Back to Journals » The Application of Clinical Genetics » Volume 12
Genetics of COPA syndrome
Authors Kumrah R, Mathew B, Vignesh P, Singh S , Rawat A
Received 29 August 2018
Accepted for publication 28 November 2018
Published 8 February 2019 Volume 2019:12 Pages 11—18
DOI https://doi.org/10.2147/TACG.S153600
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Martin Maurer
Rajni Kumrah, Babu Mathew, Pandiarajan Vignesh, Surjit Singh, Amit Rawat
Pediatric Allergy and Immunology Unit, Department of Pediatrics, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India
Abstract: Inborn errors of immunity usually not only result in immunodeficiency but may also manifest as immune dysregulation in the form of autoinflammation, autoimmunity, or sometimes malignancy. One of the most recently discovered monogenic disorder of immune dysregulation is COPA syndrome. COPA syndrome is an inherited autoimmune disorder caused by mutations in COPA gene. COPA gene encodes for α subunit of the COP1 protein, which is involved in the reverse vesicular protein transport from Golgi apparatus to the endoplasmic reticulum (ER). The inheritance pattern of COPA syndrome is autosomal dominant, and the patients typically present with interstitial lung disease with pulmonary hemorrhage and subsequently develop arthritis. Immunological features involve autoantibody formation, elevated expression of IL-1β and IL-6, and increase in the number of Th17 cells. Molecular pathophysiology of COPA syndrome is not clearly understood. However, it is known that accumulation of unfolded proteins in ER leads to ER stress, which is an indirect result of aberrant vesicular transport of proteins from Golgi apparatus to ER and defective cellular autophagy. ER stress induces inflammation and is responsible for pathogenesis of a large number of chronic inflammatory diseases. Unfolded protein response process responds to improperly folded proteins and defends against stress in ER to ensure the fidelity of the protein folding. It maintains the expression of stress-response genes and causes initiation of inflammatory signaling pathways essential for the innate immunity. Mutation in COPA gene associated with defective protein sorting to ER has unearthed a new primary immunodeficiency disease with a unique clinical phenotype. This review highlights the clinical and molecular aspects of COPA syndrome.
Keywords: COPA syndrome, endoplasmic reticulum stress, autoimmunity, autoinflammation, protein transport, interstitial lung disease, arthritis, Golgi apparatus, interleukins
Introduction
COPA syndrome has been classified under the category of autoinflammatory disorders in the 2017 Update of the Classification of Inborn Errors of Immunity by International Union of Immunological Societies (IUIS).1 In recent years, many important immune dysregulation syndromes such as autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) caused by mutated AIRE; immune dysregulation, polyendocrinopathy, and enteropathy caused by mutations in FOXP3 (IPEX); and autoimmune lymphoproliferative syndrome (ALPS) caused by mutated Fas/FasL have unveiled the role of autoimmune regulator protein, T-regulatory cells, and Fas/FasL, respectively, in immune tolerance. Having studied the role of these molecules, we now have a significantly clearer view of the underlying pathophysiology of some of the monogenic autoimmune diseases.
Autoimmune manifestations with an onset at a very early age or of familial nature are likely due to monogenic defects. Normally, non-immune tissues are mostly affected in pediatric autoimmune diseases such as skin, kidneys, joints, and endocrine organs (type 1 diabetes). Autoimmunity involving the lungs is rare in pediatric population. Granulomatosis with polyangiitis and microscopic polyangiiitis collectively called ANCA-associated vasculitis are the established cause of autoimmune pulmonary hemorrhage. Anti-myeloperoxidase and anti-proteinase 3 antibodies are found to be elevated in ANCA-associated vasculitides.2 Children having ANCA-associated vasculitis frequently present with fever, renal disease, and malaise along with pulmonary hemorrhage.3–5
Diseases such as systemic lupus erythematosus (SLE) and other immune dysregulation diseases such as juvenile dermatomyositis and scleroderma may progress into non-specific interstitial pneumonia (NSIP).6–9 Recent findings of gain of function mutations in TMEM173 causing STING-associated vasculopathy of infancy (SAVI) syndrome provide clues about interstitial lung disease (ILD) and its association with an increased production of IFNs.10,11 Patients with COPA syndrome share some of the clinical features with SAVI syndrome, such as ILD and an upregulated IFN signature.10–13
Large number of patients with STAT1 GOF mutations has chronic mucocutaneous candidiasis (CMC) and recurrent lower respiratory tract bacterial infections. Patients with germline STAT3 GOF mutations have been associated with early-onset multiorgan autoimmunity, lymphoproliferation, early-onset growth failure, and may have severe recurrent infections. Studies have also shown an association of STAT3 GOF with interstitial pneumonitis and role of STAT3 signaling in interstitial and fibrotic lung disease pathogenesis.14
The etiopathogenesis of COPA syndrome is unknown, but it is hypothesized that disturbances in protein trafficking pathway can lead to endoplasmic reticulum (ER) stress that results in unfolded protein response (UPR) activation, thereby resulting in upregulation of T helper (Th)-17 cells and hence autoimmunity.15 However, due to ubiquitous expression of COPA gene, it might have different effects on different types of cells and tissues. It might represent a mixed pattern disorder like type 1 interferonopathies having features of both autoimmunity and autoinflammation.16
Considering the fact that aforementioned diseases with confirmed mutations have overlapping clinical symptoms with the COPA syndrome, here in this review, we have explained the clinical and genetic features of the poorly understood disease.
