Back to Journals » Clinical Interventions in Aging » Volume 15
Frailty Phenotype: Evidence of Both Physical and Mental Health Components in Community-Dwelling Early-Old Adults
Authors Batko-Szwaczka A , Dudzińska-Griszek J, Hornik B , Janusz-Jenczeń M , Włodarczyk I, Wnuk B , Szołtysek J , Durmała J , Wilczyński K , Cogiel A, Dulawa J , Szewieczek J
Received 13 November 2019
Accepted for publication 27 December 2019
Published 5 February 2020 Volume 2020:15 Pages 141—150
DOI https://doi.org/10.2147/CIA.S238521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Agnieszka Batko-Szwaczka,1 Joanna Dudzińska-Griszek,1 Beata Hornik,2 Magdalena Janusz-Jenczeń,2 Iwona Włodarczyk,2 Bartosz Wnuk,3 Joanna Szołtysek,3 Jacek Durmała,3 Krzysztof Wilczyński,1 Anna Cogiel,1 Jan Dulawa,4 Jan Szewieczek1
1Department of Geriatrics, School of Health Sciences in Katowice, Medical University of Silesia, Katowice, Poland; 2Department of Internal Nursing, School of Health Sciences in Katowice, Medical University of Silesia, Katowice, Poland; 3Department of Rehabilitation, School of Health Sciences in Katowice, Medical University of Silesia, Katowice, Poland; 4Department of Internal Medicine and Metabolic Diseases, School of Health Sciences in Katowice, Medical University of Silesia, Katowice, Poland
Correspondence: Jan Szewieczek
Department of Geriatrics, GCM, Ul. Ziolowa 45/47, Katowice 40-635, Poland
Tel +48323598239
Fax +48322059483
Email [email protected]
Background: Demographic aging results in increased incidence of old-age disability. Frailty is a major factor contributing to old-age disability. The aim of this study was to investigate the prevalence of the frailty phenotype as defined by Fried et al and to estimate the need for associated preventative interventions in early-old community-dwelling inhabitants of the southern industrial region of Poland, as well as to investigate the defining components of the frailty phenotype.
Methods: The study group consisted of 160 individuals with an average age of 66.8 ± 4.2 years ( ± SD), 71 (44.4%) of study participants were women. The cohort was randomized out of over 843 thousand community-dwelling Upper Silesian inhabitants aged 60– 74 years, who agreed to participate in this project. A comprehensive geriatric assessment (CGA), frailty phenotype test (as described by Fried et al) blood tests and bioimpedance body structure analysis was completed for study participants. Functional assessment included Barthel Index of Activities of Daily Living (Barthel Index), Instrumental Activities of Daily Living Scale (IADL), Mini-Mental State Examination (MMSE), the Timed Up and Go (TUG) test, Tinetti Performance-Oriented Mobility Assessment (POMA), and Geriatric Depression Scale – Short Form (GDS-SF).
Results: Prefrailty was diagnosed in 24.4% of the subjects (95% Confidence Interval (CI) = 17.7– 31.0%; 31% in women and 19.1% in men, P=0.082) and frailty in 2.5% subjects (95% CI 0.1– 4.9%; more frequently in women: 4.2% versus 1.1% in men, P=0.046). Having one or more positive frailty criteria was positively associated with depression (odds ratio (OR)=2.85, 95% CI=1.08– 7.54, P=0.035) and negatively associated with MMSE score (OR=0.72, 95% CI=0.56– 0.93, P=0.012) and fat-free mass (OR=0.96, 95% CI=0.92– 0.99, P=0.016) in multivariate logistic regression analysis adjusted for age, sex, disease prevalence, number of medications, functional tests (Barthel Index, IADL, MMSE, GDS-SF), BMI, bioimpedance body composition score, and blood tests.
Conclusion: At least 25% of the early-old community-dwelling population would benefit from a frailty prevention program. The frailty phenotype reflects both physical and mental health in this population.
