Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Genetic polymorphisms in Sox17 associated with intracranial aneurysm in Chinese Han people: a genotype-phenotype study
Authors Li M , Wang W, Zhang L, Xin W , Zhao Y, Huan L, Yu J, Zhang H , Zhang J, Yang S, Liang D, Yang W, Yang X
Received 7 November 2018
Accepted for publication 19 February 2019
Published 2 April 2019 Volume 2019:15 Pages 779—783
DOI https://doi.org/10.2147/NDT.S193478
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Mengqi Li,1,2,* Weihan Wang,1,2,* Lin Zhang,3,* Wenqiang Xin,1,2,* Yan Zhao,1,2 Linchun Huan,4 Jianjun Yu,4 Hao Zhang,1,2 Jianning Zhang,1,2 Shuyuan Yang,1,2 Degang Liang,5,* Weidong Yang,1,2,* Xinyu Yang1,2,*
1Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China; 2Key Laboratory of Post-Neurotrauma Neurorepair and Regeneration in Central Nervous System, Ministry of Education in China and Tianjin, Tianjin Neurological Institute, Tianjin, People’s Republic of China; 3Department of Neurosurgery, Linyi People’s Hospital, Shandong, People’s Republic of China; 4Department of Cardio-Thoracic Surgery, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China; 5Department of Neurosurgery, Tianjin Fifth Central Hospital, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Purpose: Genetic factors play a vital role in intracranial aneurysm (IA) onset and development. Studying the relationship between IA and the Sox17 polymorphisms in diverse populations is essential for establishing credibility.
Patients and methods: We collected blood samples derived from a total of 596 sporadic IA patients and 600 individual controls in several medical institutes in China. We used the Sequenom MassArray system for single nucleotide polymorphisms (SNPs) genotyping after DNA extraction. The SNPs data was tested and analyzed in PLINK (version 1.9). Multiple-testing was performed in PLINK to make the statistics more rigorous and accurate.
Results: We found that the allelic G of rs1072737 (OR=1.303, genomic-control corrected P-value =0.001032) is a risk allele, while the allelic G of rs9298506 (OR=0.7253, genomic-control corrected P-value =0.01559) is a protective allele in Chinese Han people.
Conclusion: The allelic G of rs1072737 is a risk factor for IA, while the allelic G of rs9298506 serves as a protective factor for IA in Chinese Han people.
Keywords: intracranial aneurysm, Sox17, genetics, cerebral vascular disease
An Erratum had been published for this article
Introduction
Rupture of intracranial aneurysm (IA) is a contributing factor for hemorrhagic stroke which is characterized by high fatality and disability rates.1 A systematic review and meta-analysis found that the prevalence of unruptured IA is about 3.2%.2
The genetic risk factors associated with IA and the strength of their associations with IA remain uncertain.3 Thus, the underlying genetics of IA should be further explored. Moreover, IA-related genetic polymorphisms in diverse populations are not entirely the same. Many initial studies have been carried out in populations of European descent, and it is still a challenge to extend the studies to other populations, especially to populations of Asian and African descent.4 Compared with other populations, genetic studies on IAs have rarely been carried out in Chinese populations, which is ill-matched with the increasing rate of sequencing in China.5 Two genetic variations in the Sox17 gene, rs1072737 (NC_000008.11: g.54501764T>G) and rs9298506 (NC_000008.10: g.55437524A>G), have been shown to be risk factors for IA.6–8 However, there have not been any studies to test these findings in the Chinese people. Genotype-phenotype associations in different populations are essential to establish credibility; thus, it is important to study IA-related single nucleotide polymorphisms (SNPs) in Sox17 in the Chinese population. To further establish the credibility of the relationship between IA and the Sox17 risk allele in diverse populations, we studied the IA-related single nucleotide variants of Sox17, and discoveries of Sox17 SNPs associated with IA were made in Chinese Han people.
