Back to Archived Journals » Research and Reports in Forensic Medical Science » Volume 12
Forensic Odor Analysis: Current Application in Postmortem Examinations
Authors Titus KC , Gallegos SF, Prada-Tiedemann PA
Received 14 December 2021
Accepted for publication 12 April 2022
Published 19 April 2022 Volume 2022:12 Pages 1—12
DOI https://doi.org/10.2147/RRFMS.S272225
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Henrik Druid
Katherine C Titus, Shawna F Gallegos, Paola A Prada-Tiedemann
Forensic Analytical Chemistry and Odor Profiling Laboratory, Department of Environmental Toxicology, Texas Tech University, Lubbock, TX, 79416, USA
Correspondence: Paola A Prada-Tiedemann, Forensic Analytical Chemistry and Odor Profiling Laboratory, Department of Environmental Toxicology, Texas Tech University, 1207 S. Gilbert Drive, Lubbock, TX, 79416, USA, Email [email protected]
Abstract: Postmortem examinations are crucial in understanding the events surrounding death in medico-legal applications. While traditional autopsy procedures and visual examinations represent the routine processing scheme, a novel tool that has emerged in forensic applications is odor analysis. With respect to postmortem examination applications, the use of odor analysis has catapulted into understanding volatile odor signatures indicative of distinctive human decomposition stages. In turn, this quest for the decomposition odor signature has shed light into understanding the chemical characterization of target odor volatiles emitted by a range of sources available in the postmortem window. This review introduces the use of odor evidence in general forensic practices and provides a brief synopsis of the principles of odor and its subsequent laboratory analysis. A short description of the decomposition process is provided, followed by a review of recent trends in analytical studies that have embarked on using volatile odor profiles to enhance various postmortem examination perspectives.
Keywords: postmortem examinations, odor analysis, volatile organic compounds, VOCs, decomposition
Introduction
When an individual dies due to unexpected, unnatural, or suspicious circumstances an autopsy, or post-mortem examination, is commonly performed to determine the cause and manner of death. Besides cause and manner of death, a key aspect of postmortem examinations entails the estimation of the time since death, commonly known as the postmortem interval (PMI). Research has attempted to develop an accurate estimation process; however, environmental conditions, victim size, containment, presence/absence of clothing are some of the many variables that can impact the rate of decomposition and thus make PMI estimation a challenging process. Forensic practitioners rely on early postmortem changes that include the classic “signs of death”: livor mortis, rigor mortis, and algor mortis to somewhat estimate a predictable timeline pattern. Another common tool available is forensic entomology. Forensic entomologists evaluate the insects (and larva forms) present on the body to make an estimate of the time of death by studying insect populations and subsequent larval stages/development.
In the last decade, research has provided yet another tool in the practitioner’s toolbox for postmortem examinations: odor analysis. Odor analysis has many uses in forensic science applications including toxicology, drug identification and profiling, explosive and ignitable liquid residue investigations, and human odor (both live and deceased).1,2 With respect to postmortem examinations applications, the use of odor analysis has been explored for its utility in understanding volatile odor signatures indicative of distinctive human decomposition stages.3 In turn, this quest for the decomposition odor signature has shed light into understanding the chemical characterization of target odor volatiles emitted by a range of sources available in the postmortem window such as the decomposing body itself, biological fluids/tissues, internal cadaveric region, microbes, entomological specimens, and soil. Figure 1 depicts sources exploited for odor analysis that will be discussed in this review. Odor analysis of these specimens have assisted in understanding postmortem changes from the viewpoint of a “volatile chemical” perspective that not only provides a chemical pathway to understanding postmortem/decomposition processes but also contributes for detection technology development as is seen with the use of cadaver dogs for victim identification.4,5 This paper highlights the existing applications of odor analysis in general forensic practices, briefly exploring odor sources and analysis techniques. We then provide a succinct synopsis of the human decomposition process as it is key to understanding odor sampling techniques with respect to postmortem applications. A review of recent trends in sample specimens is then highlighted to represent studies that have embarked on using volatile odor profiles to enhance various postmortem analysis perspectives.
 |
Figure 1 Postmortem odor – common matrices for analysis (Graphic design by Brad Thomas). |
What is Odor Analysis and Why is It Useful to Forensic Science?
Forensic evidence is key in the investigation of crime scenes as its recovery and analysis in the laboratory can provide associative links between the crime scene, victim, and perpetrator. Therefore, it is crucial for forensic scientists to recognize the different types of evidentiary items that may be available for collection and subsequent analysis. With the rapid advancement of detection technologies, one area that has seen tremendous growth is odor evidence applications. This area of study has focused on targeting the chemical characterization of volatile organic compounds (VOCs) from different forensic specimens of interest. VOCs are low-molecular-weight organic compounds that transfer from the liquid to gaseous phase at standard room conditions, which can be further categorized by their volatility and their emission source (human/living organism, substance, environment, etc).6 The characteristic for a chemical to be identified as volatile is related to its saturated vapor pressure which is the amount of chemical in the air above its liquid/solid phase, or the “tendency” of the chemical particles to escape from the liquid/solid phase.7 The scent or odor profile of a substance can be characteristic of a particular specimen, thus making it a very powerful investigation tool for identification and discrimination purposes.
