Back to Journals » Cancer Management and Research » Volume 11
Gemcitabine, cisplatin, prednisone, and thalidomide for relapse and refractory peripheral T-cell lymphoma: a retrospective study from China
Authors Liu X, Shang Y, Li L, Zhang X, Li Z , Zhang M
Received 13 May 2019
Accepted for publication 16 August 2019
Published 9 September 2019 Volume 2019:11 Pages 8277—8284
DOI https://doi.org/10.2147/CMAR.S215585
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Xiangli Liu, YuFeng Shang, Ling Li, Xudong Zhang, Zhaoming Li, Mingzhi Zhang
Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450000, People’s Republic of China
Correspondence: Mingzhi Zhang
Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450000, People’s Republic of China
Tel +86 1 383 856 5629
Email [email protected]
Background: Peripheral T-cell lymphoma (PTCL) is often prone to relapse and progression even after formal first-line treatment, and there is no standard regimen for second-line treatment. What is more, the activity of thalidomide against this type of lymphoma is unknown.
Purpose: The objective of this study was to evaluate the efficacy and safety of GDPT regimen in the treatment of relapsed/refractory peripheral T-cell lymphoma.
Patients and methods: In this retrospective study, gemcitabine, cisplatin, prednisone, and thalidomide (GDPT) combination regimen was used as salvage protocol for PTCL that failed in first-line treatment for 29 patients and it was scheduled to give 6 cycles of GDPT therapy in order to better evaluate the efficacy unless there was evidence of disease progression, unacceptable toxicities, or refusal by the patient.
Results: After a total of 106 cycles of GDPT regimen were administered, the result showed that the disease control rate (DCR) achieved 82.8% and overall response rate (ORR) reached 69.0% with 34.5% complete remission (CR) and 34.5% partial remission (PR). The median progression-free survival (PFS) was 10.0 months (95% CI 6.6–13.4) and median OS was 28.0 months (95% CI 19.2–36.8). And the 1-year PFS rate and 1-year OS rate were 43.6% and 64.6%, respectively. Both hematologic and non-hematologic toxicities were moderate and well tolerated. There was no treatment-related death.
Conclusion: Thalidomide in combination with gemcitabine, cisplatin, prednisone regimen is a new promising approach to treating patients with relapse and refractory PTCL.
Keywords: peripheral T-cell lymphoma, salvage chemotherapy, thalidomide, gemcitabine
Introduction
Peripheral T-cell lymphoma (PTCL), also known as mature T-cell lymphoma, is a highly heterogeneous group of aggressive non-Hodgkin’s lymphatic proliferative disease derived from T-cells.1 According to the pathological morphology, immunohistochemical characteristics, and molecular genetic characteristics of the disease, the WHO classification criteria for hematopoietic and lymphoid tissue tumors divide PTCL into about 20 distinct pathological subtypes, including peripheral T-cell lymphoma, non-specific type (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), ALK-positive Anaplastic large cell lymphoma (ALK+ALCL) and ALK-negative anaplastic large cell lymphoma (ALK-ALCL), mycosis Fungoides (MF), Sezary’s syndrome (SS), etc.2 The incidence epidemiology of PTCL shows significant regional difference. PTCL accounts for approximately 25–30% non-Hodgkin’s disease in Asians, which is significantly higher than 10–15% of European and American countries.3 Because the clinical manifestations, biological behaviors, and sensitivity to chemotherapy are very different, it is difficult to carry out strict unification of the treatment regimen. The prognosis of PTCL is dismal, and the majority of patients with most subtypes of PTCL do not enjoy long-term disease-free survival in spite of aggressive chemotherapy. The most common first-line treatment regimens for initial treatment of PTCL are CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) and CHOP-like regimens with the 5-year survival rate of only 38.5%, which is associated with a high failure rate and frequent relapses.4,5 Therefore, for the treatment of relapse and refractory PTCL, the 2018 National Comprehensive Cancer Network (NCCN) treatment guidelines recommend that participating in clinical trials is suggested, and PTCL’s second-line chemotherapy regimens still have no standard treatment.6 Although stem cell transplantation is a relatively alternative second-line regimen for patients at high risk, the transplantation has a relatively small range of clinical application because most of the disease occur in the patients between the ages of 50 and 60 years with poor physical tolerance. On the other hand, the stem cell transplantation is effective for patients who are sensitive to chemotherapy because achieving complete remission (CR) through induced chemotherapy is necessary before transplantation.7,8 Recently, a number of emerging therapeutic agents and treatment regimens, such as the anti-CD30 antibody, histone deacetylase inhibitors, and immunotherapy, have improved the efficacy of some subtypes of NHL patients and changed the treatment mode of some NHL patients, showing good application prospect, but they are not widely available because of their high price and unacceptable side effects.9,10 Therefore, for these patients who are ineligible for high-dose chemotherapy with stem cell transplant or those cannot afford the new drugs, traditional effective salvage chemotherapy is still an important option. Thalidomide plays an important role in anti-tumor, immune regulation, and anti-angiogenesis. However, the application of thalidomide in PTCL still remains controversial. Therefore, we added thalidomide into the GDP (gemcitabine, cisplatin, prednisone) program. In this retrospective study, The First Affiliated Hospital of Zhengzhou University adopted the GDPT program (gemcitabine, cisplatin, prednisone, thalidomide) as the salvage chemotherapy to treat recurrent and refractory PTCL and analyzed and explored the efficacy and tolerability of GDPT regimen for relapsed or refractory PTCL patients.