Patients and methods
Ethics approval and informed consent
All the studies complied with the Declaration of Helsinki and were approved by the Institutional Review Board and Ethics Committee of Tianjin Medical University General Hospital and the collaborating hospitals’ ethics committees. All the participants reported themselves to be of Han nationality and provided written informed consent.
Patients and blood samples
This case-control study included 595 patients with sporadic IAs and 600 controls. We recruited patients with IAs between February 2014 and March 2017 from three clinical centers located in Tianjin (Tianjin Medical University General Hospital), Shandong (Linyi People’s Hospital), and Hunan (Xiangya Hospital). All IA cases were confirmed to have IA by imaging or surgery. The IA diagnosis was based on symptoms suggestive of subarachnoid hemorrhage (SAH), subarachnoid blood on computed tomography (CT), computerized tomography angiography (CTA), digital subtraction angiography (DSA), and/or surgery. All cases with confirmed IAs based on angiography were retrospectively confirmed by three independent observers, a neuroradiologist, and two highly qualified cerebrovascular neurosurgeons who were blinded to the clinical history and the imaging results. All the patients included in this research underwent either endovascular embolization or a neurosurgical operation after confirmation of the IA. All patients were asked whether they had a family history of IA. The patients with no family history of IA were included.
A total of 600 controls were randomly selected from the Physical Examination Center in the Tianjin Medical University General Hospital (TJMUGH). For an extended period, the Physical Examination Center collected the clinical information and the blood of healthy patients and built a database that provided information on the sex, age, blood pressure, smoking status, alcohol usage and other IA-related indexes for this study. In 2016, TJMUGH united 28 high-level hospitals from different regions in China and established the Chinese Multicenter Cerebral Aneurysm Database (CMAD). The CMAD is a cloud database that consists of clinical information and blood samples (http://www.cmadtj.com/). The CMAD contributed data for IA cases in this study.
Selection of SNPs
The Sox17 variants were selected based on the frequency of these variants in the Chinese population and based on the previous genome-wide association study (GWAS) on the Sox17 gene. The rate of Sox17 variants in the Chinese population was determined using the NCBI (National Center for Biotechnology Information) database (http://www.ncbi.nlm.nih.gov/SNP/).
Genotyping of SNPs
Genomic DNA was extracted from peripheral blood. All of the obtained DNA samples were stored at −80°C. All the primers were designed by MassArray Assay Design 3.1 (Sequenom Inc., San Diego, CA, USA). SNPs were genotyped via the Sequenom MassArray system (BioMiao Biotechnology (Beijing) Co. Ltd) and analyzed with the MassArray Typer 4.0 software (Sequenom).9
Statistical analysis
First, we checked the grouping information using the ID of the blood sample and the SNP data. Second, the data were tested and analyzed in PLINK (version 1.9).10 Finally, when the outcomes were determined, the data were tabulated for further discussion. Multiple-testing was performed in PLINK to make the statistics more rigorous and accurate.
Results
Case and control characteristics and statistical outcome
Characteristics of the cases and controls are shown in Table 1. The genotype distribution of the Sox17 variants rs1072737 and rs9298506 in the cases and controls are shown in Table 2. rs1072737 and rs9298506 conform to Hardy–Weinberg equilibrium (Table 3).