Keywords: frailty phenotype, early-old community-dwelling population, cognitive function, depression, disability, comprehensive geriatric assessment
Introduction
Population aging is a global phenomenon. According to the Central Statistical Office of Poland (GUS), the proportion of persons aged 60 years and older is projected to grow to 29% of the population of Poland in 2030 and to exceed 40% by the year 2050. As a result, the incidence of old-age disability is increasing along with the demand for caregiving, nursing, medical and social services. Old-age disability is often multifactorial and includes physical, mental (psychological), sensorial, and social dimensions. Based on GUS data, it is estimated that the requirement for caregiver assistance with activities of daily living ranges from over 12% in sexagenarians to more than 50% in octogenarians. Demographic trends affecting family structure, which represents the primary elder care providers for seniors in Poland, and limitations in health and social care services indicate a risk for inability to meet the needs of elderly persons in the near future. Thus, strategies to prevent old-age disability should be undertaken to address inadequacies in elder care services.1,2
Frailty has been identified among major factors contributing to old-age disability.3,4 According to the Survey of Health, Aging and Retirement in Europe (SHARE), more than 50% of the European community-dwelling adults 50 or more years of age are prefrail or frail.5 Although a consensus regarding the definition of frailty has not yet been achieved,6,7 the frailty phenotype diagnostic criteria developed by Fried and colleagues are the most widely used criteria for population-based studies.6,8 These criteria consist of five components: unintentional weight loss, exhaustion, low physical activity, slow walking speed at usual pace, and low grip strength, with 1–2 positive criteria indicating pre-frailty, and 3 or more positive criteria indicating frailty.9 Based on the Cardiovascular Health Study, Fried et al not only provided a standardized diagnostic criteria for frailty in community-dwelling older adults but also demonstrated that disability is an outcome of frailty.9 Subsequent studies confirmed the predictive value of Fried frailty phenotype definition for adverse health outcomes in community-dwelling older adults in various populations.10–13
It is broadly accepted that frailty is a syndrome of age-associated decline in physiologic reserve and function across multiple organ systems, resulting in diminished strength and endurance.1,2,7–9 In addition, organ insufficiency may compromise frailty component assessment. Thus, frailty should not be considered in isolation from the general health status of patients. Frailty among older persons is a dynamic process, characterized by frequent transitions between frailty states over time.14 There is increasing evidence that frailty is potentially reversible, with physical activity being one of the most effective interventions.15
Few data on prevalence of frailty, as assessed by frailty phenotype criteria, are available in Poland. This study was designed to evaluate the prevalence of prefrailty and frailty in early-old community-dwelling inhabitants of southern Poland and to identify conditions that may potentially be important for the prevention and treatment of frailty.
Patients and Methods
Patients
The study group consisted of 160 subjects aged 66.8±4.2 years (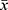 ± SD), 44.4% women. To achieve this number of participants, invitation letters were sent to 4963 persons randomized out of 843,278 community-dwelling 60–74 years old inhabitants of the Silesian Voivodeship. Data regarding relevant inhabitants of the Voivodeship were obtained from the Ministry of Digital Affairs of Poland. A response to the study invitation (sent by mail) was received from 163 invitees (a response rate of 3.28%) of whom 160 persons gave written consent for participation in the project (Figure 1).
± SD), 44.4% women. To achieve this number of participants, invitation letters were sent to 4963 persons randomized out of 843,278 community-dwelling 60–74 years old inhabitants of the Silesian Voivodeship. Data regarding relevant inhabitants of the Voivodeship were obtained from the Ministry of Digital Affairs of Poland. A response to the study invitation (sent by mail) was received from 163 invitees (a response rate of 3.28%) of whom 160 persons gave written consent for participation in the project (Figure 1).
 |
Figure 1 Recruitment of study participants. |
Measurements
A comprehensive geriatric assessment (CGA) was complemented with tests for frailty and body mass assessment. CGA included a structured interview, physical examination, functional assessment, electrocardiogram (ECG), and blood sampling.
A structured patient history was taken and included indicators of morbidity (such as pain, weakness, dyspnea, swelling, and weight loss as reported by the subject), specific signs of geriatric conditions (memory impairment, impairment of vision/glasses, impairment of hearing/hearing aid, instability, mobility disorders/assistive devices for walking, falls, incontinence), chronic disease (verified with subject’s medical records if available), pharmacological treatment, alcohol consumption, smoking, living conditions, and family or social service support.
Physical examination included general status, body build, mental status, speech, vision, hearing, gait, resting blood pressure of both arms (highest value was included in analysis), pulse, body mass, height, and waist and hip circumference.
Blood tests are specified in Table 1. Serum samples were frozen and collected for assessment of a range of cytokines and growth factors – results will be presented in a future paper after completion of analysis.