How is Odor Evidence Sampled?
The collection, extraction, and subsequent analysis of odor is extremely important when discussing this type of sample in the laboratory. The range of analytical techniques available makes the characterization of VOCs challenging due to a lack of agreement across different studies. With respect to postmortem examinations, this can be attributed to differences in type of samples collected (whole body vs tissue), species (human vs animal), decomposition stage, location, environment, and analytical techniques employed. A thorough review of analytical techniques specifically in forensic decomposition studies has been provided by Iqbal et al.8 Both static headspace (SH) and dynamic headspace (DH) techniques are routinely used for the direct analysis of volatiles from target specimens with limited disturbance or alteration of the sample.9 Once collected, trapped volatiles can be recovered from these headspace techniques for analysis via thermal desorption, solvent extraction, or collection onto sorbent traps like Tenax employing a dynamic airflow process. In recent years, odor evidence has exploited solid-phase microextraction (SPME).1 SPME is an extraction technique that involves the adsorption or absorption of target analytes onto a silica/metallic fiber support. This extraction procedure is then followed by a thermal desorption of the collected analytes in a suitable instrument.10 Instrumentation for their analysis is commonly conducted with gas chromatography coupled with mass spectrometry (GC-MS), the gold standard detection system for VOC odor profile analysis due to its large simultaneous compound capacity coupled with built-in identification software, commonly known as mass spectral libraries. GC is also advantageous as the odor components released from targeted forensic samples are mostly VOCs, which have a high vapor pressure and thus are ideal for GC separation. An upcoming new analytical trend has been observed with the introduction of multidimensional chromatographic systems such as two-dimensional gas chromatography. The separation power of GC×GC relies on the in-series combination of two capillary columns characterized by different stationary phases, by means of a transfer device called a modulator. The first column is usually the traditional GC column length while the second column is a shorter segment (~1–5m) to yield a fast separation. This double column framework enables a chromatographic separation that has increased sensitivity, selectivity, separation power, and speed.4
While analytical technology has advanced greatly in terms of low detection limits, instruments fall short when compared to biological detection, namely canine detection. The canine’s nose has a high discriminating and advantageous power as seen with its capability of being trained on multiple odors rather quickly with high degrees of reliability, regardless of environments or complex chemical backgrounds.2 In terms of postmortem examinations, canines are of tremendous assistance as is seen with human remains detection (HRD) dogs.
Human remains detection dogs are specially trained to recognize the scent of human decomposition and communicate to their handler the location of that scent.5,11 These canines are trained from an early age on a range of human decomposition material to include human blood, tissues, adipocere, body fluids, wet/dry bone and burned human remains.12 HRD dogs are a robust non-invasive resource for law enforcement in many different types of investigations including locating victims of homicides, missing persons, victims of mass disasters, and concealed remains as seen with clandestine graves.13 Due to their powerful olfactory systems and large amounts of receptor cells they can detect a wide variety of compounds making canines capable of alerting on both whole cadavers as well as smaller amounts of remains such as body parts or even body fluids. Canines are also able to distinguish between animal remains and human remains in varying stages of decomposition. This includes fresh remains that are emitting few decomposition VOCs up to skeletal remains that have been left outside for decades.11 The high-profile case of Casey Anthony highlights the challenge of the chemical characterization of the “odor of death” owing to a lack of method error rates and multiple factors affecting the decomposition odor profile. In this case, human remains detection dogs alerted the handler to the presence of human remains in the passenger and trunk areas of Casey Anthony’s vehicle, presumably her daughter’s remains. A sample of the carpet inside the trunk of the vehicle was taken and analyzed by Dr Arpad Vass and colleagues at the Oak Ridge National Laboratory. Odor evidence in this controversial case revolved around the identification of five key compounds: carbon tetrachloride, chloroform, dimethyl disulfide, dimethyl trisulfide and carbon disulfide.14 In turn, the defense’s argument emphasized that these five key compounds could not be attributed solely to human decomposition as these chemicals can also be present in household chemicals and solid waste or composting environments. Hence, this high-profile murder trial brought to light the conflicting chemical characterization of the odor profile of human decomposition and the existing research gap to fine-tune a reliable scientific definition of the odor of human death.
Brief Synopsis of the Decomposition Process
To understand the odor profile of decomposition and its utility in postmortem examinations, it is important to overcome the dynamic process of decay. Decomposition is commonly discussed by way of the predictable physical and chemical processes that occur after death. The total number of stages in the decomposition timeline in existing literature varies due to researcher preference regarding how decomposition stages should be grouped or labeled and is summarized in Figure 2.15–19
 |
Figure 2 Stages of Decomposition.15–19 |
Post-Mortem Examinations from an “Odor” Viewpoint
There is an abundance of applications for the use of odor profiling as it relates to postmortem examination perspectives including the odor analysis of decomposing tissue and/or whole body, internal cadaveric gas monitoring, soil, entomological specimens, microorganisms, as well as optimization of canine detection applications. One of the main challenges when evaluating the scent of death’s volatilome is the influence of both intrinsic and extrinsic variables, which affect the decomposition rate. These variables include factors such as victim size, wounds, drug use, geographic region, environmental conditions, containment, presence/absence of clothing, scavenging, insect activity, deposition ecosystems, etc, all of which have the potential to affect decay rates and subsequently the generated odor profile (Figure 3).
