Back to Journals » Journal of Pain Research » Volume 12
Genetic study in patients operated dentally and anesthetized with articaine-epinephrine
Authors López-Valverde N , López-Valverde A , Gómez de Diego R , Cieza-Borrella C, Ramírez JM, González-Sarmiento R
Received 7 November 2018
Accepted for publication 26 March 2019
Published 29 April 2019 Volume 2019:12 Pages 1371—1384
DOI https://doi.org/10.2147/JPR.S193745
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Nansi López-Valverde,1 Antonio López-Valverde,1 Rafael Gómez de Diego,2 Clara Cieza-Borrella,3 Juan M Ramírez,4 Rogelio González-Sarmiento3
1Dental Clinic, Department of Surgery, Biomedical Research Institute of Salamanca (IBSAL), Salamanca, Spain; 2Dental Clinic, Alfonso X El Sabio University, Madrid, Spain; 3Molecular Medicine Unit, Department of Medicine, Biomedical Research Institute of Salamanca (IBSAL), Salamanca, Spain; 4Department of Morphological Sciences, School of Medicine, University of Córdoba, Córdoba, Spain
Aims: In this study we wanted to figure out if there was a correlation between OPRM1 N40D, TRPV1 I316M, TRPV1 I585V, NOS3 −786T>C and IL6 −174C>G polymorphisms and the response to locally applied articaine-epinephrine anesthetic.
Methods: In this observational study, 114 oral cell samples of patients anesthetized with articaine-epinephrine (54 from men 60 from women), were collected from dental centers in Madrid (Spain). High molecular weight DNA was obtained from oral mucosa cells. The analysis of OPRM1 N40D (rs1799971), TRPV1 I316M (rs222747), TRPV1 I585V (rs8065080) and IL6 −174C>G polymorphism was performed through real-time PCR allelic discrimination using TaqMan probes. Polymorphism NOS3 −786T> C (rs2070744) was analyzed using RFLP-PCR.
Results: The studied polymorphisms are involved neither in the response to the anesthetic, nor in the intensity of perceived dental pain. However, in a subset of female patients we found that TRPV1 I316M was associated with a delayed onset of anesthesia.
Conclusions: There is no association among these polymorphisms and the time elapsed between the application of the anesthetic and the onset of its effect.
Keywords: Pain, polymorphism, OPRM1, TRPV1, NOS3, IL6
Introduction
Pain can be defined as an unpleasant experience associated with actual or potential tissue damage. It is classically divided into nociceptive pain, which appears as the normal response to tissue damage and pathological pain. The latter, in turn, can be divided into inflammatory, which is produced by the action of mediators of inflammation substances that act as promoters of nociception; or neuropathic, which is the result of direct damage to the central or peripheral nervous system.
Pain perception is not a simple matter and, since it is influenced by a variety of environmental and genetic factors1, individual differences in sensitivity and pain tolerance are large. The severity of pain is controlled by genetic variants affecting the expression or function of nociceptive sensory system components.2–4 However, the pain inheritance patterns are complex and phenotypes are the result of the expression of multiple differently distributed genes.
Since pain origin is polygenic, the combination of the alleles of different genes can lead to different genotypes with different degrees of vulnerability that determine individual differences in the efficiency and kinetics of analgesics.
