Back to Journals » Clinical and Experimental Gastroenterology » Volume 15
Gastrointestinal Mucormycosis-Induced Massive Lower Gastrointestinal Bleeding, Rectal Perforation, and Pulmonary Embolism: A Long Diagnostic Pathway in a Case Report
Authors Ralaizanaka BM , Razafindrazoto CI , Bolot E, Bors G, Housson-Wetzel S, Razafimahefa SH, Ramanampamonjy RM, Claude P
Received 18 May 2022
Accepted for publication 9 August 2022
Published 12 August 2022 Volume 2022:15 Pages 145—151
DOI https://doi.org/10.2147/CEG.S373728
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Anastasios Koulaouzidis
Behoavy Mahafaly Ralaizanaka,1 Chantelli Iamblaudiot Razafindrazoto,2 Eloïse Bolot,3 Georges Bors,3 Stéphanie Housson-Wetzel,3 Soloniaina Hélio Razafimahefa,1 Rado Manitrala Ramanampamonjy,2 Pierre Claude3
1Unity of Hepato-Gastroenterology, University Hospital Andrainjato, Fianarantsoa, Madagascar; 2Unity of Gastroenterology, University Hospital Joseph Raseta Befelatanana, Antananarivo, Madagascar; 3Unity of Gastroenterology, Emile Müller Hospital of Regional Hospital Group of Mulhouse South Alsace, Mulhouse, France
Correspondence: Behoavy Mahafaly Ralaizanaka, Unity of Hepato-Gastroenterology, University Hospital Andrainjato, Fianarantsoa, Madagascar, Tel +261341920148, Email [email protected]
Introduction: Mucormycosis is a rare systemic fungal infection, mainly observed in immunocompromised patients. It is responsible for surface and deep tissue destruction leading to perforations and hemorrhage. Its pathogenesis represented by an angio-invasion is at the origin of a local infarction and a vascular thrombosis. We report a case of gastrointestinal (GI) mucormycosis-induced multiple gastric ulcers, GI bleeding and rectal perforation.
Case Presentation: A 75-year-old man, with type II diabetes mellitus, was admitted to the intensive care unit for an acute abdominal pain associated with massive hematochezia. Clinical examination was that of an acute peritonitis and a hemorrhagic shock state. Abdominal and pelvic CT scan with intravenous contrast concluded to a perforation of the anterior wall of the rectum. He underwent immediate laparotomy with temporary colostomy. Several upper GI endoscopies had shown multiple gastric ulcer lesions. Lower GI endoscopy showed a fistulous orifice of the rectum on its anterior surface. Histopathology of the gastric biopsy showed acute and subacute inflammatory changes with filamentous elements suggesting mucormycosis. Histopathology of the rectal biopsy showed a subacute non-specific inflammation. Culture of the secretions from the rectal fistula orifice showed the strain Rhizopus sp. Antifungal susceptibility testing reported sensitivity to liposomal amphotericin B. The diagnosis of GI mucormycosis-induced multiple gastric ulcers, rectal perforation and pulmonary embolism in the patient with type II diabetes mellitus was retained. The outcomes were favorable after 6 weeks of treatment with liposomal amphotericin B associated with temporary colostomy and appropriate diabetes management.
Conclusion: GI mucormycosis remains a multidisciplinary diagnostic challenge, less frequent in clinical practice, with a long diagnostic pathway. This opportunistic systemic mycosis can lead to numerous GI complications including perforation, massive GI bleeding and even multiple extra-GI complications. GI mucormycosis has a good prognosis if it is treated early with medical and surgical treatment.
Keywords: gastrointestinal bleeding, gastrointestinal mucormycosis, gastrointestinal perforation, gastric ulcer
Background
Mucormycosis is an opportunistic, uncommon and potentially fatal fungal infection caused by filamentous fungi belonging to the subphylum Mucormycotina, order Mucorales, class Zygomycetes. Rhizopus species are the most common cause of infection. The main routes of entry are the sinuses, lungs, gastrointestinal (GI) tract and skin.1,2 The entire organ system can be affected, but rhino-orbital, cerebral and pulmonary infections dominate the literature.1–4 The GI form of mucormycosis is by far the least frequent.4 These fungi are responsible for both superficial and deep tissue destruction associated with angioinvasion. This pathogenesis is the cause of massive GI hemorrhage, GI perforation, disseminated systemic infection, local infarction and vascular thrombosis. Massive GI bleeding and GI perforation are the most common cause of death.2,5 The prognosis of GI mucormycosis is often poor due to delayed diagnosis because of its atypical manifestations requiring a long diagnostic process.2,4–6 Despite the intensive use of antifungal therapy and surgical debridement, the mortality rate of adult patients varies from 20% to 100%.2,5 We report a case of GI mucormycosis presenting as massive hematochezia, multiple gastric ulcers and rectal perforation in a 75-year-old man with type II diabetes mellitus.