 |
Figure 3 Variables affecting decomposition rate. |
Human cadavers are the ideal sample specimen when studying decomposition VOCs. However, ethical and legal restrictions make whole human cadavers difficult to obtain. Therefore, limited studies are available in the literature that have been able to provide a complete odor profile from the whole human cadaver.20–25 Most studies are conducted with human tissue samples or with human analogue models using either a whole carcass, or tissue samples collected at specific timepoints from the carcass. A novel shift that has helped with this limitation in forensic taphonomy research has been the establishment of decomposition facilities, or “body farms” across the globe. The first facility was established in 1980 by Dr William Bass at the University of Tennessee. Since then, another seven have opened in the US (See Figure 4), 1 in Australia and one in Holland. Within the facilities in the US alone, the geographic variation allows researchers to study decomposition variables in distinctive regions and embark on more realistic experimental designs to formulate theories.26 Studies regarding decomposition VOCs have been extensively reviewed comparing both laboratory and field environments, instrumental analysis techniques, and various sampling sources.8,18,19,27 Laboratory environments offer the unique perspective of being able to study odor without the influence of compounds from the environment in which the specimen is placed. However, field studies help to account for the effect that extrinsic variables inflict on the odor profile and are more representative of what forensic practitioners will encounter in casework applications. Both are equally useful, and a substantial research base exists utilizing different sample types to serve various post-mortem goals, which are explored further below categorized by sampling odor source.
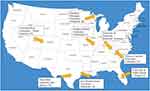 |
Figure 4 Decomposition research facilities in the United States. |
Human Cadavers
With respect to postmortem examination uses, understanding the volatile chemical profile of the cadaver is fundamental within the scope of odor analysis. The first study of decomposition VOCs was reported by Vass et al.28 The study’s aims were to identify the odor signature emanating from fresh buried human bodies to establish a “decompositional odor analysis database” as a first step in enhanced detection technology for shallow graves. Over 400 VOCs were reported across eight major chemical classes serving as a gateway experimental design for investigating the odor analysis of human remains. Statheropoulous et al followed this quest a year later, analyzing the VOCs emitted from two cadavers that had been deceased for approximately 3–4 weeks, thus accounting for an advanced stage of decomposition.20 The VOCs were analyzed utilizing thermal desorption/gas chromatography/mass spectrometry (TD-GC-MS) and more than 80 compounds were detected, with those in highest concentrations being dimethyl disulfide, toluene, hexane, benzene, 1,2,4-trimethyl, 2-propanone and 3-pentanone. In 2007, Statheropoulous et al published a similar study in which a human cadaver in the early stage of decomposition, with a PMI estimation of approximately 3 days was sampled for decomposition VOCs. The same sampling and analysis methods were utilized as the earlier experiment, with the exception in the latter where sampling took place at 0, 4, 8, and 24 hours. Analysis of VOCs revealed the largest number of compounds in the 24-hour sampling period, with exclusive compounds reported only in this sampling period. These results reflect how the decomposition odor profile, like the process of decomposition, increases in complexity over time. There were over 30 compounds detected in the experiment with all sampling periods containing the compounds ethanol, 2-propanone, dimethyl disulfide, methyl benzene, octane, 2-butanone, methyl ethyl disulfide, dimethyl trisulfide, and o-, m- and p-xylenes.21
DeGreeff et al represent the largest sample size of human cadavers studied for decomposition VOCs to date, utilizing 27 cadavers at a morgue and crematorium. This experiment was conducted with multiple research goals, two of which were to determine shared decomposition volatiles from the 27 cadavers and to compare the odor profile of living humans, deceased human remains, and animal remains. Compounds were sampled utilizing a Scent Transfer Unit (STU-100), of which the collection medium was sampled using SPME fibers, and analyzed using GC-MS. The researchers found shared odor compounds between living and deceased human samples, which is to be expected, however the overall odor signature of the samples differed substantially between the two. Differences in the complete odor profile of deceased animal and deceased human remains were also distinct. From the two cadaver locations (crematorium/morgue) used in this experiment, there were 12 shared compounds including heptadecane, octanoic acid, phenol, pentadecane, decanal, benzoic acid-methyl ester, 2-2-methoxyethoxy ethanol, benzaldehyde 2-ethyl-1-hexanol, tridecane, 1,2,3, trimethyl benzene, and styrene.22
Vass et al conducted a field study to determine the odor profile of decomposing buried remains to strengthen training aids for human remains detection dogs when locating clandestine graves.23 In this study, four human cadavers were buried at depths ranging from 1.5 to 3.5 ft and sampled over a 4-year period. This study followed the initial experimental design from the seminal experiment conducted in 2004 and utilized sorbent traps for sampling purposes from below and above the cadavers as well as at the soil surface of the graves. Analysis revealed 478 detected compounds, of which 30 were determined to be “key markers” of human decomposition.