What is COPA syndrome?
COPA syndrome is a monogenic autoimmune disease described in 2015 that usually affects the lungs and joints. Most of the patients with COPA syndrome present with DPLD or diffuse alveolar hemorrhage (DAH) and arthritis.15–17
COPA syndrome is called so because it is caused by missense mutations in the COPA gene located on chromosome 1 (1q23.2), which is inherited in an autosomal dominant mode in a heterozygous fashion. COPI is a heptameric protein associated with membranes by ARF1, a GTP-binding protein. This subunit binds to proteins having dilysine residues at carboxyl-terminal end and is involved in retrograde protein trafficking.12 Excessive ER stress in COPA syndrome is probably related to disrupted retrograde transport causing aberrant cellular autophagy leading to an impaired early endosomal function.
Earlier, these processes were not thought of causing immune dysregulation, hence, studying COPA syndrome would be highly helpful in understanding links between immune dysregulation and intracellular trafficking of vesicular proteins. Furthermore, it would also pave way for new therapeutic targets for the management of patients with COPA syndrome. COPα is expressed on all the cells of the body, but clinical phenotypes are seen only on pulmonary, joint, and renal tissues, which are supposedly more vulnerable to this mutation.
Clinical and laboratory features of COPA syndrome
COPA syndrome is associated with immune dysregulation. Clinical manifestations usually start at early childhood, with an average age of presentation at 3.5 years. Seven studies reported 32 individuals having confirmed mutations in the COPA gene.18 There is no racial predilection for COPA syndrome. It has been reported in Caucasians, Asians, African Americans, and Icelandic-Nordic population. Gender bias has been reported in the expressivity and penetrance of the disease with 13 of the 14 females with COPA mutations showing clinical signs of the disease.18 Pulmonary manifestations are present in the majority of the patients, either as ILD or DAH. The lung histopathology in these patients has revealed features similar to that of ILD in other systemic autoimmune diseases. Arthritis as the predominant clinical manifestation is seen in 95% of COPA patients (Table 1). Renal manifestations such as immune complex glomerulonephritis have been documented in four of the 21 patients in a cohort by Watkin et al.15
  | Table 1 Clinical phenotype and pattern of COPA gene mutations in different cohorts Abbreviations: ANA, anti-nuclear antibody; ANCA, anti-neutrophil cytoplasmic antibody; F, female; M, male. |
Laboratory tests have revealed elevated levels of various inflammatory markers such as rheumatoid factor, erythrocyte sedimentation rate (ESR), and CRP. Forty-three percent of cases reported autoantibodies to rheumatoid factor.15 Positive ANA titer and rheumatoid factor have been reported in most of the patients, while other antibodies such as perinuclear ANCA (pANCA), anti-myeloperoxidase antibodies, cytoplasmic ANCA (cANCA), and anti-proteinase three antibodies may also be present in few patients. Upregulation of Th17 priming cytokines (IL-17A, IL-23, IL-6, and IL-1β) with various other pro-inflammatory cytokines including IL-1β and IL-6 while downregulating IFN-γ-secreting Th1 cells has also been observed.12 Watkin et al15 demonstrated a significant change in the phenotype of Th cells toward the Th17 phenotype, which is an effector population of T-cells involved in the process of autoimmunity.19,20 Despite an increased Th17 cells, psoriasis or inflammatory bowel disease (IBD) has not been reported in COPA patients till date.