Charlson Comorbidity Index16 was used to assess comorbidity. Berlin Initiative Study (BIS) creatinine equation17 was used to estimate glomerular filtration rate (eGFR). Barthel Index of Activities of Daily Living (Barthel Index)18 and Instrumental Activities of Daily Living Scale (IADL)19 were used to determine functional independence. Mini-Mental State Examination (MMSE)20 was used to assess global cognitive performance. Geriatric Depression Scale – Short Form (GDS-SF) was used to screen for depression.21 Barthel Index scores range from 0 to 100, IADL – from 9 to 27, MMSE – from 0 to 30; higher scores indicate better functional status. GDS-SF scores range from 0 to 15 with higher scores indicating higher depression probability. Tinetti Performance-Oriented Mobility Assessment (POMA)22 and Timed Get-up and Go (TUG) test were used to evaluate fall risk.23,24 The 6-min Walk Test (6MWT) was used as an integrated measure of physical capacity and mobility and consisted of measuring the distance the subject traversed in 6 mins.25,26
Frailty was diagnosed according to Fried et al's criteria.9 Body-mass change was calculated from current weight measurement and the weight measured 12 months ago as recalled by the subject (data were verified with medical records if available). A Kern digital dynamometer was used for grip strength measurement. The subject was instructed to squeeze the dynamometer maximally three times with the dominant, resting in lap hand in the sitting position. An average grip strength value from three trials with the dominant hand was recorded. Exhaustion was assessed using two questions from the modified Center for Epidemiologic Studies Depression Scale (CES–D), as described by Fried et al.9 Usual pace walk time was assessed by instructing the subject to traverse a distance of 4.57 m at his/her usual speed, just as if he/she were walking down the street to go to the store and to pass the finish line without slowing down. The use of an assistive device for walking was accepted (but not the assistance of another person). The average of two trials was recorded. Usual pace walk time (s) was converted to usual pace walk speed (m/s). Low physical activity (weekly energy expenditure) was calculated on the basis of the modified Minnesota Leisure Time Activity Questionnaire.27,28 Polish language version of the Frailty Assessment Components: Standardized Protocols was used. We used reference values proposed by Fried et al for frailty criteria.9 MMSE, GDS-SF, TUG, 6MWT, and frailty assessment were also considered methods of functional assessment.
Body mass index (BMI) and waist-to-hip ratio (WHR) were calculated for all subjects. Body composition analysis with the use of bioimpedance method was performed in 149 subjects. Tanita BC-418MA Body Composition Analyzer was used to estimate total body fat percentage (the percentage of total body weight that is fat), total fat mass (total weight of fat mass in the body), fat-free mass (all fat-free mass of the body, including muscles, bones, other tissues, and water), and total water content (the amount of water retained in the body). Tanita Viscan Analyzer AB140 was used to estimate total abdominal fat (body fat percentage of trunk fat) and abdominal visceral fat (expressed as visceral fat rating from 1 to 35, with values higher than 13 indicating excessive level of visceral fat). Both devices have been previously validated and employed in clinical research.29–31
Subject examination was performed at the Department of Geriatrics of the Leszek Giec Upper-Silesian Medical Centre of the Silesian Medical University in Katowice on an outpatient basis. Complete home-based examination, performed by a research team which included a nurse, was offered to participants at a scheduled date who were unable to ambulate to our medical facilities. Only 3 subjects (1.9%) requested home examination.
Subjects were asked to come fasting for at least 8 hrs. A standard breakfast was served after patient interview, physical examination, and blood sampling. Functional assessment was performed one hour after breakfast. All subjects received the results of their blood tests for review by their primary care provider.
Statistical Analysis
Data were analyzed using STATISTICA version 13 (Stat Soft, Inc., Tulsa, OK, USA; Stat Soft Polska). The nonparametric Mann–Whitney U-test for quantitative variables, and chi-square test, V-square test, and Fisher’s exact test for categorical variables were used. The nonparametric Spearman’s rank correlation coefficient was used to assess relationships between frailty measures. Multivariate linear regression was used to assess measures associated with grip strength, usual pace walk speed, and physical activity. Multivariate logistic regression was performed to assess measures associated with positive frailty components. Analysis with backward elimination included variables that yielded P values of 0.1 or lower in the initial univariate analysis. Collinearity of independent variables was eliminated before odds ratios (OR) calculation. P values <0.05 were considered statistically significant.
Ethics
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Bioethical Committee of the Medical University of Silesia in Katowice, Poland (Letter KNW/0022/KB1/1/14).