With advances in analytical detection capabilities, Stefanuto et al analyzed the VOCs from both human cadavers and pig carcasses utilizing two-dimensional gas chromatography with time-of-flight mass spectrometry (GCxGC-TOFMS) for the first time.29 This study targeted the first 6 days of decomposition after deposition on field site. The cadavers had been deceased between 3 and 9 days prior to deposition. Both human and pig carcasses exhibited similar trends during the early postmortem period of tissue decomposition. More recently, Ueland et al conducted a study with six human donors over a 1-month period in a simulated mass disaster scenario.30 VOC analysis via GCxGC-TOF-MS allowed for non-destructive tracking of the decomposition process. Their results indicate the location of the body in the rubble environment impacted the decomposition process as well as the change in odor profile throughout the sample period.
Exploring additional analytical techniques, Clases et al utilized GC-ICP-MS as a novel technique to investigate VOCs from a decomposing body. Elements such as phosphorus, sulfur, and chlorine were investigated as forensically relevant elements, and depicted time-dependent concentrations, which could be developed as useful forensic biomarkers of the postmortem interval clock.24 Another crucial factor in the decomposition timeline is temperature. A recent study by Deo et al targeted seasonal variations as the focal point using five human cadavers. Although all seasonal trials highlighted variation, the identification of key VOCs could impact enhanced canine detection year round, regardless of climate effects.25
These studies provide a data baseline for what can be expected in forensic casework when human cadavers are exposed to different intrinsic and extrinsic variables, therefore, providing a foundational description of the most suitable and relative concentrations of volatiles that can be utilized for detection technology development.
Human Tissue Studies
Besides understanding the odor “snapshot” from a full body perspective, studies have also evaluated different human tissue parts to add to the knowledge of how postmortem processes affect different body parts. Hoffman et al conducted an experiment to look at the odor profile of 14 pieces of decomposing human tissue including a blood clot from a placenta, blood, muscle, a testicle, skin, body fat attached to skin, two samples of adipocere, adipose or fat tissue, vertebra bone, two samples of unspecified bone, and teeth. The study highlights the issue that while canines are generally regarded in literature to be successful detectors of remains, they are trained on many different types of tissues, and little is known about what specific odorants create a detection response. All samples were exposed to SPME fibers for 20 minutes and then analyzed using GC-MS. The researchers were unable to find a compound that was shared between all decomposing tissue; however, p-xylene was found to be in 13 of the 15 samples, and over half of the compounds from the sample were found to be present in high (67–100%) and medium frequency ranges (33–66%) across all the samples. The results of this research suggest that decomposition odors are similar across all regions of the body but combining tissue types would likely be more beneficial when training human remains detection dogs.31 In 2015, Rosier et al compared the VOC emission of six decomposing human tissues/organs from six different bodies with pig remains. Organs and tissues used for the study included blood, brain, fat, heart, intestines, kidney, liver, lung, muscle, pancreas, spleen, and stomach. The study sampled these laboratory-controlled decomposed samples for a period of up to 6 months utilizing TD-GC-MS. The study was able to separate human and pig odor volatiles based on five esters: 3-methylbutyl pentanoate, 3-methylbutyl 3-methylbutyrate, 3-methylbutyl 2-methylbutyrate, butyl pentanoate and propyl hexanoate.32
Internal Cadaveric Region
Another area that has seen the uses of odor analysis in postmortem examinations is that of locating gas reservoirs within the cadaver. Typically, this has been done with computed tomography (PMCT). In 2016, Stefanuto et al utilized two-dimensional gas chromatography coupled to time-of-flight high-resolution mass spectrometry (GCxGC-HRTOF-MS) for VOC analysis.33 Four human cadavers were used in this study where the location of each intra-cadaveric gaseous region was first identified with PMCT and consequently sampled with a syringe to remove gaseous matrix for analysis. Various locations of the body were sampled for each cadaver, with results depicting that the chemical composition of VOCs within the gas reservoirs differed between locations within a single body and across individuals. This application needs more research but opens the door to a novel approach to cadaver screening in routine medico-legal investigations.
