinic acid and methyl aminolevulinate equally effective in topical photodynamic therapy for non-melanoma skin cancers. J Eur Acad Dermatol Venereol. 2016;30:420–423. doi:10.1111/jdv.13558
77. Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309–319. doi:10.1111/bjd.16441
78. Salmivuori M, Grönroos M, Tani T, et al. Hexyl aminolevulinate, 5-aminolevulinic acid nanoemulsion and methyl aminolevulinate in photodynamic therapy of non-aggressive basal cell carcinomas: a non-sponsored, randomized, prospective and double-blinded trial. J Eur Acad Dermatol Venereol. 2020;34:2781–2788. doi:10.1111/jdv.16357
79. Genouw E, Verheire B, Ongenae K, et al. Laser-assisted photodynamic therapy for superficial basal cell carcinoma and Bowen’s disease: a randomized intrapatient comparison between a continuous and a fractional ablative CO 2 laser mode. J Eur Acad Dermatol Venereol. 2018;32:1897–1905. doi:10.1111/jdv.14989
80. Vrani F, Sotiriou E, Lazaridou E, et al. Short incubation fractional CO 2 laser-assisted photodynamic therapy vs. conventional photodynamic therapy in field-cancerized skin: 12-month follow-up results of a randomized intraindividual comparison study. J Eur Acad Dermatol Venereol. 2019;33:79–83. doi:10.1111/jdv.15109
81. Choi SH, Kim KH, Song KH. Er: YAG ablative fractional laser-primed photodynamic therapy with methyl aminolevulinate as an alternative treatment option for patients with thin nodular basal cell carcinoma: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2016;30:783–788. doi:10.1111/jdv.13453
82. Li C, Wang P, Wang D, et al. Fluorescence kinetics study of twice laser irradiation based HpD-PDT for nonmelanoma skin cancer. Lasers Surg Med. 2022;54:945–954. doi:10.1002/lsm.23538
83. Rigual N, Shafirstein G, Cooper MT, et al. Photodynamic therapy with 3-(1′-Hexyloxyethyl) pyropheophorbide a for cancer of the oral cavity. Clin Cancer Res. 2013;19:6605–6613. doi:10.1158/1078-0432.CCR-13-1735
84. Liew YCC, De Souza NNA, Sultana RG, Oh CC. Photodynamic therapy for the prevention and treatment of actinic keratosis/squamous cell carcinoma in solid organ transplant recipients: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2020;34:251–259. doi:10.1111/jdv.15852
85. Keyal U, Bhatta AK, Zhang G, Wang XL. Present and future perspectives of photodynamic therapy for cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2019;80:765–773. doi:10.1016/j.jaad.2018.10.042
86. Um W, Park J, Ko H, et al. Visible light-induced apoptosis activatable nanoparticles of photosensitizer-DEVD-anticancer drug conjugate for targeted cancer therapy. Biomaterials. 2019;224:119494. doi:10.1016/j.biomaterials.2019.119494
87. Ren Q, Yi C, Pan J, Sun X, Huang X. Smart Fe3O4@ZnO core-shell nanophotosensitizers potential for combined chemo and photodynamic skin cancer therapy controlled by UVA radiation. Int J Nanomedicine. 2022;17:3385–3400. doi:10.2147/IJN.S372377
88. Wang X, Shi L, Tu Q, et al. Treating cutaneous squamous cell carcinoma using 5-aminolevulinic acid polylactic-co-glycolic acid nanoparticle-mediated photodynamic therapy in a mouse model. Int J Nanomedicine. 2015;10:347–355. doi:10.2147/IJN.S71245
89. Mascaraque-Checa M, Gallego-Rentero M, Nicolás-Morala J, et al. Metformin overcomes metabolic reprogramming-induced resistance of skin squamous cell carcinoma to photodynamic therapy. Mol Metab. 2022;60:101496. doi:10.1016/j.molmet.2022.101496