Results
The study group was characterized by multi-morbidity (Charlson Comorbidity Index 3.38±1.50). The most common diseases were as follows: osteoarthritis, hypertension, coronary heart disease, diabetes, depression, heart failure, and osteoporosis. Mean number of oral medications was 3.79±3.16 and the number of all medical agents (including topical medications, supplements, and herbs) was 4.29±3.60. Functional status was fair (Barthel Index 98.1±8.2, MMSE score 29.0±1.5). Detailed data are presented in Table 1.
Prefrailty was diagnosed in 24.4% of the subjects (95% Confidence Interval (CI) = 17.7–31.0%; 31% in women and 19.1% in men, P=0.082) and frailty in 2.5% subjects (95% CI 0.1–4.9%; more frequently in women: 4.2% versus 1.1% in men, P=0.046). Slowness and low physical activity were the most common positive criteria for frailty. Grip strength and physical activity were lower in women, and prevalence of slowness criterion was higher in women (Table 2).
 |
Table 2 Mean and Median Values of Grip Strength, Usual Pace Walk Speed and Physical Activity ( |
Grip strength correlated negatively with female sex and osteoporosis, while positively with serum albumin concentration and MMSE score in the multivariate linear regression analysis adjusted for age, sex, disease prevalence, number of medications, functional tests (Barthel Index, IADL, MMSE, GDS-SF), height, body mass, BMI, WHR, bioimpedance body composition scores, and blood tests. Usual pace walk speed correlated negatively with total number of medications and positively with blood hemoglobin concentration, serum total protein level, and Barthel Index. Physical activity correlated positively with fat-free mass, MMSE score, and serum calcium concentration (Table 3).
 |
Table 3 Factors Associated with Quantitative Frailty Measures (Grip Strength, Usual Pace Walk Speed, and Physical Activity) in Multivariate Linear Regression Analysis |
No independent factors were associated with 12-month body-mass change. Unintentional weight loss was associated with depression, while exhaustion was negatively associated with IADL scores and positively associated GDS-SF scores in multivariate logistic analysis adjusted for age, sex, prevalent diseases, number of medications, functional tests (Barthel Index, IADL, MMSE, GDS-SF), BMI, bioimpedance body composition measures, and blood tests (Table 4).
 |
Table 4 Factors Associated with Positive Frailty Criteria (Unintentional Weight Loss and Exhaustion in Multivariate Logistic Regression Analysis) |
Compared to subjects who met no frailty criteria, subjects with one or more frailty criterion were of increased age, female sex, had a higher likelihood of osteoarthritis, depression, osteoporosis, lower fat-free body mass and total water content, lower MMSE scores and higher GDS-SF scores, lower Tinetti POMA scores and 6-min Walk Test results and lower serum creatinine concentrations (Table 1). Grip strength correlated positively with usual pace walk speed (Spearman’s rank correlation coefficient ρ=0.258; P=0.001) and physical activity (ρ=0.231; P=0.003). Usual pace walk speed was correlated with physical activity (ρ=0.156; P=0.048).
Having one or more positive frailty criteria were positively associated with depression (OR=2.85, 95% CI=1.08–7.54, P=0.035) and negatively associated with MMSE score (OR=0.72, 95% CI= 0.56–0.93, P=0.012) and fat-free mass (OR=0.96, 95% CI=0.92–0.99, P=0.016) in the multivariate logistic regression analysis adjusted for age, sex, disease prevalence, number of medications, functional tests (Barthel Index, IADL, MMSE, GDS-SF), BMI, bioimpedance body composition scores, and blood tests.
Discussion
This study was designed to estimate the prevalence of frailty among early-old adults. However, a low invitation response rate was achieved. Frailty prevalence based on data from this study, although significant, was lower than expected in comparison to other comparable studies, even when accounting for different diagnostic criteria. Manfredi et al estimated the prevalence of pre-frailty in Poland at 47.3% in adults aged 50–64 years and at 51.1% in adults aged 65–74 years, prevalence of frailty was estimated at 2.9% and 8.2%, respectively. Pre-frailty and frailty were defined in the Manfredi et al study using the SHARE operationalized version that is based on the five frailty dimensions described by Fried et al.5 Also, sex distribution (44.4% women) was reversed as compared to age-matched samples from the general population.32 Finally, the functional status of study participants was better than would be expected from other studies, such as the PolSenior Study.33 These results suggest sampling bias, despite randomization. It would seem that healthy-aging individuals were more inclined to participate in the study, despite no cost examinations being offered both in the hospital and home setting. Thus, this study’s patient population is most likely not representative of early-old community-dwelling inhabitants of the Silesian region.