Materials and methods
Eligible patients
The study began in June 2011. The eligibility criteria for patients in this study included: (1) patients who were pathologically diagnosed with PTCL. All pathological specimens were reviewed in pathology department to confirm the diagnosis of PTCL based on the WHO classification in our hospital, (2) at least one measurable lesion, (3) received chemotherapy first-line chemotherapy regimens including CHOP, CHOPE. Besides, all patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2 at the beginning of treatment and female patients were prohibited pregnancy. Given many other available treatment options such as brentuximab vedotin and so on about the ALK+ALCL, and the heterogeneity in response and survival rates across PTCL subgroups, we did not focus our analysis on patients if there was evidence of central nervous system involvement with lymphoma and patients with ALK+ALCL in this study. Prior to the treatment, participating patients were fully informed of the adverse reactions and risks and signed an informed consent for chemotherapy. The implementation of the scheme was carried out according to Good Clinical Practice Guidelines and the Declaration of Helsinki. The study was approved by the Local Ethics Committee of Zhengzhou University and the Scientific Council of the Faculty of Medicine.
Therapy regimen
The specific GDPT regimen included gemcitabine 800 mg/m2, intravenous infusion (IV), 30 mins, day 1, 8; cisplatin 25 mg/m2, intravenous infusion (IV), days 1–3; prednisone 60 mg/m2, orally (PO), days 1–5; thalidomide, 200 mg/day, orally (PO), continued use to the termination of treatment. Taking the toxicity and tolerance into account, we gradually increased the dose from the 50 mg dose to 200 mg for maintenance and then continued until the end of chemotherapy. Every 21 days is a cycle and patients were scheduled to receive up to 6 cycles of GDPT therapy unless there was evidence of disease progression, unacceptable toxicities, or refusal by the patient.
Treatment response and toxicity assessment
The efficacy evaluation was performed every 2 cycles according to CT or PET-CT, and the bone marrow examination should also be repeated for the patients with bone marrow involvement. Treatment responses were evaluated according to modified Cheson criteria.11 CR was defined as disappearance of all previously measurable lesions and absence of any new tumor lesions. Partial remission (PR) was defined as a decrease of at least 50% in size. Progressive disease (PD) was defined as greater than 25% increase in the product of the two diameters of at least one tumor or as the presence of a newly developed lesion. Stable disease (SD) was defined as any response that did not fall into the other defined categories. Those patients who suffer from advanced disease may consider changing regimen or participating in clinical trials.
Treatment-related adverse reactions were monitored by routine physical or biochemical examination such as full blood count, liver function tests, and so on prior to each treatment and during treatment interval. The severity of toxicity including non-hematological and non-biochemical toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0.12
Statistical analysis
The primary clinical endpoint was disease control rate (DCR) including patients who have reached CR, PR, and SD, and overall response rate (ORR) including patients achieving CR and PR. Secondary endpoints included safety, 1-year progression-free survival (PFS), and 1-year overall survival (OS). OS was calculated from the first day of GDPT administration to the date of last follow-up or death due to any cause. PFS was defined as the period from the first day of GDPT administration to the date of disease progression, last follow-up, or death from any cause. The response between relapse patients and refractory patients was measured using Pearson’s chi-square test or Fisher’s exact test. OS and PFS curves were calculated according to the Kaplan-Meier method. All data were analyzed with the Statistical Package for the Social Sciences (SPSS) 21.