The effect of pain relief drugs is modulated by different factors such as the clinical course, severity and individual perception of pain, factors that alter the pharmacokinetic mechanisms controlling the local availability of analgesic molecules at their site of action, factors involved in the interaction of analgesic molecules with their target structures influencing the intensity of pain, factors that modulate opioid dosage requirements by conferring a risk of drug addiction and side effects,3 and variations in genes coding for proteins involved in all stages of drug interaction with the body.5
Dental surgery induces transient nociceptive pain, and different genes have been involved either in its perception or in its response to analgesic drugs. For example, opioid peptides, which function as neuromodulators of pain, and their receptors, among which is the μ-Opioid receptor (OPRM1), whose polymorphism cause variability in receptor density and function, may explain the variation of responses among patients6 and has been related to nociception.7 Transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor, is involved in neuropathic pain8 as well as nociception.9 Nitric oxide synthases (NOS), which are enzymes that synthesize nitric oxide (NO) one of the most abundant neurotransmitters in the regulatory processes of nociceptive stimulus, are also involved in nociception, neuropathic and inflammatory pain.10–14 Interleukin-6 (IL-6) is one of the pro-inflammatory cytokines that modulate the presence of extracellular and intracellular mediators that are activated during transduction, conduction and transmission of painful stimuli and it has been involved in nociception.15,16 As far as we know these polymorphisms are not involved in articaine metabolism, and there is compelling evidence indicating that they play a major role in pain response. Therefore, these alleles are good candidates that require further study. To this end, we analyzed, for the first time, the possible association of anesthesia effectiveness with the OPRM1 N40D polymorphism, whose amino acid change in the extracellular domain modifies its affinity for endogenous and exogenous ligands such as β-endorphin.17 Moreover, the OPRM1 rs11799971 (c.118A>G; p. N40D) allele G is associated with lower gene expression and a decreased number of cell surface receptors.18,19 The polymorphisms of the TRPV1 gene I315M and I585V, whose amino acids changes apparently do not alter the structure or function of the receptor20 (allele M) have been associated with a higher response to capsaicin, while TRPV1 rs8065080 (c.1191A>G; p.I585V) allele G has been associated with a higher tolerance to pain and a lower capsaicin response.21 The IL6 −174C>G polymorphism changes protein expression levels22 and the NOS3 −786T>C polymorphism, which regulates the transcription rate of the NOS3 gene and has the ability to change enzyme levels, has been associated with less promoter activity and protein expresion.23,24
We believe that for clinical practice it is of utmost importance to understand the mechanism behind pain production in the patients and thus in the future be able to predict and avoid or reduce the possible pain produced by the oral interventions. With this aim in mind, we recruited both male and female healthy patients for carrying out an observational study. Thus, we think that the aforementioned four genes are strong candidates to play a role in nociception and pain, so that the aim of our study was to analyze OPRM1 N40D, TRPV1 I316M, TRPV1 I585V, IL6 −174C>G and NOS3 −786T>C polymorphisms and their possible relationship with the articaine-epinephrine oral anesthesia’s delayed time of onset and with pain perception.
Patients and methods
Patients
This observational study was carried out once informed signed consent had been obtained from the participants. In total, 114 oral cell samples (54 from men 60 from women) were collected from dental centers in Madrid (Spain) from May to December 2009. The ages of the patients included in the study ranged between 18 and 92, with an average age of 47.99±15 and a median of 48. The patients were apparently healthy, of both sexes and had good oral hygiene. The exclusion criteria were as follows: patients with an active systemic infection or any other severe uncontrolled systemic disease, diabetics and those with thyroid endocrinopathy or fibromyalgia, patients who had received head and neck radiation therapy and/or chemotherapy, lactating women, and individuals with cognitive impairment. The patients who had undergone orthognathic, oral or implant surgery less than 1 year prior to the onset of the study or with trauma in the orofacial territory and/or associated structures were excluded from the study. This also included patients who had received more than 3 years of treatment with oral bisphosphonates or less than 3 years of concomitant treatment with immunosuppressant. Exclusion criteria also included patients who smoked, and in particular those who consumed more than 10 cigarettes a day.
This study was approved by the ethics board of University of Salamanca and the University Hospital of Salamanca and was in accordance with the Helsinki declaration.
Samples were collected by unilateral exfoliative cytology using simple, dry (without medium) and sterile swabs. Then, the amide oral infiltrative anesthetic articaine with epinephrine, was applied in the corresponding oral region under intervention (Table S1).
Subsequently, subjects were operated on using different oral surgery techniques for dental implants and extraction. A protocol to collect clinical and demographic data was developed, and surgery was performed in the mandibular and maxillary area, numbered according to the sextants defined by the International Dental Federation (IDF).