Nonetheless, an analysis of associations between frailty components and demographic, clinical and functional factors on the basis of this sample seemed reasonable. Our study indicates that at least 25% of the early-old community-dwelling inhabitants of our region would benefit from a frailty prevention program. The study sample consists of well-matched groups of women and men with respect to age, BMI, total number of comorbidities and medications, cognitive function (MMSE scores), and functional status assessed by IADL scores.
Our finding of higher prevalence of frailty among women was also observed in other studies. Saum et al found an increased prevalence of frailty in community-dwelling women as compared to men aged 59+ in Germany using two different frailty phenotype diagnostic methods.10 In addition, women were more likely to report exhaustion and to have unintentional weight loss, lower mean grip strength and physical activity.10 Meta-analysis of data from five studies using the Frailty Index seems to confirm this pattern of sex differences throughout varied populations and older adult age strata. Simultaneously, mortality risk was higher for men at every level of frailty and age group.34 This phenomenon has been called a “male-female health-survival paradox”,34 as frailty is a risk factor for mortality.35 Sex-dependent differences in body build and structure, which were consistent with our data (Table 1), promote better results for frailty assessment components in men, especially with respect to grip strength. To compensate these differences and make frailty criteria universal for both sexes, Fried et al defined lower threshold values for women for three out of five frailty criteria (weakness, slowness, and low physical activity).9 Despite adjustments in frailty assessment components for sex, higher frailty prevalence is still observed in women in most studies. This discrepancy between increased frailty prevalence among women and higher mortality among men suggests that the frailty phenotype omits factors important for prediction of adverse health events. Studies have shown a decrease in the contribution of traditional cardiovascular risk factors on mortality with increasing age. Frailty was shown to be a strong risk factor for mortality in older-old subjects (aged 80+ years), while traditional cardiovascular risk factors were not associated with increased mortality in this age strata.36 However, male sex, smoking, high blood pressure, high glucose, and elevated creatinine levels, but not total cholesterol, LDL cholesterol, and HDL cholesterol levels, were still associated with increased 5-year mortality risk in the community-dwelling early-old adults (aged 65+ years).37 Independent predictors of 5-year mortality in this population included also relative poverty, low physical activity, indicators of frailty, and disability.37 Age, male sex, smoking, and type 2 diabetes mellitus were independent risk factors for mortality in persons aged 70–78 years, while total cholesterol, HDL-cholesterol, and systolic blood pressure were no longer associated with increased mortality in this age strata.38 Polypharmacy and patient apathy were identified as new relevant predictors of mortality.38 Our data suggest that male sex is associated with higher prevalence of ever-smoking, higher blood pressure and higher prevalence of diabetes. Regular alcohol consumption was also strongly associated with male sex, which consequently may result in findings of negative associations of alcohol consumption with frailty. Meta-analysis by Stockwell et al disputed a beneficial effect of moderate alcohol consumption on mortality risk.39 On the other hand, female sex in our sample was associated with higher prevalence of depression, osteoarthritis, and osteoporosis – factors which may compromise results of the assessment of frailty components, that are also associated with increased mortality.40–42
Our results indicate that in contrast to the frailty phenotype analyzed in older-old adults in other studies, the frailty phenotype in early-old adults does not encompass all significant risk factors for mortality and therefore may not be considered an index of global health. Thus, as other studies have also concluded,43,44 frailty phenotype assessment adds important information to the comprehensive geriatric assessment, but it may not substitute for the CGA as a method of global health assessment in early-old community-dwelling adults. Notably, frailty risk in our sample was associated with depression and lower cognitive function. Fried frailty phenotype is commonly identified with physical frailty.1,45 Our findings, along with other studies,46 indicate that the frailty phenotype is associated with both physical and psychological conditions. Other studies, using different methodological approaches, demonstrated that frailty is also related to socioeconomic factors.47,48 It is postulated that individually tailored interventions should be delivered to preserve an individual’s independence, physical function, and cognition.49
Our findings support the opinion that frailty prevention and treatment plans should include psychological and social support along with a comprehensive physical activity program.
