Back to Journals » International Medical Case Reports Journal » Volume 13
From Reactive Lymphadenopathy to Systemic Vasculitis, the Importance of Providing Sufficient Clinical Information to Optimize Pathological Interpretation, a Case Report
Authors Soleimani N , Mokhtari M, Mohammadzadeh S
Received 29 September 2019
Accepted for publication 20 December 2019
Published 9 January 2020 Volume 2020:13 Pages 1—5
DOI https://doi.org/10.2147/IMCRJ.S232867
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Neda Soleimani, Maral Mokhtari, Sahand Mohammadzadeh
Department of Pathology, Shiraz University of Medical Sciences, Shiraz, Iran
Correspondence: Sahand Mohammadzadeh
Department of Pathology, Shiraz University of Medical Sciences, Shiraz, Iran
Tel +98 9173078147
Fax +98 71323017
Email [email protected]
Introduction: Pathology must aim at a correct diagnosis, which is complete and useful for clinicians. However, in routine practice, there are multiple sources of errors in the pathology results, which have several impacts on the patient’s treatment and outcome.
Case presentation: Our patient is a 66 years old man, case of rheumatoid arthritis with lymphadenopathy due to vasculitis, which was underdiagnosed due to lack of complete clinical data during pathologic examination. Since the patient was extremely ill, and the workup was inconclusive, the pathology slides were sent to our center for consultation and molecular study to rule out lymphoma. The slide review was done with complete access to the patient’s history and status. In addition to reactive follicular hyperplasia, there was inter-follicular/paracortical plasma cell infiltration and remarkable leukocytoclastic vasculitis of small vessels.
Discussion: Most frequent errors in the laboratories are preanalytical, due to clinical failures (wrong clinical procedure, inappropriate ordering, erroneous, incomplete or misleading clinical information), and specimen transportation and delivery. Surgical pathology by its nature depends heavily on the input of clinicians and surgeons who are fully aware of patient condition.
Conclusion: This case clearly shows the importance of communication between the pathologist and clinicians and the impact on patient care. Clinicians should also provide complete clinical data for the pathologist. Full access to clinical information improves the pathologist’s ability to make an accurate diagnosis.
Keywords: rheumatoid arthritis, clinical data, communication in pathology
Introduction
Pathology must aim at a correct diagnosis, which is complete and useful for clinicians. However, in routine practice, there are multiple sources of errors in the pathology results, which have several impacts on the patient’s treatment and outcome. Diagnostic errors or incomplete diagnoses may cause harm to the patient by delaying appropriate treatment. The pathologist should be aware of patient’s clinic. These data, along with specific microscopic features and ancillary studies, help the pathologist to form an accurate and complete diagnosis.1
Rheumatoid arthritis (RA) is a chronic autoimmune disease that causes pain, swelling, and stiffness of joints. The characteristic feature is symmetrical and erosive arthritis of small peripheral joints. Extra-articular manifestations develop in 40% of patients and contribute to significant disease-related morbidity and mortality. Among these, systemic rheumatoid vasculitis, characterized by inflammation of mid-size arteries and capillaries, is associated with a particularly dire outcome.2,3 We want to report a case of rheumatoid arthritis with lymphadenopathy due to vasculitis, which was underdiagnosed due to lack of complete clinical data during pathologic examination.
Case Report
A 66 years old man referred to our center at Shiraz University of Medical Sciences, Iran, complaining of fever, severe weight loss, and malaise for several months. He had a long-term history of uncomplicated RA with total hip joint replacement following a car accident 2 years ago.
His physical examination was significant for temperature: 38°C (orally) and axillary lymphadenopathy.
Laboratory investigation showed mild normochromic normocytic anemia with lymphocyte dominancy in differential WBC count. Serum protein electrophoresis was in favor of polyclonal gammopathy, and bone marrow study with immune-phenotyping revealed normocellular marrow with increased polyclonal plasma cells. Other significant laboratory test results in admission time are listed in Table 1.
 |
Table 1 Laboratory Test Results of Patient in Hospital Admission |
Patient disease activity score was low (DAS 28:2.9), and patient previous lab data were negative in terms of ANA (anti-nuclear antibody), Anti-dsDNA (Anti-double Stranded DNA), and ANCA (Anti-neutrophil cytoplasmic antibody).4
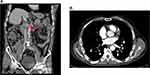
Chest CT scan shows multiple lymph nodes in aortopulmonary window and also sub-carina. More lymph nodes were identified at para-aortic, para iliac, celiac axis, and peri-pancreatic area in abdominopelvic spiral CT scan (Figure 1). The patient was referred to an oncologist and lymph node excisional biopsy was done for him with the impression of Hodgkin’s lymphoma, but the final report was just reactive follicular hyperplasia (Figure 2).