Molecular genetics
All COPA mutations identified till date are missense mutations in exons that encode WD40 domain of the COPA protein. Size of the COPA gene is 54 kb (54,978 bp), with mRNA sequence of 5,666 bp. Size of genomic DNA is 37 kb, while its coding sequence is of 3,702 bp. It has 33 exons ranging from 67 to 611 bp in size with 32 introns present in the gene ranging from 80 bp to 4 kb (Figure 1).The untranscribed and non-coding portions of the 5′ of the gene are rich in GC content consistent with its being a housekeeping gene.21
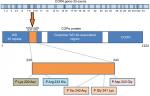  | Figure 1 Schematic representation of COPA gene with annotated exons and protein structure describing the respective domains with most reported mutations. |
Studies have reported COPA gene to be highly constrained, which had low scores in both missense and loss of function mutations.22,23 The predicted deleterious effects due to the loss-of-function mutations in COPA gene and missense mutations in the WD40 domain appears to be same.27 Watkin et al have reported four different heterozygous missense mutations affecting WD5 and WD6 repeats in WD40 domain of COPA gene. These mutations were identified in 30 individuals from five different families who were affected with autoimmune interstitial lung, joint, and kidney disease.15 WES and targeted sequencing were done for the identification of unique mutations in these families. Hot spot mutations reported include Arg233His, Asp243Gly, Glu241Lys, and Lys230Asn.15 Prediction algorithms (SIFT and Polyphen-2 scores) predicted all the mutations to be damaging and disease causing. Most common mutation reported is (p.Arg233His) followed by (p.Glu241 Lys).
Tsui et al17 reported that all of these missense mutations occur in the eighth and ninth exon of the COPA gene that encodes C’ of the WD40 domain in COPα protein. Impaired binding to proteins targeted for retrograde transport has been shown in two mutant variants in in vitro studies. All identified variants were found to be deleterious by various prediction tools and were located in 14 amino acid sequence in the highly conserved WD40 region of COPA (Figure 1). WD repeats are highly conserved units involved in cell division, determination of cell fate, and vesicular trafficking.24–26 Another WES study in six family members (three affected and three unaffected) revealed recurrent missense mutations with heterozygous pattern (p.Glu241Lys) in WD40 domain of COPA gene in an Icelandic family.27 Another study revealed (p.W240R) variant in the COPA gene in a 12-year-old boy who was presented with dyspnea, clubbing, and fatigue.28 Volpi et al reported c.698G>A mutation by targeted next-generation sequencing. Mutation was confirmed by Sanger sequencing in the patient and her mother.13
Quantitative PCR and immunoblots were carried out for determining the expression level of the COPA transcript and its protein patterns.15 Results revealed no significant differences between COPA expression in patients and controls.15 Normal distribution of COPA protein was demonstrated by imaging analysis throughout the cell, indicating that the variant did not cause mislocalization of COPA to impair its function.15,29
ER stress and UPR
Synthesis of protein and its folding takes place in ER. Cells have molecular chaperones (BiP-binding immunoglobulin protein) for folding of the polypeptide chain into a specific conformation so as to avoid inappropriate interactions.30 Properly folded proteins are destined to secretory pathway through Golgi apparatus, while misfolded proteins are degraded by ER-associated degradation (ERAD). Physiological states that demand more protein folding or cause disruption of protein folding by various stimuli or states in which the number of proteins entering the ER exceeds its folding capacity causes imbalance and hence resulting in improperly folded proteins to gather in the ER – a phenomenon termed as ER stress. Eukaryotic cells have various intracellular signaling paths that respond to improperly folded proteins and defend against stress in ER to ensure the fidelity of protein folding. These processes are called UPR that causes alterations at RNA and protein level to cope with ER stress and sort out protein folding defects.31,32