Human Analogue Studies
As mentioned previously, obtaining human cadavers or body tissues is a challenge within decomposition-focused research. To circumvent this limitation in odor analysis studies, human analogues are routinely used with the most accepted being the domestic pig (Sus scrofa domesticus L.) due to similarities in internal anatomy, fat distribution, hair density, and gut fauna.34 Studies generally involve the use of the whole carcass, however research has explored the capability to obtain decomposition VOCs from other by-products of pig decay such as fluids.35,36 Additionally, other research points to chicken remains as a non-suitable analogue for studying decomposition VOCs.37 Due to these differences and that it is easier to obtain human analogues for research purposes, multiple studies have been conducted to expand on the knowledge regarding the odor of death. While various analogs are used to model human decomposition processes, it should be noted that recent studies have found this may not be the case further indicating the need for expanded research studies to verify the transferability of animal proxy results with human cadavers.38 This “transferability” with respect to animal modeling lies in the need for standardization of experimental parameters when comparing across species and studies. These include variables such as number of carcasses, distances between carcasses, clothing/wrapping, caging, to name a few. These crucial variables can set the platform for species comparisons going forward and enhance the value of the results obtained for practical trend extrapolation and analysis.39
Due to the postmortem interval being one of the most important pieces of evidence to establish, research has been conducted to explore the ability to determine the decomposition stage as well as PMI based on detected compounds. Armstrong et al determined that in the first 72 hours following death, patterns can be seen between time elapsed postmortem and predominant compounds. In the first 23 hours after death the most abundant compounds were esters and ketone containing compounds. Sulfur containing compounds were most prominent in the 43–49-hour period, many of which continued into the 69–75-hour period where carboxylic acids were also detected.40 Paczkowski et al explored the ability to correlate compounds and PMI to determine what volatiles are considered core volatiles to the decomposition process. It was found that aldehydes were mostly present in the fresh stage and sulfur compounds in the bloat stage. These compounds decrease throughout active and advanced decay stages and in turn are replaced with an increase in butan-1-ol and phenol. It was observed that all of the identified compounds occurred in varying overlap between decomposition stages. Seven compounds were determined to be core volatiles of the bloated, active, and advanced decay decomposition stages, which included dimethyl-disulfide, dimethyl-trisulfide, heptanal, phenol, undecane, acetophenone, and butan-1-ol.41 Similarly, other studies have attempted to identify potential target odors of decomposition and their repeatability across multiple years.42 One of the most frequently seen compounds in decomposition studies are polysulfides including dimethyl sulfide and dimethyl trisulfide.41,42
It has been established that multiple variables have the capability to influence the decomposition process, most notably is temperature which can vary greatly by location and subsequent climate.15 As a result of odor studies being performed in various locations around the world including Europe17,20,21,41,43,44 Canada,42 Australia25,40,45–50 and the US,22,23,31,37 researchers can begin to piece together how the postmortem odor profile may change due to environmental variables introduced by location. However, few studies have been conducted to directly study these variables, especially temperature. Forbes et al bridge this research gap by conducting an experiment to determine how decomposition VOCs are affected by season by comparing the odor profiles of decaying pig carcasses in both summer and winter, with 256 and 116 compounds detected, respectively. This was attributed by the researchers to be caused by the faster decomposition rate and increased insect activity typical of warmer months.45 In support of previously mentioned decomposition studies, sulfur compounds were predominantly detected in the summer trial, specifically dimethyl disulfide and dimethyl trisulfide. The largest abundance of compounds, regardless of season, was found in the onset of the active decay stage41,42,45 suggesting this stage has the strongest odor signature. Additional studies have revealed how ecosystems located in the same area can influence the odor profile. Dekeirsschieter et al conducted a study to analyze decomposition VOCs in three different ecosystems: a forest site, an agricultural site, and an urban site in Belgium. A total of 57 VOCs were identified in the urban site, 85 in the forest site, and 90 in the agricultural site with all specimens decomposing differently in each targeted ecosystem. The researchers attributed the differences in odor profile to climate, environmental microorganisms, and entomological species respective of these climates.17
Along with natural environments and given the current world climate, mass disaster environments such as building collapse should also be considered. Agapiou et al conducted a study looking at the VOCs of pig carcasses that had been simulated to mimic deceased victims trapped under collapsed buildings. The study utilized three carcasses placed in various scenarios at a search and rescue operational field terrain. One of the pigs was placed in a concrete tunnel in an open body bag, another in a closed body bag, and one in a concrete tunnel buried under soil. Decomposition VOCs varied substantially between the three-sampling subjects which the researcher attributed to the differences in entrapment the specimens were subjected to. Moreover, that decomposition VOCs likely differed due to diffusion of compounds into the soil or tunnel air, respective of the method of entrapment, and that these surfaces or substances interact with the odor profile.44 Ueland et al conducted a simulated trapped building collapse scenario as part of a law enforcement victim recovery exercise using human cadavers, adding to the body of knowledge and the forensic “realism” of such occurrence. The study depicted how the decomposition timeline affected the bouquet of volatiles detected thus aiding canine detections in better training regimens based on these observed odor fluctuations.30 Due to bodies being discovered in many ways, decomposition odor analysis would benefit greatly from additional studies to evaluate how the odor profile is influenced by various forms of concealment and entrapment.
Microbes
A new approach in postmortem investigations that has captured the attention of various research groups is exploiting microbial communities to estimate PMI. While the genomic content has been the primary focal point in this area to demonstrate shifts in microbial community composition during the terrestrial decomposition process, odor analysis has also been added as a tool in this postmortem application.51 Three postmortem bacterial isolates (Bacillus subtilis, Ignatzschineria indica, I. ureiclastica) were sampled via headspace SPME arrow extraction under controlled laboratory conditions. Results of this foundational study highlighted different VOC profiles for each species that encompassed common decomposition odor volatiles.52 This unique link of microorganism contribution to the chemical odor profile can improve the prediction of VOC trends in the decomposition timeline and enhance postmortem examinations.