90. Anand S, Rollakanti KR, Brankov N, et al. Fluorouracil enhances photodynamic therapy of squamous cell carcinoma via a p53-independent mechanism that increases protoporphyrin IX levels and tumor cell death. Mol Cancer Ther. 2017;16(6):1092–1101. doi:10.1158/1535-7163.MCT-16-0608
91. Choi SH, Kim KH, Song KH. Effect of methyl aminolevulinate photodynamic therapy with and without ablative fractional laser treatment in patients with microinvasive squamous cell carcinoma: a randomized clinical trial. JAMA Dermatol. 2017;153:289–295. doi:10.1001/jamadermatol.2016.4463
92. Zhou P, Li J, Song C, Lou Y, Fu B. The application of Wood’s lamp combined with 5-aminolevulinic acid for defining tumor margins in patients with extramammary Paget’s disease. Photodiagnosis Photodyn Ther. 2021;35:102490. doi:10.1016/j.pdpdt.2021.102490
93. Lin JD, Li MH, Wu TH, Chang CH. Combined methyl aminolevulinate-based photodynamic therapy and imiquimod in a patient with perianal extramammary Paget’s disease. Photodiagnosis Photodyn Ther. 2021;35:102407. doi:10.1016/j.pdpdt.2021.102407
94. Shim PJ, Zeitouni NC. Photodynamic therapy for extramammary Paget’s disease: a systematic review of the literature. Photodiagnosis Photodyn Ther. 2020;31:101911. doi:10.1016/j.pdpdt.2020.101911
95. Li X, Zhao C, Kou H, et al. PDD-guided tumor excision combined with photodynamic therapy in patients with extramammary Paget’s disease. Photodiagnosis Photodyn Ther. 2022;38:102841. doi:10.1016/j.pdpdt.2022.102841
96. Wang D, Wang P, Li C, et al. Efficacy and safety of HpD-PDT for Extramammary Paget’s Disease refractory to conventional therapy: a prospective, open-label and single arm pilot study. Photodiagnosis Photodyn Ther. 2022;37:102670. doi:10.1016/j.pdpdt.2021.102670
97. Li C, Guo L, Wang P, et al. ALA-PDT combined with holmium laser therapy of postoperative recurrent extramammary Paget’s disease. Photodiagnosis Photodyn Ther. 2019;27:92–94. doi:10.1016/j.pdpdt.2019.05.011
98. Apalla Z, Lallas A, Tsorova A, et al. Complete response of extramammary Paget’s disease with imiquimod and PDT: report of two cases. Photodermatol Photoimmunol Photomed. 2018;34:273–275. doi:10.1111/phpp.12386
99. Gao Y, Zhang X-C, Wang W-S, et al. Efficacy and safety of topical ALA-PDT in the treatment of EMPD. Photodiagnosis Photodyn Ther. 2015;12(1):92–97. doi:10.1016/j.pdpdt.2014.11.004
100. Wang HW, Lv T, Zhang -L-L, et al. A prospective pilot study to evaluate combined topical photodynamic therapy and surgery for extramammary paget’s disease. Lasers Surg Med. 2013;45:296–301. doi:10.1002/lsm.22142
101. Chen M, Chen X, Dai Y, et al. Excision combined with photodynamic therapy for scrotal Paget’s disease in patients aged over 60 years. Aging Male. 2020;23(5):854–859. doi:10.1080/13685538.2019.1607284
102. Shen S, Zhang G, Wang P, et al. ALA-PDT as palliative care in a patient with secondary perineum EMPD. Photodiagnosis Photodyn Ther. 2018;22:166–168. doi:10.1016/j.pdpdt.2018.04.002
103. Rioli DI, Samimi M, Beneton N, et al. Efficacy and tolerance of photodynamic therapy for vulvar Paget’s disease: a multicentric retrospective study. Eur J Dermatol. 2018;28:351–355. doi:10.1684/ejd.2018.3289
104. Vicentini C, Carpentier O, Lecomte F, et al. Treatment of a vulvar Paget’s disease by photodynamic therapy with a new light emitting fabric based device. Lasers Surg Med. 2017;49:177–180. doi:10.1002/lsm.22631