Anesthesia
A carpule of Ultracain® with epinephrine 40/0.01 mg/ml containing 4% articaine vasoconstrictor epinephrine 1:100.000 (Normon S.A.) was applied to different anatomical regions of the oral cavity. Articaine is less toxic than other drugs belonging to the same family due to the presence of an additional ester group that is rapidly hydrolyzed by plasma esterases, and this type of anesthesia was chosen because of the latency, potency and duration of its effect on soft tissues compared to others with similar chemical structures.25 The presence of a vasoconstrictor in the solution, usually 1:200.000 adrenaline or 1:100.000 epinephrine, decreases the rate of absorption, resulting in lower latency and increased duration of action, so that lower doses are required and local hemostasis is promoted.26,27
Pain scales
We performed an objective and a subjective analysis of pain post-anesthetic administration. In all of the patients who underwent surgery, pain measurements were made by a single examiner. Onset time of the anesthesia was determined objectively with a stesiometer (Table 1). Objective pain (Algometry) was assessed using a pressure algometer (Von Frey’s Esthesiometer).28,29 Esthesiometer measure the exteroceptive sensory. Designed by Sidney Weinstein30 in the decade of the fifties of the last century, it is formed by a set of 20 nylon monofilaments of equal length and different amplitude that provide a logarithmic scale of the applied real force and a linear scale of perceived intensity. To normalize the distribution of the dependent variable, the designer of the esthesiometer designated the logarithm for the numerical value of the pressure force as Log10 F (mg). The numerical values obtained in the range of 1.65 to 6.65 mm in diameter, equal to 0.008 and 300 force of pressure expressed in grams respectively. Subjective pain was carried out using a visual analog scale (VAS) with a range of 0 to 10, where 0 was nothing, 1–3 few, 4–7 much, and 8–10 unbearable. Huskisson EC originally referenced VAS in 1974.31 This method, evaluates in one person the intensity of pain and its evolution over time. As the pain analyzed is subjective, it is not useful to compare the intensity of pain among different people, since each individual has a different perception of pain. It consists of a line of 10 cm that represents the continuous spectrum of the painful experience; the left end means “no pain“ and the right ”the worst pain imaginable.” In this scale the patient must indicate the intensity of his painful sensation, which will be measured, with a ruler, by the examiner from his left end to the point indicated.
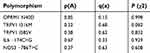 | Table 1 Allelic frequencies of each polymorphism in patients under study |
Thus, we also searched for a relationship between polymorphisms and subjective pain at different times: 15 seconds and 45 seconds from the application of the anesthetic. Subjects were grouped by pain ranges based on VAS values below or over 2. In the last case, those subjects in whom the anesthetic had taken effect were removed from the study. Thus, the frequency of genotypes of different polymorphisms in each of the other groups was studied to see whether there were any polymorphisms involved in the individual’s susceptibility to pain.
DNA extraction
High molecular weight DNA was obtained from oral mucosa cells. Isolation and cell lysis were carried out using buffer Fornace (0.25 M Sucrose, 50 mM Tris-HCl pH: 7.5, 25 mM KCl, 5 mM MgCl2), chelating agent EDTA (0.5M, pH=8), proteinase K (Boehringer Mannheim, 20 mg/mL) and SDS (1%). In total 118 patients were recruited, but for 4 of them we were unable to genotype the four genes owing to technical problems.
The mixture was incubated at 55 °C for 16 hrs and the process of DNA extraction and purification was performed using phenol-chloroform, isoamyl-alcohol, absolute ethanol and 70% ethanol. The DNA was resuspended in sterile ddH2O and frozen at −80 ºC until used. Some of the extracted samples showed very low DNA concentrations, requiring genomic DNA amplification using Illustra™ V2 DNA Amplification Kit GenomiPhi (GE Healthcare, Amersham Biosciences).