Soil
Limited studies have been conducted to understand the relationship between soil and decomposition VOCs. Characteristics and composition of soil such as temperature, moisture level, humidity, etc, all have the potential to affect the way bodies decompose. Vice versa, when bodies decompose on the surface of soil, they can influence the underlying soil by producing changes in pH, microbial activity, and microorganisms, all of which are capable of depositing VOCs characteristic of the decomposition process into the soil. The relationship between bodies and the soil they are placed in has the potential to impact the decomposition odor profile which has resulted in studies being conducted to understand these interrelationships in greater depth. Brasseur et al analyzed postmortem VOCs emitted into soil by studying buried decaying pig carcasses over a 6-month period. VOCs were sampled using absorbent filters and analyzed using GCxGC-TOFMS. Twenty compounds were found exclusively in the soil samples that were obtained from directly below the carcass and 34 methyl-branched alkanes were found in all soil samples at varying depths.43 Similar studies have been conducted to understand the interactions between decomposing tissue and soil, including longitudinal studies of how decomposition VOCs change over time, how they vary by season, contribute to the odor profile, and what VOCs are deposited even after remains have been removed.47,49,50,53 Studies have also been conducted to optimize sampling and analysis techniques including comparison of commonly used sampling materials in decomposition odor studies, such as sorbent tubes and solid-phase microextraction, as well as finding ways to improve the elimination of background VOCs present in soil leading to enhanced odor profile resolutions.48,50 These studies help to understand decomposition from the perspective of the deposition site, depicting nutrient fluxes into the soil matrix which could yield information in terms of residual odor implications (ie, how long ago has a body been removed) or simply how similar is the soil odor profile to the decomposing tissue odor profile and the potential of using soil as a possible training aid when no human remains are available.
Entomological Specimens
The use of entomological species in forensic science has many applications including assisting in time since death estimations based on insect succession patterns and species identification.54,55 Insects arrive at decomposing bodies in a predictable fashion, usually in patterns associated with decomposition stages, making them a useful tool for forensic practitioners in calculating PMI. A substantial amount of research has been conducted analyzing the hydrocarbon composition of puparia from necrophagous flies to date the puparia and subsequently estimate PMI based on hydrocarbon composition patterns.56–60 Studies analyzing the hydrocarbon composition of puparia have also been conducted with the research goal of assisting in taxonomic differentiation and identification of fly species.59,60 The chemical composition of fatty acids derived from puparia has also been explored for PMI estimation application as well as the hydrocarbon composition and fatty acids from blowfly eggs for species identification.57,61
While most studies conducted to date involve rearing entomological specimens in laboratory environments with meat samples, Blanar et al sampled blowfly maggots from decomposing human analogues in a field environment. Previous studies focused on specific functional groups; however, this study explored whether larval mass samples collected along the decomposition timeline could yield odor signatures indicative of the distinctive decomposition stage it was collected in. The study compared both entomological and tissue samples taken from human analogues and determined that the putrefactive processes inherent to decomposition are capable of transmitting decomposition VOCs to the larval masses.54 This study showcases the potential of a new matrix that can be collected from decomposing carcasses to contribute to postmortem processing as it relates to understanding important odor biomarkers characteristic of specific decomposition stages.
Human Remains Detection (HRD) Dogs
With respect to detection technology capabilities available for the recovery of human remains, canines are a practical and widely used tool across law enforcement agencies worldwide. In cases of missing victims, suspected homicides or for natural/man-made disasters, canines can reliably locate cadavers in various operational scenarios. Odor analysis in the area of postmortem investigation has revolved around the chemical characterization of available synthetic training aids used for animal training,62,63 textile evaluation for novel training aids,64 effect of soil characteristics on canine response65 and evaluation of soil residual decomposition odor traces for canine detection.66 Understanding the odor composition of training materials as well as the odors recognized by HRD dogs when making the positive alerts ultimately improves the training quality and thereby enhances the performance and efficacy of this biological detection tool essential in locating a body.