Results
Clinical features of the patient
During June 2011 to December 2018, a total of 29 patients were enrolled in the study and about 12 patients were not included. Of the 12 people excluded, 2 lost follow-up, 5 participated in the clinical trial of Chidamide tablets, and 5 used the GDP treatment program. The baseline characteristics of enrolled 29 patients are as follows in Table 1. Twenty-nine PTCL patients including 12 PTCL-NOS patients (41.4%), 9 ALK-ALCL patients (31.0%, 7 AITL patients (24.1%), and 1 EATL patient (3.40%) in the First Affiliated Hospital of Zhengzhou University, China, were retrospectively analyzed in this study. Eighteen refractory patients and 11 relapse patients received GDPT chemotherapy. The median age of the patients was 52 years (range from 24 to 70 years), and there was a male predominance, with a male-to-female ratio of 1.4:1. When first diagnosed, 6 patients (20.7%) had stage II lesions,10 patients (34.5%) had stage III disease, and 13 patients (44.2%) had stage IV disease. Bone marrow invasion was confirmed in 5 cases through routine bone marrow aspiration and biopsy. B symptoms can be observed in 12 patients (41.4%). The elevation of serum lactate dehydrogenase (LDH) levels was observed in 9 patients (31.0%) and 16 of the patients (55.2%) had elevated β2 microglobulin (β2MG). As far as the first-line therapy, cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) regimen was used in almost all patients in the first line, except for 2 cases using CHOPE (CHOP+ etoposide) regimen and 1 case using etoposide, cytarabine, cisplatin, methylprednisolone (ESHAP) regimen.
 |
Table 1 The baseline characteristics of 29 patients were as follows |
Treatment response
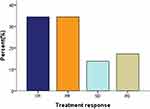
The selected patients completed at least two cycles of GDPT and could be evaluated for efficacy. Twelve patients used PET-CT to evaluate the efficacy and the remaining 17 patients used CT to assess the efficacy of the treatment. A total of 106 cycles of GDPT regimen were administrated. An average of 3.7 cycles were completed and the median number of cycles was 4 (range 2–6 cycles). For patients who completed 2–3 cycles, most of them were due to stable condition change or disease progression. Twelve patients completed 4 cycles of chemotherapy, among which 4 patients obtained CR, 7 patients developed PR, and 1 patient developed PD. Two patients completed 5 cycles, of which 1 patient had CR and 1 patient had PR. Four patients completed 6 cycles, of which 3 had CR and 1 had PR. At the end of treatment, 10/29 patients (34.5%) achieved CR, 10/29 patients (34.5%) had PR, and 4 patients (13.8%) had no obvious response to treatment, showing the DCR of 82.8% and the ORR of 69%. In addition, there was disease progression in 5 patients (17.2%) (Figure 1). Besides, we have also showed the response to treatment between relapse patients and refractory patients. As can be seen from Table 2, the ORR of refractory patients was higher than that of relapse patients (70.6% vs 45.4%, P=0.048), but the CR rate was not statistically significant.
 |
Table 2 Response to treatment between relapse patients and refractory patients |
Survival
The follow-up time was up to October 2018, with the median follow-up time of 26.0 months (range 3–84 months). The median PFS was 10.0 months (95% CI 6.6–13.4) and median OS was 28.0 months (95% CI 19.2–36.8). The 1-year PFS rate and 1-year OS were 43.6% and 64.6%, respectively (Figures 2 and 3).
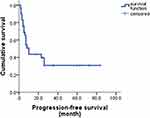 |
Figure 2 Progression-free survival of GDPT with relapsed/refractory PLCL.Abbreviation: GDPT, gemcitabine, cisplatin, prednisone, and thalidomide. |
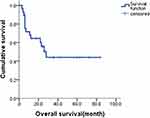 |
Figure 3 Overall survival of GDPT with relapsed/refractory PLCL. Abbreviation: GDPT, gemcitabine, cisplatin, prednisone, and thalidomide. |
Adverse effects
As we can see in Table 3, in all 29 patients with GDPT chemotherapy, both hematologic and non-hematologic toxicities were moderate and well tolerated. Although no patient died from treatment‐related adverse effects, some adverse reactions still existed in the GDPT regimen. The most common adverse reactions were hematological toxicities such as myelosuppression, including leucopenia, thrombocytopenia, and anemia. Grade 3/4 leucopenia occurred in 11 patients (37.9%), grade 3/4 thrombocytopenia was found in 9 patients (31.0%), as well as grade 3/4 anemia in 7 patients (24.1%). Compared with the hematologic toxicities, most non‐hematological toxicities were tolerable and manageable, including mild digestive tract such as nausea and vomiting, and transient liver dysfunction which mainly manifested in elevated transaminase in 2 cases (6.9%). Besides, it was worth mentioning that 2 patients had peripheral nerve damage such as numbness of the hands and feet, paresthesia, and drowsiness occurred in 5 patients. No rash and thrombosis was observed in any of the patients.