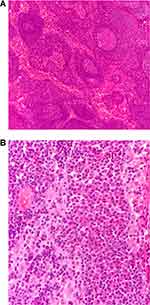 |
Figure 2 (A) Reactive follicular hyperplasia (H&E stain, ×100). (B) Plasma cell infiltration in inter-follicular areas (H&E stain, ×400). |
Since the patient was extremely ill, and the workup was inconclusive, the pathology slides were sent to our center for consultation and molecular study to rule out lymphoma. We are a referral center, and it is not surprising that there was also a serum specimen from that patient at the same time in our clinical laboratory, and he was recalled to get information about his present and past medical history. The slide review was done with complete access to the patient’s history and status. In addition to reactive follicular hyperplasia, there was inter-follicular/paracortical plasma cell infiltration as well as remarkable leukocytoclastic vasculitis of small vessels (Figure 3).
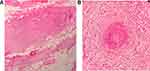 |
Figure 3 (A) Leukocytoclastic vasculitis in a medium-sized vessel in lymph node (H&E stain, ×100). (B) Leukocytoclastic vasculitis in small vessel in lymph node (H&E stain, ×400). |
P-ANCA (perinuclear anti-neutrophil cytoplasmic antibody) was also positive with an atypical pattern indirect IF study. The patient was visited by an expert rheumatologist and treated with a rheumatoid vasculitis diagnosis. Prednisone 60 mg daily was started for the patient, then tapered and after that Methotrexate 15 mg weekly was started with folic acid 1 mg daily.
In a follow-up visit three months later, the patient showed improvement in his symptoms. Repeated inflammatory markers showed a C reactive protein of 6 mg/L and a sedimentation rate of 29 mm/hour. The response to treatment was rapid and satisfactory, and now the patient has a favorable condition.
The patient has provided written informed consent for publication of this case report including images. Our institutional approval is not required to publish the case details.
Discussion
Vasculitis is an inflammatory process that affects the vessel wall as the primary site of inflammation. This process causes damage to the vessel wall. Fibrinoid necrosis and thrombosis lead to the narrowing of the lumen due to thickening or total occlusion.5,6 Classification of vasculitis is based on the size of the primary type of vessel involved in a given disease or other factors like clinical data, organ tropism, the presence of granulomatous inflammation, the role of immune complexes in pathophysiology and the association of autoantibodies with some forms of vasculitis.7
Systemic vasculitis is regarded as primary when no etiological factor is identified or secondary to infections, drugs, systemic autoimmune diseases (systemic lupus erythematosus, RA, and Sjögren’s syndrome), or to malignancies. Recognition of manifestations suggestive of systemic vasculitis is essential since a delay in diagnosis may impact the outcome significantly. Due to the multisystemic nature of vasculitis, a multidisciplinary approach is usually necessary. The presentation may vary from subacute non-specific complaints to life-threatening features. Suspicion of systemic vasculitis is generally raised when constitutional symptoms (e.g., fever, malaise, or weight loss) are present in association with a combination of different organs and systems involved. However, the diagnosis needs to be confirmed by tissue biopsy, imaging studies, and specific serological markers (i.e., PR3- or MPO-ANCA, anti-GBM antibodies, and cryoglobulins).8,9
Individual vasculitis cases with nonspecific presentations may still be difficult to assign to a specific disease entity.10 Lymphadenopathy is a rare presentation of systemic vasculitis. Kawasaki disease, a medium vessel vasculitis most commonly seen in childhood, may cause lymphadenopathy (hence the synonym mucocutaneous lymph node syndrome) but is also associated with a rash, fever and in severe cases coronary artery vasculitis.11
The exact cause of RA is unknown, but many possible etiologies include environmental factors like hormonal, genetic, infectious. Extra-articular manifestations of RA occur in about 40% of patients, either in the beginning or during clinical course of their disease. They are mostly related to vasculitis, and often a reflection of longstanding inflammation. Extra-articular RA is a serious condition, and RA patients with extra-articular manifestations should be aggressively treated and monitored. Most organs can be involved.12
Although lymphadenopathy is consistently recognized among the possible extra-articular manifestations of the disease, generalized painless lymphadenopathy with the involvement of central lymph nodes (para-aortic, para iliac, celiac axis and peripancreatic area) is not seen commonly, and lymph node biopsy was done in our case to rule out lymphoproliferative disorders.13
Most common errors in the laboratories are preanalytical, due to clinical failures (wrong clinical procedure, inappropriate ordering, erroneous, incomplete or misleading clinical information), and specimen transportation and delivery.14 The best pathology report in the world is worthless if the diagnosis is inaccurate.15
Surgical pathology by its nature depends heavily on the input of clinicians and surgeons who are fully aware of patient condition. They should know that a pathologic diagnosis is a subjective evaluation that acquires full meaning only when the pathologist is fully aware of the essential clinical data, surgical findings, and type of surgery”, Juan Rosai, MD said.16
According to the Laboratory Accreditation Program of the 1998 Laboratory Checklist, and the Joint Commission on Accreditation of Healthcare Organizations 1998 Standard, each surgically removed specimen should be accompanied by pertinent clinical information and, by the preoperative and postoperative diagnosis.17
Even though some communication exists between pathologists and their clinical colleagues, it is often circumstantial and usually occasioned by clinicopathological discrepancies which are generally recognized by the clinician.11,16 In one study published in a College of American Pathologists Jurnal, by Nakhleh RE and coworkers, about 2.4% of cases submitted to surgical pathology had no clinical history.15,19
Inappropriate and Incomplete communication between the clinician and the pathologist may make diagnosis difficult or impossible and is also dangerous for the patient with potentially enormous medical, legal, and financial consequences. So close rapport between the pathologist and clinician is, therefore, a necessary step in minimizing this ill.13,19
This case clearly shows the importance of communication between the pathologist and clinicians and the impact on patient care. Clinicians should also provide complete clinical data for the pathologist. It is also essential to review the pathologic diagnosis before setting any treatment plans. New communication technologies allow sharing patient’s information in developed health services worldwide. But there are still health-care services, which these communication systems like voice mail or electronic mail are not available.20
Conclusion
Systemic vasculitis is a secretive disease with poor outcome (if untreated), which should be in mind in patients with constitutional symptoms, at least in whom with known autoimmune diseases. Rheumatoid vasculitis should be regarded as a potential cause for any lump (including lymphadenopathy) in advanced rheumatoid arthritis patients. Full access to clinical information improves the pathologist’s ability to make an accurate diagnosis. This access to clinical data is almost always impossible unless each specimen is supported by a formal pathology requisition form which is filled by the clinician who is most familiar with the case and not by the most junior member of the team.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Santana MF, Ferreira LC. Diagnostic errors in surgical pathology. J Bras Patol Med Lab. 2017;53(2):124–129.