Conclusions
This review discusses the use of odor analysis in various forensic applications within postmortem examinations. Current research has begun to account for the many influences on decomposition VOCs of tissues including decomposition stage, climatic variables, and varying scene scenarios. Odor analysis has explored the potential for other matrices of study and how in turn these matrices can provide knowledge in the chemical characterization of volatile biomarkers that can provide indication of putrefactive state as well as provide scientific foundations for both instrumental and canine detection developments. While a comprehensive knowledge of the odor of death is far from being complete, foundational understanding on the odor signatures from cadavers, human analogues, insects, microorganisms, and soil has led to innovative information that offers potential for more accurate PMI determination from not only the cadaver itself but also from variety of sources that are available on scene. Additionally, analytical results and trends from these studies can be directly applied to detection tools such as human remains detection dog teams. The research studies presented highlight how the dynamic decomposition process yields volatile odor compounds of varying chemical classes from a wide range of matrices. Priorities for further research include incorporating larger sampling sizes, extending collection timelines, comparing instrumental approaches, expanding, and comparing geographical locations, to name a few. The high variation in the results obtained showcases the vast number of variables in this area of study area and highlights the importance for forensic practitioners to understand the limitations and capabilities associated with odor analysis. Postmortem examinations are crucial in any criminal investigation, and odor analysis has proven to be a viable form of evidence. However, only with further research can this new tool progress and fully develop.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Prada A, Furton KG. Recent Advances in Solid-Phase Microextraction for Forensic Applications. In: Comprehensive Sampling and Sample Preparation. Elsevier; 2012:877–891. doi:10.1016/B978-0-12-381373-2.00117-4
2. Furton KG, Caraballo NI, Cerreta MM, Holness HK. Advances in the use of odour as forensic evidence through optimizing and standardizing instruments and canines. Phil Trans R Soc B. 2015;370(1674):20140262. doi:10.1098/rstb.2014.0262
3. Ioan BG, Manea C, Hanganu B, Statescu L, Gheuca Solovastru L, Manoilescu I. The chemistry decomposition in human corpses. Rev Chim. 2017;68(6):1352–1356. doi:10.37358/RC.17.6.5672
4. Zanella D, Focant J, Franchina FA. 30th Anniversary of comprehensive two‐dimensional gas chromatography: latest advances. Analytical Sci Adv. 2021;2(3–4):213–224. doi:10.1002/ansa.202000142
5. Komar D. The use of cadaver dogs in locating scattered, scavenged human remains: preliminary field test results. J Forensic Sci. 1999;44(2):14474J. doi:10.1520/JFS14474J
6. Giannoukos S, Agapiou A, Brkić B, Taylor S. Volatolomics: a broad area of experimentation. J Chromatography B. 2019;1105:136–147. doi:10.1016/j.jchromb.2018.12.015
7. Goss KU. The physical chemistry of odors — consequences for the work with detection dogs. Forensic Sci Int. 2019;296:110–114. doi:10.1016/j.forsciint.2019.01.023
8. Iqbal MA, Nizio KD, Ueland M, Forbes SL. Forensic decomposition odour profiling: a review of experimental designs and analytical techniques. TrAC Trends Analytical Chem. 2017;91:112–124. doi:10.1016/j.trac.2017.04.009
9. Biniecka M, Caroli S. Analytical methods for the quantification of volatile aromatic compounds. TrAC Trends Analytical Chem. 2011;30(11):1756–1770. doi:10.1016/j.trac.2011.06.015
10. Sajid M, Khaled Nazal M, Rutkowska M, Szczepańska N, Namieśnik J, Płotka-Wasylka J. Solid phase microextraction: apparatus, sorbent materials, and application. Critical Rev Analytical Chem. 2019;49(3):271–288. doi:10.1080/10408347.2018.1517035
11. Rebmann AJ, David E, Sorg MH, Koenig M. Cadaver Dog Handbook Forensic Training and Tactics for the Recovery of Human Remains. Boca Raton: Fla.: CRC Press; 2000. http://www.crcnetbase.com/isbn/9781420039634.
12. SWGDOG SC8– SUBSTANCE DETECTOR DOGS Human Remains Detection (HRD) Land and Water. Available from: https://ifri.fiu.edu/research/detector-dogs/swgdog/_assets/sc8_human_remains.pdf.
13. Martin C, Maesen P, Minchilli D, Francis F, Verheggen F. Forensic taphonomy: characterization of the gravesoil chemistry using a multivariate approach combining chemical and volatile analyses. Forensic Sci Int. 2021;318:110569. doi:10.1016/j.forsciint.2020.110569
14. Ensiminger J. Was there a body in the trunk: volatile organic compounds in the trial of casey anthony and the evolving search for a chemical profile for human decomposition. SMU Sci. 2016;19:275.
15. Vass AA. Beyond the grave – understanding human decomposition. Microbiology Today. 2001;28:190–193.
16. Goff LM. Early post-mortem changes and stages of decomposition in exposed cadavers. Exp Appl Acarol. 2009;49(1–2):21–36. doi:10.1007/s10493-009-9284-9
17. Dekeirsschieter J, Verheggen FJ, Gohy M, et al. Cadaveric volatile organic compounds released by decaying pig carcasses (Sus domesticus L.) in different biotopes. Forensic Sci Int. 2009;189(1–3):46–53. doi:10.1016/j.forsciint.2009.03.034