Real-time PCR allelic discrimination
The analysis of OPRM1 N40D (rs1799971), TRPV1 I316M (rs222747) and TRPV1 I585V (rs8065080) polymorphisms was performed through real-time PCR allelic discrimination using TaqMan probes. The final volume of the reaction consisted of 10μl, and commercial mixtures of primers and TaqMan MGB® probes (C_89500741, C_1093688 and C_11679656) (Applied Biosystems), alongside the mixture TaqMan® Universal PCR Master Mix No AmpErase® UNG (Applied Biosystems) containing the DNA polymerase, were used. The amplification program for both polymorphisms involved 40 one-minute cycles at an annealing temperature of 60 ºC. The PCR reactions were carried out using the TaqMan© universal PCR Master Mix in a Step-One Plus Real-time PCR system, following the manufacturer’s instructions. To assess reproducibility, 5% of the samples were randomly selected and re-genotyped, and all genotypes matched those originally obtained. The following probes were obtained from Thermofisher®:rs11799971: C__3204138_10, rs222747: C__1093688_20, rs8065080: C__11679656_10, rs2070744: C_15903863_10.
The allelic discrimination of IL6 −174C>G (rs1800795) polymorphism was performed by real time PCR using designed probes and primers. The forward primer’s sequence was 5´-TGACGACCTAAGCTGCACTTTTC-3´ and that of the reverse primer was 5´-GGGCTGATTGGAAACCTTATTAAGA-3´. Probes were VIC: TCTTGCGATGCTAAA and FAM: TCTTGCCATGCTAAA, and the annealing temperature was 57ºC for 40 one-minute cycles. Genotyping was performed at the Biomedical Research Institute of Salamanca (IBSAL) in small batches.
Allelic discrimination by RFLP-PCR
NOS3 −786T>C (rs2070744) polymorphism was analyzed using RFLP-PCR. PCR amplification reactions were performed in a volume of 25 µl and consisted of 0.6 U Taq DNA polymerase, 400 µM of each dNTP, 3.0mM MgCl2, 5 pmol of each forward primer 5‘-TCTACAGTCCCCCTTGCCGT-3‘ and reverse primer 5’-CTGACCGTGCAAGTCACAGA-3’. The amplification program consisted of 35 cycles involving denaturation at 95 °C for 30 seconds, annealing of primers at 60 °C for 30 seconds and primer extension at 72 °C for 30 seconds. All amplification reactions were carried out in an automatic thermocycler Abi verity 96 well (Applied Biosystem). The correct amplification of 180 bp fragments was
confirmed by horizontal electrophoresis using 2% agarose gel. The PCR product was digested with 1U of the restriction enzyme PdiI (Fermentas), which recognizes the cleavage site in the sequence generated by the nucleotide change. Digestion was carried out for 8–10 hrs at 37 ºC. DNA fragments were resolved by horizontal electrophoresis using 3% agarose gel, generating three different patterns for the three possible genotypes: (C/C) fragments of 87 bp and 93 bp; (T/T) one fragment of 180 bp; and (C/T) three fragments of 180 bp, 87 bp and 93 bp.
Statistical analysis
Sample size was estimated using the software Gpower 3.1.9.2 for correlation analysis, assuming an alpha and beta error of 0.5 and 0.95, respectively, with a side effect of 0.32. In addition, the minimum number of 100 samples was needed for carrying out the study. The statistical contingency test (χ2) analysis was performed using SPSS v.18 software for Windows. Differences in genotypic and allelic distribution among different groups and Hardy-Weinberg equilibrium were determined using the chi-square test (χ2). P-values (p) less than 0.05 were considered significant.
Results
We initially set out to test whether the groups included in this study were in accordance with the Hardy-Weinberg equilibrium, which involved conducting a χ2 test. Table 1 shows the allelic frequencies of our population and the p-value associated with the χ2 test. All probabilities were above of 0.05 therefore our groups were in accordance with this law.
Initial algometry in all patients, measured with Von Frey esthesiometer, was 6.65 (mm). We established three periods of anesthesia corresponding to three kinds of patients with respect to their pain response: fast responders (up to 45 seconds), the medium responders (up to 50 seconds) and the low responders (up to 65 seconds). Analyzing all the patients, none of the five polymorphisms showed significant differences when comparing the frequency of genotypes between the different groups and the onset of anesthesia after administration of Ultracain® (Table 2). Thus, we concluded that there was no association between polymorphism and the onset anesthesia time. Additionally it was tested whether gender had an influence on the onset of anesthesia in combination with polymorphism presence. Results are shown in Tables 3 and 4 for men and female respectively. It was found that in women there was a significant association between the genotype TPRVC315 and longer response times to anesthesia. In particular, there were more patients with anesthesia time greater than 65 seconds than was expected (10 vs 5.9).