105. Su L, Chen Y, Huo H, et al. NIR-II ratiometric chemiluminescent/fluorescent reporters for real-time monitoring and evaluating cancer photodynamic therapy efficacy. Small. 2022;18(41):e2202551. doi:10.1002/smll.202202551
106. Xu FZ, Zhu L, Han H-H, et al. Molecularly engineered AIEgens with enhanced quantum and singlet-oxygen yield for mitochondria-targeted imaging and photodynamic therapy. Chem Sci. 2022;13(32):9373–9380. doi:10.1039/d2sc00889k
107. Heerfordt IM, Wulf HC. Daylight photodynamic therapy of actinic keratosis without curettage is as effective as with curettage: a randomized clinical trial. J Eur Acad Dermatol Venereol. 2019;33:2058–2061. doi:10.1111/jdv.15744
108. Nene LC, Magadla A, Nyokong T. Enhanced mitochondria destruction on MCF-7 and HeLa cell lines in vitro using triphenyl-phosphonium-labelled phthalocyanines in ultrasound-assisted photodynamic therapy activity. J Photochem Photobiol B. 2022;235:112553. doi:10.1016/j.jphotobiol.2022.112553
109. Tyrrell J, Paterson C, Curnow A. Regression analysis of protoporphyrin IX measurements obtained during dermatological photodynamic therapy. Cancers. 2019;11(1):72. doi:10.3390/cancers11010072
110. Kaw U, Ilyas M, Bullock T, et al. A regimen to minimize pain during blue light photodynamic therapy of actinic keratoses: bilaterally controlled, randomized trial of simultaneous versus conventional illumination. J Am Acad Dermatol. 2020;82(4):862–868. doi:10.1016/j.jaad.2019.09.010
111. Jansen MHE, Kessels JPHM, Merks I, et al. A trial-based cost-effectiveness analysis of topical 5-fluorouracil vs. imiquimod vs. ingenol mebutate vs. methyl aminolaevulinate conventional photodynamic therapy for the treatment of actinic keratosis in the head and neck area performed in the Netherlands. Br J Dermatol. 2020;183:738–744. doi:10.1111/bjd.18884
112. Heppt MV, Steeb T, Niesert AC, et al. Local interventions for actinic keratosis in organ transplant recipients: a systematic review. Br J Dermatol. 2019;180(1):43–50. doi:10.1111/bjd.17148
113. Li J, Xue Y, Tian J, et al. Fluorinated-functionalized hyaluronic acid nanoparticles for enhanced photodynamic therapy of ocular choroidal melanoma by ameliorating hypoxia. Carbohydr Polym. 2020;237:116119. doi:10.1016/j.carbpol.2020.116119
114. Li X, Tan L, Kou H, et al. Ocular preservation through limited tumor excision combined with ALA-PDT in patients with periocular basal cell carcinoma. Photodiagnosis Photodyn Ther. 2019;27:291–294. doi:10.1016/j.pdpdt.2019.06.016
115. van Doeveren TEM, van Veen RLP, van den Boom F, et al. Intra-cavity Photodynamic Therapy for malignant tumors of the paranasal sinuses: an in vivo light dosimetry study. Photodiagnosis Photodyn Ther. 2020;32:101972. doi:10.1016/j.pdpdt.2020.101972
116. Liao C, Zhang G, Wang P, Sun X, Wang X. Combination curettage and modified ALA-PDT for multiple basal cell carcinomas of the face and head. Photodiagnosis Photodyn Ther. 2021;35:102393. doi:10.1016/j.pdpdt.2021.102393
117. Jhanker Y, Mbano MN, Ponto T, et al. Comparison of physical enhancement technologies in the skin permeation of methyl amino levulinic acid (mALA). Int J Pharm. 2021;610:121258. doi:10.1016/j.ijpharm.2021.121258
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