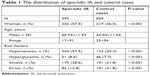  | Table 1 The distribution of sporadic IA and control cases |
  | Table 2 Genotype statistics |
  | Table 3 Hardy–Weinberg equilibrium |
The allele G of rs1072737 is a risk factor for IA, while the allele G of rs9298506 is a protective factor for IA. Multiple-testing correction was performed to verify the risk effect of rs1072737 and the protective effect of rs9298506 with. Both rs1072737 and rs9298506 passed the multiple-testing method “genomic control” (GC) (Table 4).11 The results were clear and definite in the chi-squared test (rs1072737: OR 1.306, genomic-control corrected P-value =0.001599; rs9298506: OR 0.7121, genomic-control corrected P-value =0.02359) and in the allelic gene logistic regression (rs1072737: OR: 1.303, genomic-control corrected P-value =0.01559; rs9298506: OR: 0.7253, genomic-control corrected P-value =0.001032) (Table 4). Additionally, rs9298506 passed other multiple-test methods such as the Bonferroni adjusted method and the Benjamini and Hochberg adjusted method (Table 4).12,13
  | Table 4 Chi-squared test and logistic regression (add model) with multiple-testing correction |
Discussion
In our study, we found that allele G of rs1072737 is a risk factor for IA, while allele G of rs9298506 is a protective factor for IA. This finding is based on the multiple-testing correction.
A previous GWAS showed that rs1072737 and rs9298506 the Sox17 were associated with IA.6–8 However, the present study indicates that they have different roles in IA in Chinese Han population: the allelic C of rs1072737 is a risk allele, while the allelic G is a protective allele in Chinese Han people (Table 4). The GWAS showed rs9298506 (P=1.3×10−12, OR: 1.28, posterior probability of association >0.9999) to be significantly associated with IA.6,8 Additionally, rs9298506 was reported to have an IA-related risk effect in another meta-analysis.3 Nonetheless, in our study, allele G of rs9298506 was a protective factor for IA in Chinese Han people. Recently, the relationship between Sox17 gene polymorphisms and IA was investigated in a homogeneous Korean population.15 It was shown that allele G of rs1072737 is a protective factor (OR: 0.69, 95%CI: 0.49–0.96, P=0.03) and that allele G of rs9298506 has no statistical significance. The results of this study in the Korean population are contrary to our results and others.
Some initial findings cannot be replicated in the follow-up studies due to some issues.14,15 For risk alleles and the haplotypes, initial studies and follow-up studies may not be the same in different populations.16–20 Therefore, the confidence would be higher if we evaluated the genotype-phenotype association in people of diverse ancestries, regardless of whether the genotype-phenotype association is derived from candidate-gene studies or genome-wide association studies.4 Concordant findings in similar studies are necessary to confirm the genotype-phenotype associations.4
Sox17 is primarily expressed in the arterial endothelial cells in the early stages of embryonic development. This expression pattern and distribution continues into adulthood.21 It suggests that the change in Sox17 expression leads to abnormalities in intracranial artery differentiation.21 A study demonstrated that IA could be generated through induced hypertension and circle of Willis damage by elastase in Sox17-knockdown mice, which further supports the theory that hereditary and acquired risk factors facilitate IA formation.22 It was shown that Sox17 has specific expression in the cerebral artery. Furthermore, compared with that in the normal cerebral artery, the Sox17 expression in IA is reduced and the endothelial junctions are destablized.22 Therefore, we assume that gene Sox17 variations lead to the function changes of Sox17, cause damage to cerebral artery, and finally promote IA formation. However, molecular biology experiments and a reliable animal model are required to explore the specific mechanisms further.
Conclusion
The allelic G of rs1072737 is a risk factor for IA while the allelic G of rs9298506 serves as a protective factor for IA in Chinese Han people.
Acknowledgments
The authors thank BioMiao Biotechnology (Beijing) Co. Ltd for providing SNP sequencing and bioinformatics analysis services. The authors appreciate the Chinese Multicenter Cerebral Aneurysm Database (CMAD), which contributed clinical data and blood samples to this study. The authors thank the National Natural Science Foundation of China (81571144) and (81501073), and the Natural Science Foundation of Tianjin City (16JCZDJC35700) and (15ZXLCSY00060) for supporting this work.
Disclosure
The authors report no conflicts of interest in this work.