Case Presentation
A 75-year-old man was admitted to the intensive care unit for an acute abdominal pain associated with massive hematochezia. He had a history of type II diabetes mellitus, hypertension, hypercholesterolemia, weaned smoking, unstable angina with triple coronary artery bypass grafting, macular retinal degeneration, and benign prostatic hypertrophy. He underwent endarterectomy for left carotid stenosis. General examination reported a blood pressure of 60/40 mmHg, a heart rate of 180 bpm, a respiratory rate of 34/min, an oxygen saturation of 89% and a temperature of 38.3°C. Physical examination had reported abdominal tenderness. He was immediately treated in the intensive care unit as an acute peritonitis. The patient was intubated and received fluid resuscitation, vasopressor drugs (norepinephrine), empiric antibiotic therapy (piperacillin/tazobactam) and high dose of proton pump inhibitor. Abdominal and pelvic computed tomography (CT scan) with intravenous contrast showed a perforation of the anterior wall of the rectum (Figure 1). The patient underwent immediate laparotomy that failed to find the perforation. Temporary colostomy was performed. He had a hemoglobin level of 9.3 g/dL after transfusion of 5 units of packed red blood cells. There was a hyperleukocytosis of 14,760 elements/mm3 and a C-reactive protein of 101 mg/L. QuantiFERON-tuberculosis assay and human immunodeficiency virus (HIV) serology were negative. Several upper GI endoscopies showed multiple ulcerated lesions along the greater gastric tuberosity, on the anterior wall, 3 to 4 cm in diameter, surrounded by a budding edematous mucosa, and fragile on contact (Figure 2). Lower GI endoscopy showed a productive fistulous orifice with a whitish filamentous discharge at the anterior aspect of the rectum (Figure 3). Histopathological examination of gastric biopsy showed acute and subacute inflammatory remodeling mixed with filamentous elements suggestive of mucormycosis (Figure 4). Rectal biopsy histology was fibro-edematous remodeling, lymphoid nodular cluster, and non-specific subacute inflammation with negative Periodic Acid-Schiff (PAS). Fungal culture of secretions from the rectal fistula orifice identified the strain Rhizopus sp. Antifungal susceptibility testing reported sensitivity to liposomal amphotericin B. Chest CT scan during the hospitalization revealed bilateral pleural effusion and atelectasis. Lung scintigraphy showed recent segmental pulmonary embolisms of the right upper lobe. The diagnosis of GI mucormycosis-induced multiple gastric ulcers, rectal perforation and pulmonary embolism was retained. The patient was treated with liposomal Amphotericin B 5mg/kg/day intravenously for 6 weeks and anticoagulation with low-molecular-weight heparin and insulin therapy. Upper gastrointestinal endoscopy after 4 weeks showed healing of the gastric ulcers (Figure 5). After 6 weeks, the outcome was favorable without recurrence or other complications.