 | Table 2 Genotype association with anesthesia onset |
 | Table 3 Genotype association in men with anesthesia onset in men |
 | Table 4 Genotype association with anesthesia onset in female |
We are aware that pain is a subjective feeling; therefore for the purpose of normalization, two time points were established for comparing the feeling of pain in all groups (15 and 45 seconds). In this comparison we also did not find any relationship between the polymorphisms being analyzed and the feeling of pain, assessed subjectively using VAS scales as shown in Table 5. Furthermore, no associations were found among the feeling of pain, the sex of the patient and the various polymorphisms (Tables 6 and 7).
 | Table 5 Genotype association with pain at 15 and 45 seconds from anesthesia administration |
 | Table 6 Genotype association with pain at 15 and 45 seconds from anesthesia administration in females |
 | Table 7 Genotype association with pain at 15 and 45 seconds from anesthesia administration in females |
Discussion
Pain is a complex process that involves numerous factors. It is known that age, gender, or psychological and ethnic factors may modify pain perception,32–34 and there are several studies on the interaction between an individual’s genetic basis and their response to painful stimuli.1,3,35
Articaine is a short-acting local anesthetic belonging to the amide group. Epinephrine acts as a vasoconstrictor to promote local hemostasis and reduce systemic absorption of local anesthetic. Therefore, their combination induces anesthesia within 1 to 6 mins and its effect stands for several hours.26 In our study, patients showed different times of onset of action of anesthesia and different pain sensation levels. This variability is due to individual differences, and its origin is yet unclear, since there are very few genetic studies that provide data on the onset and duration of anesthesia. Thus, we analyzed OPRM1 N40D, TRPV1 I316M, TRPV1 I585V, IL6 −174C>G and NOS3 −786T>C polymorphisms in subjects treated with local anesthetic articaine with epinephrine before dental surgery.
According to our findings, there is no association between these polymorphisms and the time elapsed between the application of the anesthetic and the onset of its effect. There are no differences between patients before and after 45, 50, and 65 seconds. Likewise, subjective pain intensity, measured by VAS, bears no relationship with them, because no differences were found in the frequency of appearance of the genotypes. However, it is true that in women an association was found between TPRVC315 G/G and a delayed time of anesthesia. At present, the reason for why this occurs is unknown, but we believe that new studies with larger numbers of patients may corroborate this finding.
As far as we know, this is the first attempt to find a relationship between local oral anesthesia and genetic variability. Regarding polymorphism related to OPRM1 and sensitivity to analgesic, we have only found a previous study by Fukuda et al (2010)36 in which the authors reported that SNP A118G, which was related to response to morphine doses in cancer patients, was responsible for differences in sensitive to fentanyl. In the case of TRPV1 (rs8065080) our results agree with a previous study were authors failed in finding any association between this polymorphism and chronic postoperative pain in patients of thoracic cosmetic surgery.37
On the other hand, we are aware that the limitation of this study is the number of patients and perhaps in the future a study designed with more patients might find any association between the studied polymorphisms and the anesthesia onset. We also are aware that comparing the pain feeling is difficult between patients given the differences places of anesthesia application and probably the different diffusion of the drug in each patient. For these reasons, we tried to sort the patients out with an arbitrary VAS of 2 in 15 and 45 seconds. However, we also failed to find any correlation with this aggrupation.