References
O’Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761–775. doi:10.1016/S0140-6736(16)30506-2 | ||
Vlak MHM, Algra A, Brandenburg R, Rinkel GJE. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10(7):626. doi:10.1016/S1474-4422(11)70109-0 | ||
Alg VS, Sofat R, Houlden H, Werring DJ. Genetic risk factors for intracranial aneurysms: a meta-analysis in more than 116,000 individuals. Neurology. 2013;80(23):2154–2165. doi:10.1212/WNL.0b013e318295d751 | ||
Chanock SJ, Manolio T, Boehnke M, et al. Replicating genotype-phenotype associations. Nature. 2007;447(7145):655–660. doi:10.1038/447655a | ||
Cyranoski D. China’s bid to be a DNA superpower. Nature. 2016;534(7608):462. doi:10.1038/534462a | ||
Bilguvar K, Yasuno K, Niemela M, et al. Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat Genet. 2008;40(12):1472–1477. doi:10.1038/ng.240 | ||
Foroud T, Koller DL, Lai D, et al. Genome-wide association study of intracranial aneurysms confirms role of Anril and SOX17 in disease risk. Stroke. 2012;43(11):2846–2852. doi:10.1161/STROKEAHA.112.656397 | ||
Yasuno K, Bilguvar K, Bijlenga P, et al. Genome-wide association study of intracranial aneurysm identifies three new risk loci. Nat Genet. 2010;42(5):420–425. doi:10.1038/ng.563 | ||
Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet. 2009;60(1): 2.12.1–2.12.18. | ||
Purcell S, Neale B, Toddbrown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi:10.1086/519795 | ||
Bacanu SA, Devlin B, Roeder K. The power of genomic control. Am J Hum Genet. 2000;66(6):1933–1944. doi:10.1086/302929 | ||
Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279–284. | ||
Ranstam J. Multiple P-values and Bonferroni correction. Osteoarthritis Cartilage. 2016;24(5):763–764. doi:10.1016/j.joca.2016.01.008 | ||
Yeager M, Orr N, Hayes RB, et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39(5):645–649. doi:10.1038/ng2022 | ||
Colhoun HM, McKeigue PM, Davey Smith G. Problems of reporting genetic associations with complex outcomes. Lancet. 2003;361(9360):865–872. | ||
Straub RE, Jiang Y, MacLean CJ, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71(2):337–348. doi:10.1086/341750 | ||
Van Den Bogaert A, Schumacher J, Schulze TG, et al. The DTNBP1 (dysbindin) gene contributes to schizophrenia, depending on family history of the disease. Am J Hum Genet. 2003;73(6):1438–1443. doi:10.1086/379928 | ||
Bray NJ, Preece A, Williams NM, et al. Haplotypes at the dystrobrevin binding protein 1 (DTNBP1) gene locus mediate risk for schizophrenia through reduced DTNBP1 expression. Hum Mol Genet. 2005;14(14):1947–1954. doi:10.1093/hmg/ddi199 | ||
Funke B, Finn CT, Plocik AM, et al. Association of the DTNBP1 locus with schizophrenia in a U.S. population. Am J Hum Genet. 2004;75(5):891–898. doi:10.1086/425279 | ||
Mutsuddi M, Morris DW, Waggoner SG, Daly MJ, Scolnick EM, Sklar P. Analysis of high-resolution HapMap of DTNBP1 (Dysbindin) suggests no consistency between reported common variant associations and schizophrenia. Am J Hum Genet. 2006;79(5):903–909. doi:10.1086/508942 | ||
Corada M, Orsenigo F, Morini MF, et al. Sox17 is indispensable for acquisition and maintenance of arterial identity. Nat Commun. 2013;4:2609. doi:10.1038/ncomms3609 | ||
Lee S, Kim IK, Ahn JS, et al. Deficiency of endothelium-specific transcription factor Sox17 induces intracranial aneurysm. Circulation. 2015;131(11):995–1005. doi:10.1161/CIRCULATIONAHA.114.012568 | ||
Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. JSTOR, Series B (Methodological). 1995;57(1):289–300. | ||
Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. The Annals of Statistics. 2001;29(4):1165–1188. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