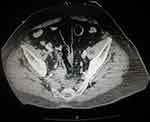 |
Figure 1 Abdominal and pelvic CT scan of a 75-year-old man showing a perforation of the anterior wall of the rectum. |
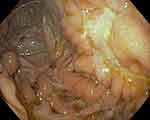 |
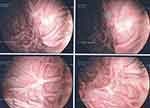
Figure 2 Upper gastrointestinal endoscopy showed suspicious ulcerated lesions along the large anterior gastric tuberosity, surrounded by a budding mucosa. |
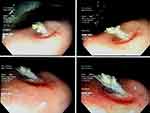 |
Figure 3 Recto-sigmoidoscopy of a 75-year-old man showing uncommon fistulous orifice with leakage on the anterior rectal wall. |
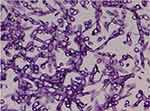 |
Figure 4 Histopathology of the gastric biopsies had shown a typical of Muchorales image, suggestive mucormycosis. |
Discussion
Mucormycosis is a severe, opportunistic, angio-invasive fungal infection of the Mucorales. It is a common filamentous fungus, of the class Mucormycetes. It is the third most common invasive fungal infections after aspergillosis and candidiasis.7,8 An increasing annual incidence with age was noted from 1997 to 2006.9,10 The disease can be transmitted by inhalation or direct inoculation of spores through injured skin or mucosa. Mucormycetes are characterized by the presence of large aseptate hyphae (coenocytic mycelia) and the formation of zygospores.7 The genera Rhizopus are by far the most frequently found.1–8,11 Mucormycosis can present with different clinical manifestations: rhino-cerebral (39%), pulmonary (24%), cutaneous (19%) and the disseminated form (23%).1–8,11 Among these manifestations, the GI form remains uncommon and rarely diagnosed in living patients. This GI form represents only 7% of reported cases.1–8,11,12 Diseases of the GI tract are usually acquired by ingestion of contaminated food or by infection of an implanted device. Within the GI tract, the stomach is the most common site of infection (67%), followed by the colon (21%), small intestine (4%) and esophagus (2%).1,2,6 For our patient, the locations were a gastric and colonic. Given the rapid evolution of GI mucormycosis, the prognosis is poor with a very high mortality rate, approximately 85%.5–7,11,12 Many patients with mucormycosis had presented with risk factors for immunosuppression, including diabetes (9%), persistent neutropenia, hematologic malignancies (44%), HIV infection, prematurity malnutrition and undernutrition, long-term systemic corticosteroid therapy, immunosuppressive therapy following organ or stem cell transplantation, and increased serum iron levels.1,2,4–8,11,12 Our patient had type II diabetes mellitus. The diagnosis of GI mucormycosis often remains late because of the nonspecific presentation.1–8,11–14 Only 25% of cases are diagnosed ante-mortem.12 The most common symptoms are abdominal pain and distension, associated with nausea or vomiting and sometimes GI bleeding. The definitive diagnosis of mucormycosis is histologic evidence of fungal invasion of tissue. In the case of GI mucormycosis, endoscopy or even surgery is usually unavoidable for the diagnosis, as in the case of our patient.1–8,11,12 There was also the angio-invasive complication with pulmonary embolism. These are clinical presentations reported by several authors.1–4,6 It is advised that treatment of mucormycosis include urgent surgical resection of infected area whenever feasible parallel to systemic antifungal therapy to prevent disease dissemination.6 The medical/surgical and multidisciplinary management should be early for an excellent outcome.15 Management involves both the risk factors present that may be decompensated, rapid appropriate antifungal treatment and early surgical debridement.1,2,4–7,11,12,15,16 The survival rate of medical/surgical treatment is as high as 70%, whereas it is 61% with antifungal treatment alone.11 Song et al reported that 121 of the 174 patients they reviewed (69.5%) received a combination of antifungal and surgical treatment and had a higher survival rate than patients who underwent only surgery and patients who only received antifungal treatment (70.2% versus 36.4% versus 32.4%).16 Almyroudis et al reported that 73.7% of patients underwent surgery. The mortality rate of patients who were only treated with amphotericin B was higher than that of patients who received the treatment combination of amphotericin B and surgery (62.5% vs 34.3%).17 Amphotericin B, posaconazole, and isavuconazole are the only currently available antifungal treatment active against Mucorales species. Liposomal amphotericin B is the recommended first-line treatment for mucormycosis. Posaconazole has generally been recommended as maintenance and salvage therapy.6,11,18 Isavuconazole, a novel triazole with available oral and intravenous formulations, showed at least partial activity against different strains of Mucorales.19 A recently conducted open-label Phase 3 study evaluated 37 cases of mucormycosis (21 patients receiving primary therapy, 11 for refractory disease and 5 for intolerance); 31% of overall response, 32% in the primary treatment group, and 36% in patient’s refractory to other antifungal treatments. The survival rate was 0.53 at day 180, which was consistent with published data on amphotericin B and posaconazole.20 Our patient had received appropriate diabetes treatment, surgical treatment and antifungal treatment. The evolution was satisfactory with healing of the GI ulcers.