Thus, we concluded that there was not association among the polymorphism under study and anesthesia onset. However, we also believe that it cannot be ruled out that variations in other genes may be involved in individual susceptibility to local anesthesia with articaine-epinephrine.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc Natl Acad Sci U S A. 1999;96(14):7744–7751. doi:10.1073/pnas.96.14.7744
2. Hyungsuk K, Raymond A. Genetics, pain, and analgesia. In: pain IA for the S of, ed. PAIN Clinical Updates. 2005;1–4.
3. Lötsch J, Geisslinger G, Tegeder I. Genetic modulation of the pharmacological treatment of pain. Pharmacol Ther. 2009;124(2):168–184. doi:10.1016/j.pharmthera.2009.06.010
4. Lacroix-Fralish ML, Mogil JS. Progress in genetic studies of pain and analgesia. Annu Rev Pharmacol Toxicol. 2009;49(1):97–121. doi:10.1146/annurev-pharmtox-061008-103222
5. Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286(5439):487–491. doi:10.1126/science.286.5439.487
6.. Morgan B, Aroke EN, Dungan J. The role of pharmacogenomics in anesthesia pharmacology. Annu Rev Nurs Res. 2017;35(1):241–256. doi:10.1891/0739-6686.35.241
7. Sun Y, Sahbaie P, Liang D, et al. DNA methylation modulates nociceptive sensitization after incision. Taylor B, ed. PLoS One. 2015;10(11):e0142046. doi:10.1371/journal.pone.0142046
8. Kringel D, Geisslinger G, Resch E, et al. Machine-learned analysis of the association of next-generation sequencing-based human TRPV1 and TRPA1 genotypes with the sensitivity to heat stimuli and topically applied capsaicin. Pain. 2018;159(7):1366–1381. doi:10.1097/j.pain.0000000000001222
9. Frias B, Merighi A. Capsaicin, Nociception and Pain. Molecules. 2016;21(6):797. doi:10.3390/molecules21060797
10. Cheng H-YM, Pitcher GM, Laviolette SR, et al. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002;108(1):31–43. doi:10.1016/S0092-8674(01)00629-8
11. Keilhoff G, Schröder H, Peters B, Becker A. Time-course of neuropathic pain in mice deficient in neuronal or inducible nitric oxide synthase. Neurosci Res. 2013;77(4):215–221. doi:10.1016/j.neures.2013.08.008
12. Staunton CA, Barrett-Jolley R, Djouhri L, Thippeswamy T. Inducible nitric oxide synthase inhibition by 1400W limits pain hypersensitivity in a neuropathic pain rat model. Exp Physiol. 2018;103(4):535–544. doi:10.1113/EP086764
13. Ahlawat A, Rana A, Goyal N, Sharma S. Potential role of nitric oxide synthase isoforms in pathophysiology of neuropathic pain. Inflammopharmacology. 2014;22(5):269–278. doi:10.1007/s10787-014-0213-0
14. Finkel J, Guptill V, Khaibullina A, et al. The three isoforms of nitric oxide synthase distinctively affect mouse nocifensive behavior. Nitric Oxide. 2012;26(2):81–88. doi:10.1016/j.niox.2011.12.004
15. Murphy PG, Ramer MS, Borthwick L, Gauldie J, Richardson PM, Bisby MA. Endogenous interleukin-6 contributes to hypersensitivity to cutaneous stimuli and changes in neuropeptides associated with chronic nerve constriction in mice. Eur J Neurosci. 1999;11(7):2243–2253. doi:10.1046/j.1460-9568.1999.00641.x
16. De Jongh RF, Vissers KC, Meert TF, Booij LHDJ, De Deyne CS, Heylen RJ. The role of interleukin-6 in nociception and pain. Anesth Analg. 2003;96(4):1096–1103. table of contents. doi:10.1213/01.ANE.0000055362.56604.78
17. LaForge KS, Yuferov V, Kreek MJ. Opioid receptor and peptide gene polymorphisms: potential implications for addictions. Eur J Pharmacol. 2000;410(2–3):249–268. doi:10.1016/S0014-2999(00)00819-0
18. Kroslak T, Laforge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J Neurochem. 2007;103(1):77–87. doi:10.1111/j.1471-4159.2007.04738.x
19. Zhang Y, Wang D, Johnson AD, Papp AC, Sadée W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280(38):32618–32624. doi:10.1074/jbc.M504942200