BiP (molecular chaperone) is responsible for appropriate folding of polypeptide chains in ER and provides shielding from inappropriate interactions.34 During stress, ER stress sensors get activated through sequestration of BiP to improperly folded polypeptide chains.35 However, in non-stressed cells, BiP binds to IRE1α, PERK, and ATF6 stress sensors resulting in their inactivation. Three UPR signaling pathways are activated by the three ER stress sensors: PERK, IRE1α, and ATF6.31,32 These transmembrane proteins sense unfolded proteins through their ER-luminal domain and target them to the ER membrane. IRE1α,/XBP-1 arm of UPR pathway is responsible for induction of IL-6 during differentiation of plasma cells while the function is taken over by PERK/ATF4 pathway during glucose insufficiency in cancer cells.33 Activated IRE1α selectively causes splicing of intron of 26 bp from XBP1 mRNA and causes its nuclear translocation and hence upregulating the expression of target genes related to UPR signaling pathway.31,32 During ER stress, BiP removal activates PERK causing the units to dimerize and resulting in trans-autophosphorylation. Kinase activity of PERK causes phosphorylation of eIF2α and reduces the translation initiation. However, phosphorylated eIF2α specifically translates ATF4 mRNA and increases the expression of UPR-related genes.36,37 ATF6 after release from BiP gets cleaved by (S1P and S2P proteases) in Golgi apparatus, giving an active ATF6 p50 that upregulates UPR-related gene expression. Cleaved ATF6 induces the transcription of genes that promote proper protein folding, their maturation, and finally their secretion.38,39 However, if UPR fails to solve protein folding problems, it will initiate apoptosis mediated by CHOP, thereby protecting the organism. In addition to anterograde transport, some proteins require ER export chaperones or receptors for their exit into transport vesicles.
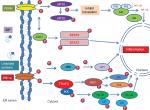
ER stress and inflammation
Recent studies evidenced that molecular relation exists between ER stress and inflammation. Reactive oxygen species production, calcium release from ER, NF-κB activation, and proteins like p38 and c-Jun-N-terminal kinase cause initiation of inflammatory pathways.38 NF-κB has an important role in regulation of transcription and is an important mediator of inflammation.39 Activation of NF-κB is responsible for inflammatory gene expression, which is caused by ER stress by driving the production of various adhesion molecules, pro-inflammatory cytokines and chemokines.42 NF-κB signaling gets activated by UPR through PERK–eIF2α-mediated weakening of translation response to ER stress (Figure 2). This increases in NF-κB to IκB ratio, thereby causing its nuclear localization.40 In the absence of an activation signal, NF-κB binds to IκB and exists in an inactive dimeric form. However, activation signal causes phosphorylation of IκB, thereby causing its proteasomal degradation and hence translocates NF-κB to nucleus. NF-κB signaling pathway gets activated by all three arms of UPR.41 Also, IRE1 interacts with TRAF2 and IκB kinase, which increases the expression of inflammatory genes by activating NF-κB.42 Studies have shown that PERK disruption can nullify ER stress-induced STAT3 activation and hence their subsequent gene expression.
Functional tests have also been performed for the assessment of enhanced ER stress in COPA patients. Protein expression and mRNA level (qPCR) of COPA in thapsigargin-treated B lymphoblastoid cell line (BLCL) was determined. Results revealed significantly elevated levels of BiP in COPA patients compared to control.15 In addition, siRNA knockdown experiments in human embryonic kidney (HEK) cells were performed for analyzing the involvement and the potential role of mutant COPA in ER stress. Results indicate increased ER stress due to decreased COPA expression marked by increased BiP expression-ER stress marker.15 When mutant and wild type COPA were overexpressed in the human embryonic kidney cells (HEK), increased expression of BiP was seen in COPA mutants as compared to wild type.15 Impaired binding of yeast Wbp1 to the mutant COPA (E241K) was demonstrated in a study.15 When E241K mutant COPA cell line was transfected with a reporter protein having dilysine retrieval signal, impaired binding of E241K variant COPA was demonstrated by immunoprecipitation assay. This provides evidence for improper binding of variant COPA and dilysine tagged proteins, showing defective protein trafficking.43
ROS, ER stress, and UPR activation
ER stress is related to accumulation of intracellular ROS that leads to oxidative stress. Intramolecular and intermolecular disulphide bond formation requires oxidizing environment leading to generation of ROS.44 Excessive protein folding in ER can cause ROS accumulation, thereby eliciting an inflammatory response.38 Excessive ROS then targets calcium channels present on ER, resulting in upregulation of calcium release from ER into the cytosol, which then gets concentrated in the mitochondrial matrix disrupting the electron transport chain (ETC), thereby further increasing ROS production.38 This ROS will further aggravate calcium release from ER and can disrupt protein folding process causing ER stress, UPR induction, and further enhances ROS production.38
ER stress in lung diseases and arthritis