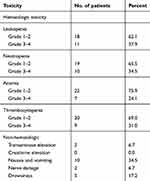 |
Table 3 Treatment-related toxicities |
Discussion
So far, our study is the first attempt to combine thalidomide with a gemcitabine-based chemotherapy protocol incorporating cisplatin, prednisone to illustrate whether the potential efficacy existed in the treatment for patients with relapsed or refractory PTCL. PTCL is a high incidence of non-Hodgkin lymphoma in China, which is a highly heterogeneous group of diseases.3 The present study retrospectively evaluated the efficacy and safety of GDPT regimen in the treatment of relapsed/refractory PLCL and the results we presented here showed the excellent antitumor effect of GDPT regimen.
Gemcitabine with broad spectrum antitumor activity is a common cytosine nucleoside derivative of cytarabine widely used in solid tumors. In recent years, gemcitabine has also shown significant efficacy in hematopoietic tumors, especially in relapse and refractory lymphoma.13 Zinzani et al used gemcitabine as a single agent to treat T-cell lymphoma, and obtained 51% ORR and 23% complete response rate.14 However, the efficacy of gemcitabine alone is still insufficient in most clinical studies. Besides, gemcitabine is a cell-cycle-specific agent, with lymphoma cells in S phase being sensitive to it, which has also been proven to be effective in blocking the transition process of cell proliferation from the G1 phase to the S phase.15 According to the theory of tumor cell proliferation kinetics, the combination of gemcitabine and cisplatin, a cell-cycle non-specific drug, has enormous clinical potential and may have a more comprehensive killing effect on tumor cells; many in vitro studies have also shown that the combination of the two drugs has a synergistic effect on the tumor cells.16,17
Thalidomide is an oral sedative with broad spectrum characteristics of suppressing inflammation, inhibiting angiogenesis, promoting apoptosis and immunomodulation, which may have direct effect on tumor cells and their microenvironment, providing an alternative strategy.18 Several clinical trials have also investigated the antitumor activity of thalidomide in solid and hematologic malignancies such as breast cancer, mantle cell lymphoma and Hodgkin’s lymphoma (HL), and so on, especially for first-line treatment of multiple myeloma (MM).19,20 As we all know, anti-angiogenesis and immunomodulatory are an important part in tumor proliferation and metastatic dissemination. Single-agent thalidomide has demonstrated limited efficacy in monotherapy when compared to multiple drug combination. Pro, B et al reported their experience with thalidomide used as a single agent in patients with recurrent/refractory non‐HL and HL. The study showed that only 1 patient (5%) with recurrent gastric mucosa-associated lymphoid tissue lymphoma achieved CR, and 3 patients (16%) achieved SD in 19 patients who had been treated with escalating doses of thalidomide.21 Kaufmann, H reported that rituximab plus thalidomide were used in relapsed/refractory MCL and 13 patients (81%) experienced an objective response, with 5 complete responders (31%).22 Garcia-Sanz, R et al report two highly refractory patients with HL treated with thalidomide, cyclophosphamide, and dexamethasone achieved sustained CR.23 However, although its immunomodulatory effect could be pivotal in the process of anti-tumor, especially for ATCL, in which the immune system and angiogenesis is extensively involved, experience with thalidomide in PTCL is very limited and remain controversial. But these clinical studies above included only B-cell lymphoma and HL, not PTCL, and therefore, it is too early to conclude whether thalidomide‐based therapy will be of clinical value for patients with relapse and refractory PTCL. Compared to the previous gemcitabine-based scheme regimen, oral thalidomide during the chemotherapy interval after the end of intravenous administration may prevent from the progression of the disease, which can explain why the GDPT regimen in our study achieved the DCR with 82.8% and the longer PFS reported compared with other literature.24 A previous prospective study conducted by our center which was registered at www.clinicaltrials.gov as NCT01664975 showed that ORR of people who were initially treated achieve 67%, with CR and PR rates of 52% and 15% for GDPT therapy, suggesting that the GDPT regimen were also effective for newly diagnosed PTCL and the underlying mechanism may be the suppression of nuclear factor (NF)-κB and NF-κB-regulated gene products.25 When it comes to thalidomide, we have to discuss the lenalidomide, which has also been studied in MM, B-cell lymphoma, PTCL, and so on. Both of them belong to the immunomodulator drugs and have anti-tumor effects.26 As a derivative of thalidomide, lenalidomide is a new generation of immunomodulator which has better efficacy and fewer adverse reactions, but its expensive price limits its clinical application. So we chose Thalidomide as a drug in the chemotherapy program.