Conclusion
At least 25% of the early-old community-dwelling population would benefit from a frailty prevention program. The frailty phenotype reflects both physical and mental health in this population.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dent E, Morley JE, Cruz-Jentoft AJ, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. 2019;23:771–787. doi:10.1007/s12603-019-1273-z
2. Lorenzo-López L, López-López R, Maseda A, Diego-Díez C, Gómez-Caamaño S, Millán-Calenti JC. Prevalence and clinical characteristics of prefrailty in elderly adults: differences according to degree of urbanization. J Am Geriatr Soc. 2016;64:221–223. doi:10.1111/jgs.13908
3. Liu HX, Ding G, Yu WJ, et al. Association between frailty and incident risk of disability in community-dwelling elder people: evidence from a meta-analysis. Public Health. 2019;175:90–100. doi:10.1016/j.puhe.2019.06.010
4. Vermeiren S, Vella-Azzopardi R, Beckwée D, et al.; Gerontopole Brussels Study group. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17:
5. Manfredi G, Midão L, Paúl C, Cena C, Duarte M, Costa E. Prevalence of frailty status among the European elderly population: findings from the survey of health, aging and retirement in Europe. Geriatr Gerontol Int. 2019;19:723–729. doi:10.1111/ggi.v19.8
6. Kojima G, Liljas AEM, Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23–30. doi:10.2147/RMHP.S168750
7. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi:10.1016/S0140-6736(19)31786-6
8. Rodríguez-Mañas L, Féart C, Mann G, et al.; FOD-CC group (Appendix 1). Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol a Biol Sci Med Sci. 2013;68:62–67. doi:10.1093/gerona/gls119
9. Fried LP, Tangen CM, Walston J, et al.; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci. 2001;56A:M146–M156. doi:10.1093/gerona/56.3.M146
10. Saum KU, Müller H, Stegmaier C, Hauer K, Raum E, Brenner H. Development and evaluation of a modification of the Fried frailty criteria using population-independent cutpoints. J Am Geriatr Soc. 2012;60(11):2110–2115.
11. Aguilar-Navarro S, Gutiérrez-Robledo LM, García-Lara JM, Payette H, Amieva H, Avila-Funes JA. The phenotype of frailty predicts disability and mortality among mexican community-dwelling elderly. J Frailty Aging. 2012;1:111–117. doi:10.14283/jfa.2012.18
12. Lahousse L, Maes B, Ziere G, et al. Adverse outcomes of frailty in the elderly: the Rotterdam study. Eur J Epidemiol. 2014;29:419–427. doi:10.1007/s10654-014-9924-1
13. Thompson MQ, Theou O, Tucker GR, Adams RJ, Visvanathan R. Recurrent measurement of frailty is important for mortality prediction: findings from the north west adelaide health study. J Am Geriatr Soc. 2019;67:2311–2317. doi:10.1111/jgs.16066
14. Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi:10.1001/archinte.166.4.418
15. Negm AM, Kennedy CC, Thabane L, et al. Management of frailty: a systematic review and network meta-analysis of randomized controlled trials. J Am Med Dir Assoc. 2019;20:1190–1198. doi:10.1016/j.jamda.2019.08.009
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi:10.1016/0021-9681(87)90171-8
17. Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157:471–481. doi:10.7326/0003-4819-157-7-201210020-00003
18. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:56–61.
19. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. doi:10.1093/geront/9.3_Part_1.179
20. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6
21. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. doi:10.1300/J018v05n01_09
22. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34:119–126. doi:10.1111/jgs.1986.34.issue-2
23. Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil. 1986;67:387–389.
24. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi:10.1111/j.1532-5415.1991.tb01616.x
25. Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837–841. doi:10.1016/S0003-9993(99)90236-8
26. Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: six-minute walk test, berg balance scale, timed up & go test, and gait speeds. Phys Ther. 2002;82:128–137. doi:10.1093/ptj/82.2.128
27. Taylor HL, Jacobs DR
28. Siscovick DS, Fried L, Mittelmark M, Rutan G, Bild D, O’Leary DH. Exercise intensity and subclinical cardiovascular disease in the elderly. The Cardiovascular Health Study. Am J Epidemiol. 1997;145:977–986. doi:10.1093/oxfordjournals.aje.a009066
29. Franssen FM, Rutten EP, Groenen MT, Vanfleteren LE, Wouters EF, Spruit MA. New reference values for body composition by bioelectrical impedance analysis in the general population: results from the UK Biobank. J Am Med Dir Assoc. 2014;15:
30. Mateo-Gallego R, Bea AM, Jarauta E, Perez-Ruiz MR, Civeira F. Age and sex influence the relationship between waist circumference and abdominal fat distribution measured by bioelectrical impedance. Nutr Res. 2012;32:466–469. doi:10.1016/j.nutres.2012.05.004
31. Zwierzchowska A, Głowacz M, Batko-Szwaczka A, et al. The body mass index and waist circumference as predictors of body composition in post CSCI wheelchair rugby players (Preliminary investigations). J Hum Kinet. 2014;43:191–198. doi:10.2478/hukin-2014-0105
32. Central Statistical Office of Poland. Available from: https://stat.gov.pl/obszary-tematyczne/ludnosc/ludnosc/ludnosc-stan-i-struktura-w-przekroju-terytorialnym-stan-w-dniu-30-06-2019,6,26.html.