The lungs and gut share embryological origins, but most of the studies done so far had concentrated on the gut. Animal studies demonstrated inflammation of colon in IRE1β knockout mouse.45 Studies have been done on ER stress in alveolar epithelium, but the involvement of particular cells that involves ER stress in lungs is still not clear. Studies revealed the involvement of COPA gene mutations in arthritis and ILD with UPR activation observed in the epithelium of lungs.15 Studies on CHOP (bZip transcription factor) expression revealed its significant high levels in the presence of mutant COPA or when COPA expression was reduced.46 Microarray analysis in patients with myositis has revealed the increased expression of glucose-regulated protein of 78 kDa (GRP78), suggesting the involvement of the ER response in the skeletal muscle damage in autoimmune myositis.47
Renal manifestations
Studies demonstrated an increased risk for renal disease in patients with COPA syndrome with age of onset from mid to late teenage. Glomerular disease (44%) with or without immune complex deposition, proteinuria, and decreased kidney function have been documented in patients with COPA.12 Renal histology showed glomerulopathy. Another study reported acquired renal disease in a subset of patients (21%).17 Different types of glomerular lesions were present in patients with COPA syndrome. IgA nephropathy with necrotizing lesions has been reported in some patients, while others had mesangial hypercellularity. Necrotizing lesions were present in 75% of patients with renal disease. Recent study documented clear cell renal carcinoma, renal cysts, nephrolithiasis, and pyelonephritis as important clinical manifestations of disease.48 A study reported a patient with chronic renal disease who undergo kidney transplantation. Early recognition of COPA might be able to reduce risk of glomerular injury.12 Immune-mediated kidney disease in combination with DAH has also been documented in three patients.17 However, normal kidney function has been reported in a study of two families carrying the p.Glu241Lys mutation, suggesting its non-deleterious effects on renal function.15
ER stress in central nervous system (CNS)
Accumulation of improper proteins in ER of neuroglia and neurons is the pathological hallmark of large number of neurodegenerative disorders that results in the clinical manifestations of various CNS diseases. ER stress is reported in Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. The UPR pathway induced in response to ER stress brings about changes at transcriptional and protein level to minimize stress and to reduce protein misfolding. However, prolonged ER stress causes disruption of the protective role of UPR, resulting in activation of inflammatory pathway and other apoptotic signals, hence contributing to neuronal dysfunction. ER stress in astrocytes of murine activates PERK-mediated inflammatory pathway in vitro, suggesting that the role of astrocytes in neurotoxic inflammation during ER dysfunction.49–51 However, ER stress resulting in CNS phenotype has not been reported in any of the COPA patients thus far.
ER stress in gut and skin
The epithelial cells of gut are exposed to complex microbiota, antigens, and various bacterial toxins. During bacterial infection, the excessive production of MUC2 or defensins exerts a significant burden on ER in intestinal epithelial cells that poses threat to protein folding capacity and hence causes ER stress. Recent studies reported the association of ER stress and UPR with susceptibility to IBD, Crohn’s disease, and ulcerative colitis.52,53 Genetic defects in UPR-related genes are also associated with IBD due to unfolded proteins in ER and overt immune response in epithelial cells.54 An increased ER stress also results in epidermal differentiation so that it might be involved in psoriasis.55,56 Studies reported the role of ER stress in damage of melanocytes in vitiligo patients and results in cell degeneration.57 However, the contribution of ER stress response in disease pathology remains to be elucidated.
Conclusion
Improper protein folding in ER is associated with the pathogenesis of a large number of disorders. In addition, stress in ER can result in lung diseases by triggering inflammatory signaling and by modulating differentiation status. Discovery of a molecular relationship between a defective COPI protein transport pathway and autoimmunity establishes a new aim for understanding the involvement of intracellular transport in autoimmunity and as a potential therapeutic option in a large number of diseases. Future studies are needed to explain that how mutant COPA is associated with ER stress and induction of autoinflammatory diseases. This new approach will help in the development of new therapeutic strategies for combating stress in cells and inflammation.