Conclusion
Mucormycosis is the third potentially fatal systemic fungal infection caused by Mucorales, mainly affecting immunocompromised patients. Diagnosis is often delayed due to an atypical clinical presentation and a long diagnostic process. Colonic opportunistic infections such as mucormycosis should be considered in the presence of GI ulcers, severe GI bleeding and GI perforations in immunocompromised patients. Early medical and surgical treatment of mucormycosis is associated with a good prognosis. Evaluation and treatment of vascular complications of mucormycosis contribute to an excellent outcome.
Informed Consent Statement
Informed written consent was obtained from the patient for publication of this report. There is no institutional approval is required to publish the case details.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Wotiye AB, Ks P, Ayele BA. Invasive intestinal mucormycosis in a 40-year old immunocompetent patient - a rarely reported clinical phenomenon: a case report. BMC Gastroenterol. 2020;20(1):61. doi:10.1186/s12876-020-01202-5
2. Huang H, Xie L, Zheng Z, et al. Mucormycosis-induced upper gastrointestinal ulcer perforation in immunocompetent patients: a report of two cases. BMC Gastroenterol. 2021;21:311. doi:10.1186/s12876-021-01881-8
3. Martinello M, Nelson A, Bignold L, Shaw D. “We are what we eat!” Invasive intestinal Mucormycosis: a case report and review of the literature. Med Mycol Case Rep. 2012;1:52–55. doi:10.1016/j.mmcr.2012.07.003
4. Bhat V, Thomas SA, Kanavi JV, Thomas A. Intestinal perforation secondary to mucormycosis associated with puerperal sepsis. Cureus. 2014;13(8):e17428. doi:10.7759/cureus.17428.
5. Zilberberg MD, Shorr AF, Huang H, Chaudhari P, Paly VF, Menzin J. Hospital days, hospitalization costs, and inpatient mortality among patients with mucormycosis: a retrospective analysis of US hospital discharge data. BMC Infect Dis. 2014;14(1):310. doi:10.1186/1471-2334-14-310
6. Chiang TH, Lee YW, Tan JH, Kao CC, Chang CC, Fang KC. Mucormycosis causing massive lower gastrointestinal bleeding: a case report. BMC Gastroenterol. 2021;21(1):272. doi:10.1186/s12876-021-01846-x
7. Bala K, Chander J, Handa U, Punia RS, Attri AK. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Med Mycol. 2015;53(3):
8. Lacarrière E, Lacaze L, Schwarz L, Huet E, Lemoine F, Scotté M. First case of gastrointestinal mucormycosis in an immunocompromised patient with gallbladder and duodenum involvement. Infection. 2011;39(6):
9. Bitar D, Lortholary O, Dromer F, Coignard B, Che D. Mycoses invasives en France métropolitaine, PMSI 2001 2010: incidence, létalité et tendances. Bulletin Épidémiologie Hebdomadaire. 2013;12-13:109–114.
10. Bitar D, Cauteren DV, Lanternier F, et al. Increasing Incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis. 2009;15(9):1395–1401. doi:10.3201/eid1509.090334
11. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):
12. Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl_1):S23–34. doi:10.1093/cid/cir866
13. Prakash H, Ghosh AK, Rudramurthy SM, et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol. 2019;57(4):
14. Patel AK, Patel KK, Patel K, Gohel S, Chakrabarti A. Mucormycosis at a tertiary care centre in Gujarat, India. Mycoses. 2017;60(6):
15. Termos S, Othman F, Alali M, et al. Total gastric necrosis due to mucormycosis: a rare case of gastric perforation. Am J Case Rep. 2018;19:
16. Song Y, Qiao J, Giovanni G, et al. Mucormycosis in renal transplant recipients: review of 174 reported cases. BMC Infect Dis. 2017;17(1):283. doi:10.1186/s12879-017-2381-1
17. Almyroudis NG, Sutton DA, Linden P, et al. Zygomycosis in solid organ transplant recipients in a tertiary transplant center and review of the literature. Am J Transplant. 2006;6:2365–2374. doi:10.1111/j.1600-6143.2006.01496.x
18. Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):
19. Verweij PE, González GM, Wiedrhold NP, et al. In vitro antifungal activity of isavuconazole against 345 Mucorales isolates collected at study centers in eight countries. J Chemother. 2009;21(3):272–281. doi:10.1179/joc.2009.21.3.272
20. Marty FM, Perfect JR, Cornely OA, et al. An open-label phase 3 study of isavuconazole (VITAL): focus on mucormycosis [Abstract]; 2014. Available from: https://idsa.confex.com/idsa/2014/webprogram/Paper45645.html.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.