33. Pac A, Tobiasz-Adamczyk B, Błędowski P, et al. Influence of sociodemographic, behavioral and other health-related factors on healthy ageing based on three operative definitions. J Nutr Health Aging. 2019;23:862–869. doi:10.1007/s12603-019-1243-5
34. Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40. doi:10.1016/j.exger.2016.12.021
35. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi:10.1016/j.jamda.2013.03.022
36. Vaes B, Depoortere D, Van Pottelbergh G, Matheï C, Neto J, Degryse J. Association between traditional cardiovascular risk factors and mortality in the oldest old: untangling the role of frailty. BMC Geriatr. 2017;17:234. doi:10.1186/s12877-017-0626-x
37. Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi:10.1001/jama.279.8.585
38. van Bussel EF, Richard E, Busschers WB, et al. A cardiovascular risk prediction model for older people: development and validation in a primary care population. J Clin Hypertens (Greenwich). 2019;21:1145–1152. doi:10.1111/jch.13617
39. Stockwell T, Zhao J, Panwar S, Roemer A, Naimi T, Chikritzhs T. Do “Moderate” drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and all-cause mortality. J Stud Alcohol Drugs. 2016;77:185–198. doi:10.15288/jsad.2016.77.185
40. Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. Br J Psychiatry. 2013;202:22–27. doi:10.1192/bjp.bp.112.112169
41. Dragomirescu I, Llorca J, Gómez-Acebo I, Dierssen-Sotos T. A join point regression analysis of trends in mortality due to osteoporosis in Spain. Sci Rep. 2019;9:4264. doi:10.1038/s41598-019-40806-0
42. Veronese N, Cereda E, Maggi S, et al. Osteoarthritis and mortality: a prospective cohort study and systematic review with meta-analysis. Semin Arthritis Rheum. 2016;46:160–167. doi:10.1016/j.semarthrit.2016.04.002
43. Chainani V, Shaharyar S, Dave K, et al. Objective measures of the frailty syndrome (hand grip strength and gait speed) and cardiovascular mortality: a systematic review. Int J Cardiol. 2016;215:487–493. doi:10.1016/j.ijcard.2016.04.068
44. Leong DP, Teo KK, Rangarajan S, et al. Prospective urban rural epidemiology (PURE) study investigators. Prognostic value of grip strength: findings from the prospective urban rural epidemiology (PURE) study. Lancet. 2015;386:266–273. doi:10.1016/S0140-6736(14)62000-6
45. Verlaan S, Ligthart-Melis GC, Wijers SLJ, Cederholm T, Maier AB, de van der Schueren MAE. High prevalence of physical frailty among community-dwelling malnourished older adults-a systematic review and meta-analysis. J Am Med Dir Assoc. 2017;18:374–382. doi:10.1016/j.jamda.2016.12.074
46. Vaughan L, Corbin AL, Goveas JS. Depression and frailty in later life: a systematic review. Clin Interv Aging. 2015;10:1947–1958. doi:10.2147/CIA.S69632
47. Poli S, Cella A, Puntoni M, et al. Frailty is associated with socioeconomic and lifestyle factors in community-dwelling older subjects. Aging Clin Exp Res. 2017;29:721–728. doi:10.1007/s40520-016-0623-5
48. van der Linden BWA, Sieber S, Cheval B, et al. Life-course circumstances and frailty in old age within different European welfare regimes: a longitudinal study with SHARE. J Gerontol B Psychol Sci Soc Sci. 2019. doi:10.1093/geronb/gbz140
49. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376–1386. doi:10.1016/S0140-6736(19)31785-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.