In order to explore and verify the effectiveness of the combined use of the above-mentioned individual drugs, a lot of research has also been done on the combined regimen, such as GDP (gemcitabine, cisplatin, dexamethasone), GEM-P (gemcitabine, cisplatin, methylprednisolone), and so on in clinical work, but it also shows great difference in efficacy. For example, the ORR about GDP regimens for recurrent and refractory PTCL could get up to 72%, ranging from 30% to 72%, and the median PFS is about 4–11 months.24,27 In general, the response rate is higher and the duration of PFS seems superior to what is observed with other regimens. Besides, compared to relapse patients, the ORR of refractory patients was higher. To some extent, the GDPT chemotherapy program may be more effective in relapse patients.
When it comes to adverse reactions, except for some common hematologic toxicity, it was observed that patients using the GDPT regimen of 6 cycles were more likely to suffer from toxicity associated with thalidomide such as drowsiness, numbness in the hands and feet presented sleeve-like changes, which may significantly relate to the individual cumulated dose.21 Besides, it was worth mentioning that we should pay attention to the problem of thrombosis caused by thalidomide during the period of GDPT regimen. In this group of patients, we did not observe the appearance of thrombosis, probably because of our early prevention work depending on the patient’s platelet status. The details were as follows: if the platelet count was more than 70×109/L, patients needed to take one aspirin per day, that is, 100 mg per day. If the platelet count was less than 70×109/L, patients needed to stop taking aspirin. Therefore, when using thalidomide, we should focus on the adverse reactions caused by it and take timely treatment measures. In order to avoid teratogenicity caused by thalidomide, this adverse effect should have been fully informed before treatment and pregnancy was strictly prohibited.
Although the number of cases in our study is insufficient and further verification is needed, the therapeutic effect and toxicity of GDPT regimen in our study revealed were acceptable, suggesting GDPT could be a valuable and alternative salvage chemotherapy regimen for patients with relapse and refractory PTCL.
Acknowledgment
This study was supported by National Natural Science Foundation of China (81570203), Scientific and Technological Project from Health and Family Planning Commission (201702047) and Department of Science and Technology of Henan province (182102310114).
Author contributions
All authors contributed to data analysis, drafting, and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. doi:10.1200/JCO.2008.16.4558
2. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
3. Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138(3):429–434. doi:10.1309/AJCP7YLTQPUSDQ5C
4. Briski R, Feldman AL, Bailey NG, et al. The role of front-line anthracycline-containing chemotherapy regimens in peripheral T-cell lymphomas. Blood Cancer J. 2014;4:e214. doi:10.1038/bcj.2014.34
5. Abouyabis AN, Shenoy PJ, Sinha R, Flowers CR, Lechowicz MJ. A systematic review and meta-analysis of front-line anthracycline-based chemotherapy regimens for peripheral T-cell lymphoma. ISRN Hematol. 2011;2011:623924. doi:10.5402/2011/623924
6. Schmitz N, de Leval L. How I manage peripheral T-cell lymphoma, not otherwise specified and angioimmunoblastic T-cell lymphoma: current practice and a glimpse into the future. Br J Haematol. 2017;176(6):851–866. doi:10.1111/bjh.14473
7. Fossard G, Broussais F, Coelho I, et al. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: an analysis of patients from LYSA centers. Ann Oncol. 2018;29(3):715–723. doi:10.1093/annonc/mdx787