Abbreviations
ATF6, activating transcription factor 6; BiP, binding immunoglobulin protein; CHOP, C/EBP homologous protein; COPA, coatomer protein subunit alpha; DPLD, diffuse parenchymal lung disease; eIF2α, eukaryotic translation-initiation factor 2α; ER, endoplasmic reticulum; IL-6, interleukin 6; IRE1α, inositol-requiring 1α; NF-κB, nuclear factor-κB; PERK, protein kinase (PKR)-like ER kinase; ROS, reactive oxygen species; TRAF2, TNF receptor-associated factor 2; UPR, unfolded protein response; WES, whole exome sequencing.
Disclosure
The authors report no conflicts of interest in this work.
References
Bousfiha A, Jeddane L, Picard C, et al. The 2017 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. 2018;38(1):129–143. | ||
Brown KK. Pulmonary vasculitis. Proc Am Thorac Soc. 2006;3(1):48–57. | ||
Sacri AS, Chambaraud T, Ranchin B, et al. Clinical characteristics and outcomes of childhood-onset ANCA-associated vasculitis: a French nationwide study. Nephrol Dial Transplant. 2015;30 Suppl 1:104–112. | ||
Siomou E, Tramma D, Bowen C, Milford DV. ANCA-associated glomerulonephritis/systemic vasculitis in childhood: clinical features-outcome. Pediatr Nephrol. 2012;27(10):1911–1920. | ||
Morishita K, Li SC, Muscal E, et al. Assessing the performance of the birmingham vasculitis Activity Score at diagnosis for children with antineutrophil cytoplasmic antibody-associated vasculitis in A Registry for Childhood Vasculitis (ARChiVe). J Rheumatol. 2012;39(5):1088–1094. | ||
Barile-Fabris L, Hernández-Cabrera MF, Barragan-Garfias JA. Vasculitis in systemic lupus erythematosus. Curr Rheumatol Rep. 2014;16(9):440. | ||
Kobayashi N, Takezaki S, Kobayashi I, et al. Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology. 2015;54(5):784–791. | ||
Burns NS, Stevens AM, Iyer RS. Shrinking lung syndrome complicating pediatric systemic lupus erythematosus. Pediatr Radiol. 2014;44(10):1318–1322. | ||
Valeur NS, Stevens AM, Ferguson MR, Effmann EL, Iyer RS. Multimodality thoracic imaging of juvenile systemic sclerosis: emphasis on clinical correlation and high-resolution CT of pulmonary fibrosis. AJR Am J Roentgenol. 2015;204(2):408–422. | ||
Liu Y, Jesus AA, Marrero B, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med Overseas Ed. 2014;371(6):507–518. | ||
Jeremiah N, Neven B, Gentili M, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. 2014;124(12):5516–5520. | ||
Vece TJ, Watkin LB, Nicholas S, et al. Copa syndrome: a novel autosomal dominant immune dysregulatory disease. J Clin Immunol. 2016;36(4):377–387. | ||
Volpi S, Tsui J, Mariani M, et al. Type I interferon pathway activation in COPA syndrome. Clin Immunol. 2018;187:33–36. | ||
Fabre A, Marchal S, Forbes LR, et al. STAT3 gain of function: a new Kid on the block in interstitial lung diseases. Am J Respir Crit Care Med. 2018;197(11):e22–e23. | ||
Watkin LB, Jessen B, Wiszniewski W, et al. COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat Genet. 2015;47(6):654–660. | ||
Volpi S, Picco P, Caorsi R, Candotti F, Gattorno M. Type I interferonopathies in pediatric rheumatology. Pediatr Rheumatol Online J. 2016;14(1):35. | ||
Tsui JL, Estrada OA, Deng Z, et al. Analysis of pulmonary features and treatment approaches in the COPA syndrome. ERJ Open Res. 2018;4(2):00017-2018. | ||
Taveira-Dasilva AM, Markello TC, Kleiner DE, et al. Expanding the phenotype of COPA syndrome: a kindred with typical and atypical features. J Med Genet. 2018:jmedgenet-2018-105560. | ||
Leipe J, Grunke M, Dechant C, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62(10):2876–2885. | ||
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–898. | ||
Quek HH, Chow VT. Genomic organization and mapping of the human HEP-COP gene (COPA) to 1q. Cytogenet Cell Genet. 1997;76(3-4):139–143. | ||
ExAC. Exome Aggregation Consortium (ExAC) browser; 2015. Available from: http://exac.broadinstitute.org. Accessed Jan 6, 2016. | ||
Samocha KE, Robinson EB, Sanders SJ, et al. A framework for the interpretation of de novo mutation in human disease. Nat Genet. 2014;46(9):944–950. | ||
Eugster A, Frigerio G, Dale M, Duden R. COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. Embo J. 2000;19(15):3905–3917. | ||