20. Xu H, Tian W, Fu Y, Oyama TT, Anderson S, Cohen DM. Functional effects of nonsynonymous polymorphisms in the human TRPV1 gene. Am J Physiol Renal Physiol. 2007;293(6):F1865–76. doi:10.1152/ajprenal.00347.2007
21. Binder A, May D, Baron R, et al. Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PLoS One. 2011;6(3):e17387. doi:10.1371/journal.pone.0017387
22. Vickers MA, Green FR, Terry C, et al. Genotype at a promoter polymorphism of the interleukin-6 gene is associated with baseline levels of plasma C-reactive protein. Cardiovasc Res. 2002;53(4):1029–1034. doi:10.1016/S0008-6363(01)00534-X
23. Oliveira-Paula GH, Lacchini R, Tanus-Santos JE. Endothelial nitric oxide synthase: from biochemistry and gene structure to clinical implications of NOS3 polymorphisms. Gene. 2016;575(2 Pt 3):584–599. doi:10.1016/j.gene.2015.09.061
24. Augeri AL, Tsongalis GJ, Van Heest JL, Maresh CM, Thompson PD, Pescatello LS. The endothelial nitric oxide synthase −786 T>C polymorphism and the exercise-induced blood pressure and nitric oxide responses among men with elevated blood pressure. Atherosclerosis. 2009;204(2):e28–e34. doi:10.1016/j.atherosclerosis.2008.12.015
25. Paterakis K, Schmitter M, Said Yekta-Michael S. Efficacy of epinephrine-free articaine compared to articaine with epinephrine (1:100 000) for maxillary infiltration, a randomised clinical trial. J Oral Rehabil. 2018;45(6):467–475. doi:10.1111/joor.12637
26. Nizharadze N, Mamaladze M, Chipashvili N, Vadachkoria D. Articaine - the best choice of local anesthetic in contemporary dentistry. Georgian Med News. 2011;(190):15–23.
27. Martin M, Nusstein J, Drum M, Reader A, Beck M. Anesthetic efficacy of 1.8 mL versus 3.6 mL of 4% articaine with 1:100,000 epinephrine as a primary buccal infiltration of the mandibular first molar. J Endod. 2011;37(5):588–592. doi:10.1016/j.joen.2011.01.001
28. Kostek M, Polaski A, Kolber B, Ramsey A, Kranjec A, Szucs K. A protocol of manual tests to measure sensation and pain in humans. J Vis Exp. 2016;(118). doi:10.3791/54130
29. Kroenke K. Pain measurement in research and practice. J Gen Intern Med. 2018;33(S1):7–8. doi:10.1007/s11606-018-4363-4
30. Weinstein S. Intensive, extensive aspects of tactile sensitivity as a function of body part, sex and laterality. In: Kenshalo D, editor. The Skin Senses. Springfield, Ill; 1968:195–222.
31. Huskisson EC. Measurement of pain. Lancet (London, England). 1974;2(7889):1127–1131. doi:10.1016/S0140-6736(74)90884-8
32. Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101(1–2):155–165. doi:10.1016/S0304-3959(02)00324-X
33. Edwards RR, Fillingim RB, Yamauchi S, et al. Effects of gender and acute dental pain on thermal pain responses. Clin J Pain. 1999;15(3):233–237. doi:10.1097/00002508-199909000-00011
34. Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113(1):20–26. doi:10.1016/j.pain.2004.08.013
35. Little J, Higgins JP, Ioannidis JP, et al. STrengthening the REporting of Genetic Association Studies (STREGA)— an extension of the STROBE statement. PLoS Med. 2009;6(2):e1000022. doi:10.1371/journal.pmed.1000022
36. Fukuda K, Hayashida M, Ikeda K, Koukita Y, Ichinohe T, Kaneko Y. Diversity of opioid requirements for postoperative pain control following oral surgery–is it affected by polymorphism of the μ-opioid receptor? Anesth Prog. 2010;57(4):145–149. doi:10.2344/0003-3006-57.4.145
37. Dimova V, Lötsch J, Hühne K, et al. Association of genetic and psychological factors with persistent pain after cosmetic thoracic surgery. J Pain Res. 2015;8:829–844. doi:10.2147/JPR.S90434
Supplementary material
 |  |  |  | Table S1 Anesthesia region, onset time, and VAS evaluation for each patient |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