8. Gooneratne LV, Gamage V, De Silva M, Karunanayake P. Relapsed, refractory peripheral T-cell lymphoma not otherwise specified successfully treated with allogeneic haematopoietic stem cell transplantation. Ceylon Med J. 2018;63(2):78–79. doi:10.4038/cmj.v63i2.8672
9. Hildyard C, Shiekh S, Browning J, Collins GP. Toward a biology-driven treatment strategy for peripheral T-cell lymphoma. Clin Med Insights Blood Disord. 2017;10:1179545X17705863. doi:10.1177/1179545X17705863
10. Ma H, Davarifar A, Amengual JE. The future of combination therapies for peripheral T cell lymphoma (PTCL). Curr Hematol Malig Rep. 2018;13(1):13–24. doi:10.1007/s11899-018-0432-3
11. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi:10.1200/JCO.2006.09.2403
12. Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. doi:10.1016/S1053-4296(03)00031-6
13. Dong M, He XH, Liu P, et al. Gemcitabine-based combination regimen in patients with peripheral T-cell lymphoma. Med Oncol. 2013;30(1):351. doi:10.1007/s12032-012-0351-4
14. Zinzani PL, Venturini F, Stefoni V, et al. Gemcitabine as single agent in pretreated T-cell lymphoma patients: evaluation of the long-term outcome. Ann Oncol. 2010;21(4):860–863. doi:10.1093/annonc/mdp508
15. Zinzani PL, Magagnoli M, Bendandi M, et al. Therapy with gemcitabine in pretreated peripheral T-cell lymphoma patients. Ann Oncol. 1998;9(12):1351–1353. doi:10.1023/a:1008409601731
16. Peters GJ, Bergman AM, Ruiz VHV, Veerman G, Kuiper CM, Braakhuis BJ. Interaction between cisplatin and gemcitabine in vitro and in vivo. Semin Oncol. 1995;22(4 Suppl 11):72–79.
17. Aviles A, Neri N, Huerta-Guzman J, Fernandez R. Gemcitabine and cisplatin in refractory malignant lymphoma. Oncology. 2004;66(3):197–200. doi:10.1159/000077995
18. Raje N, Anderson KC. Thalidomide and immunomodulatory drugs as cancer therapy. Curr Opin Oncol. 2002;14(6):635–640.
19. Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(7):719–734. doi:10.1002/ajh.24402
20. Iacopetta D, Carocci A, Sinicropi MS, et al. Old drug scaffold, new activity: thalidomide-correlated compounds exert different effects on breast cancer cell growth and progression. ChemMedChem. 2017;12(5):381–389. doi:10.1002/cmdc.201600629
21. Pro B, Younes A, Albitar M, et al. Thalidomide for patients with recurrent lymphoma. Cancer. 2004;100(6):1186–1189. doi:10.1002/cncr.20070
22. Kaufmann H, Raderer M, Wohrer S, et al. Antitumor activity of rituximab plus thalidomide in patients with relapsed/refractory mantle cell lymphoma. Blood. 2004;104(8):2269–2271. doi:10.1182/blood-2004-03-1091
23. Garcia-Sanz R, Gonzalez-Lopez TJ, Vazquez L, Hermida G, Graciani IF, San MJ. The combination of thalidomide, cyclophosphamide and dexamethasone is potentially useful in highly resistant Hodgkin’s lymphoma. Eur J Haematol. 2010;84(3):266–270. doi:10.1111/j.1600-0609.2009.01375.x
24. Park BB, Kim WS, Suh C, et al. Salvage chemotherapy of gemcitabine, dexamethasone, and cisplatin (GDP) for patients with relapsed or refractory peripheral T-cell lymphomas: a consortium for improving survival of lymphoma (CISL) trial. Ann Hematol. 2015;94(11):1845–1851. doi:10.1007/s00277-015-2468-y
25. Li L, Duan W, Zhang L, et al. The efficacy and safety of gemcitabine, cisplatin, prednisone, thalidomide versus CHOP in patients with newly diagnosed peripheral T-cell lymphoma with analysis of biomarkers. Br J Haematol. 2017;178(5):772–780. doi:10.1111/bjh.14763
26. Toumishey E, Prasad A, Dueck G, et al. Final report of a phase 2 clinical trial of lenalidomide monotherapy for patients with T-cell lymphoma. Cancer. 2015;121(5):716–723. doi:10.1002/cncr.29103
27. Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32(31):3490–3496. doi:10.1200/JCO.2013.53.9593
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.