Neer EJ, Schmidt CJ, Nambudripad R, Smith TF. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371(6495):297–300. | ||
Li D, Roberts R. WD-repeat proteins: structure characteristics, biological function, and their involvement in human diseases. Cell Mol Life Sci. 2001;58(14):2085–2097. | ||
Jensson BO, Hansdottir S, Arnadottir GA, et al. COPA syndrome in an Icelandic family caused by a recurrent missense mutation in COPA. BMC Med Genet. 2017;18(1):12. | ||
Noorelahi R, Perez G, Otero HJ. Imaging findings of Copa syndrome in a 12-year-old boy. Pediatr Radiol. 2018;48(2):279–282. | ||
Popoff V, Adolf F, Brügger B, Wieland F. COPI budding within the Golgi stack. Cold Spring Harb Perspect Biol. 2011;3(11):a005231. | ||
Dufey E, Sepúlveda D, Rojas-Rivera D, Hetz C. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 1. An overview. Am J Physiol Cell Physiol. 2014;307(7):C582–C594. | ||
Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. | ||
Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. | ||
Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2(6):326–332. | ||
Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167(1):27–33. | ||
Yaman I, Fernandez J, Liu H, et al. The zipper model of translational control: a small upstream ORF is the switch that controls structural remodeling of an mRNA leader. Cell. 2003;113(4):519–531. | ||
Yamamoto K, Sato T, Matsui T, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev Cell. 2007;13(3):365–376. | ||
Wu J, Rutkowski DT, Dubois M, et al. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13(3):351–364. | ||
Zhang K, Kaufman RJ, Randal J. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–462. | ||
Rius J, Guma M, Schachtrup C, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453(7196):807–811. | ||
Deng J, Lu PD, Zhang Y, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24(23):10161–10168. | ||
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. | ||
Eugster A, Frigerio G, Dale M, Duden R. COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. Embo J. 2000;19(15):3905–3917. | ||
Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164(3):341–346. | ||
Bertolotti A, Wang X, Novoa I, et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107(5):585–593. | ||
Wang XZ, Lawson B, Brewer JW, et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Mol Cell Biol. 1996;16(8):4273–4280. | ||
Nagaraju K, Casciola-Rosen L, Lundberg I, et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 2005;52(6):1824–1835. | ||
Taveira-Dasilva AM, Markello TC, Kleiner DE, et al. Expanding the phenotype of COPA syndrome: a kindred with typical and atypical features. J Med Genet. 2018:jmedgenet-2018-105560. | ||
Sprenkle NT, Sims SG, Sánchez CL, Meares GP. Endoplasmic reticulum stress and inflammation in the central nervous system. Mol Neurodegener. 2017;12(1):42. | ||
Meares GP, Liu Y, Rajbhandari R, et al. PERK-dependent activation of JAK1 and STAT3 contributes to endoplasmic reticulum stress-induced inflammation. Mol Cell Biol. 2014;34(20):3911–3925. | ||
Guthrie LN, Abiraman K, Plyler ES, et al. Attenuation of PKR-like ER Kinase (PERK) signaling selectively controls endoplasmic reticulum stress-induced inflammation without compromising immunological responses. J Biol Chem. 2016;291(30):15830–15840. | ||
Cao SS, Zimmermann EM, Chuang BM, et al. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology. 2013;144(5):989–1000.e6. | ||
Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. | ||
Kaser A, Lee AH, Franke A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134(5):743–756. | ||
Celli A, Mackenzie DS, Crumrine DS, et al. Endoplasmic reticulum Ca2+ depletion activates XBP1 and controls terminal differentiation in keratinocytes and epidermis. Br J Dermatol. 2011;164(1):16–25. | ||
Sugiura K, Muro Y, Futamura K, et al. The unfolded protein response is activated in differentiating epidermal keratinocytes. J Invest Dermatol. 2009;129(9):2126–2135. | ||
Li S, Zhu G, Yang Y. Oxidative stress drives CD8+ T-cell skin trafficking in patients with vitiligo through CXCL16 upregulation by activating the unfolded protein response in keratinocytes. J. Allergy Clin. Immunol. 2017;140(1):177–189.e9. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.