2. Makol A, Crowson CS, Wetter DA, Sokumbi O, Matteson EL, Warrington KJ. Vasculitis associated with rheumatoid arthritis: a case-control study. Rheumatology (Oxford). 2014;53(5):890–899. doi:10.1093/rheumatology/ket475
3. Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62–67.
4. Soleimani N, Hosseinzadeh M, Habibagahi Z. Value of serum glucose-6-phosphate isomerase in patients with rheumatoid arthritis and correlation with disease activity: a case-control study. J Educ Health Promot. 2019;8(1):125.
5. Watts RA, Scott DG. Recent advances in the classification and assessment of vasculitis. Best Pract Res Clin Rheumatol. 2009;23:429–443. doi:10.1016/j.berh.2008.12.004
6. Jennette JC, Falk RJ. Small-vessel vasculitis. N Eng J Med. 1997;337:1512–1523. doi:10.1056/NEJM199711203372106
7. Saleh A, Stone JH. Classification and diagnostic criteria in systemic vasculitis. Best Pract Res Clin Rheumatol. 2005;19(2):209–221. doi:10.1016/j.berh.2004.09.001
8. Waller R, Ahmed A, Patel I, Luqmani R. Update on the classification of vasculitis. Best Pract Res Clin Rheumatol. 2013;27:3–17. doi:10.1016/j.berh.2012.12.002
9. Gross WL, Trabandt A, Reinhold-Keller E. Diagnosis and evaluation of vasculitis. Rheumatology (Oxford). 2000;39:245–252. doi:10.1093/rheumatology/39.3.245
10. Mahr A, de Menthon M. Classification and classification criteria for vasculitis: achievements, limitations and prospects. Curr Opin Rheumatol. 2015;27(1):1–9. doi:10.1097/BOR.0000000000000134
11. Uddin A, West K, Barratt J. Lumps, bumps and vasculitis. J R Soc Med. 2009;102(1):29–31. doi:10.1258/jrsm.2008.070391
12. Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD, Tanasescu R. Extra-articular manifestations in rheumatoid arthritis. Maedica (Buchar). 2010;5(4):286–291.
13. Suleiman DE. Pathologist-clinician collaboration: a marriage of necessity toward improving the quality of patient care. Ann Nigerian Med. 2015;9:1–3. doi:10.4103/0331-3131.163325
14. Sirota RL. Error and error reduction in pathology. Arch Pathol Lab Med. 2005;129:1228–1233. doi:10.1043/1543-2165(2005)129[1228:EAERIP]2.0.CO;2
15. Nakhleh RE. What is quality in surgical pathology? J Clin Pathol. 2006;59(7):669–672. doi:10.1136/jcp.2005.031385
16. Rosai J. Introduction. In: Rosai J, editor. Ackerman’s Surgical Pathology. St Lou- is, Mo: Mosby; 1996:1–12.
17. Commission on Laboratory Accreditation. Anatomic pathology. In: 1997 Inspection Checklist. Section Northfield, Ill: College of American Pathologists; 1997:41-44
18. Anne C. Belanger. Comprehensive Accreditation Manual for Pathology and Laboratory Services. Oakbrook Terrace, Ill: Joint Commission on Accreditation of Health- care Organizations; 1998.
19. Nakhleh RE, Zarbo RJ. Amended reports in surgical pathology and implications for diagnostic error detection and avoidance: a College of American Pathologists Q-Probes study of 1667547 accessioned cases in 359 laboratories. Arch Pathol Lab Med. 1998;122:303–309.
20. Powsner SM, Costa J, Homer RJ. Clinicians are from Mars and pathologists are from Venus: clinician interpretation of pathology reports. Arch Pathol Lab Med. 2000;124(7):1040–1046.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.