18. Martin C, Verheggen F. Odour profile of human corpses: a review. Forensic Chem. 2018;10:27–36. doi:10.1016/j.forc.2018.07.002
19. Verheggen F, Perrault KA, Megido RC, et al. The Odor of Death: an Overview of Current Knowledge on Characterization and Applications. BioScience. 2017;67(7):600–613. doi:10.1093/biosci/bix046
20. Statheropoulos M, Spiliopoulou C, Agapiou A. A study of volatile organic compounds evolved from the decaying human body. Forensic Sci Int. 2005;153(2–3):147–155. doi:10.1016/j.forsciint.2004.08.015
21. Statheropoulos M, Agapiou A, Spiliopoulou C, Pallis G, Sianos E. Environmental aspects of VOCs evolved in the early stages of human decomposition. Sci Total Environ. 2007;385(1–3):221–227. doi:10.1016/j.scitotenv.2007.07.003
22. DeGreeff LE, Furton KG. Collection and identification of human remains volatiles by non-contact, dynamic airflow sampling and SPME-GC/MS using various sorbent materials. Anal Bioanal Chem. 2011;401(4):1295–1307. doi:10.1007/s00216-011-5167-0
23. Vass AA, Smith RR, Thompson CV, Burnett MN, Dulgerian N, Eckenrode BA. Odor Analysis of Decomposing Buried Human Remains. J Forensic Sci. 2008;53(2):384–391. doi:10.1111/j.1556-4029.2008.00680.x
24. Clases D, Ueland M, Gonzalez de Vega R, Doble P, Pröfrock D. Quantitative speciation of volatile sulphur compounds from human cadavers by GC-ICP-MS. Talanta. 2021;221:121424. doi:10.1016/j.talanta.2020.121424
25. Deo A, Forbes SL, Stuart BH, Ueland M. Profiling the seasonal variability of decomposition odour from human remains in a temperate Australian environment. Australian J Forensic Sci. 2020;52(6):654–664. doi:10.1080/00450618.2019.1637938
26. Wescott DJ. Recent advances in forensic anthropology: decomposition research. Forensic Sci Res. 2018;3(4):278–293. doi:10.1080/20961790.2018.1488571
27. Swann LM, Forbes SL, Lewis SW. Analytical separations of mammalian decomposition products for forensic science: a review. Anal Chim Acta. 2010;682(1–2):9–22. doi:10.1016/j.aca.2010.09.052
28. Vass AA, Smith RR, Thompson CV, et al. Decompositional odor analysis database. J Forensic Sci. 2004;49(4):1–10. doi:10.1520/JFS2003434
29. Stefanuto PH, Perrault KA, Stadler S, et al. GC × GC–TOFMS and supervised multivariate approaches to study human cadaveric decomposition olfactive signatures. Anal Bioanal Chem. 2015;407(16):4767–4778. doi:10.1007/s00216-015-8683-5
30. Ueland M, Harris S, Forbes SL. Detecting volatile organic compounds to locate human remains in a simulated collapsed building. Forensic Sci Int. 2021;323:110781. doi:10.1016/j.forsciint.2021.110781
31. Hoffman EM, Curran AM, Dulgerian N, Stockham RA, Eckenrode BA. Characterization of the volatile organic compounds present in the headspace of decomposing human remains. Forensic Sci Int. 2009;186(1–3):6–13. doi:10.1016/j.forsciint.2008.12.022
32. Rosier E, Loix S, Develter W, Van de Voorde W, Tytgat J, Cuypers E. The search for a volatile human specific marker in the decomposition process. PLoS One. 2015;10(9):e0137341. doi:10.1371/journal.pone.0137341
33. Stefanuto PH, Perrault K, Grabherr S, Varlet V, Focant JF. Postmortem internal gas reservoir monitoring using GC×GC-HRTOF-MS. Separations. 2016;3(3):24. doi:10.3390/separations3030024
34. Stefanuto PH, Rosier E, Tytgat J, Focant JF, Cuypers E. Profiling Volatile Organic Compounds of Decomposition. In: Schotsmans EMJ, Márquez-Grant N, Forbes SL, editors. Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment. Chichester, UK: John Wiley & Sons, Ltd; 2017:39–52. doi:10.1002/9781118953358.ch3
35. Swann L, Forbes S, Lewis SW. Observations of the temporal variation in chemical content of decomposition fluid: a preliminary study using pigs as a model system. Australian J Forensic Sci. 2010;42(3):199–210. doi:10.1080/00450610903258102
36. Swann L, Chidlow GE, Forbes S, Lewis SW. Preliminary studies into the characterization of chemical markers of decomposition for geoforensics. J Forensic Sci. 2010;55(2):308–314. doi:10.1111/j.1556-4029.2009.01263.x
37. Cablk ME, Szelagowski EE, Sagebiel JC. Characterization of the volatile organic compounds present in the headspace of decomposing animal remains, and compared with human remains. Forensic Sci Int. 2012;220(1–3):118–125. doi:10.1016/j.forsciint.2012.02.007
38. Knobel Z, Ueland M, Nizio KD, Patel D, Forbes SL. A comparison of human and pig decomposition rates and odour profiles in an Australian environment. Australian J Forensic Sci. 2019;51(5):557–572. doi:10.1080/00450618.2018.1439100
39. Matuszewski S, Hall MJR, Moreau G, Schoenly KG, Tarone AM, Villet MH. Pigs vs people: the use of pigs as analogues for humans in forensic entomology and taphonomy research. Int J Legal Med. 2020;134(2):793–810. doi:10.1007/s00414-019-02074-5
40. Armstrong P, Nizio KD, Perrault KA, Forbes SL. Establishing the volatile profile of pig carcasses as analogues for human decomposition during the early postmortem period. Heliyon. 2016;2(2):e00070. doi:10.1016/j.heliyon.2016.e00070
41. Paczkowski S, Nicke S, Ziegenhagen H, Schütz S. Volatile emission of decomposing pig carcasses (Sus scrofa domesticus L.) as an indicator for the postmortem interval. J Forensic Sci. 2015;60:S130–S137. doi:10.1111/1556-4029.12638
42. Stadler S, Desaulniers JP, Forbes SL. Inter-year repeatability study of volatile organic compounds from surface decomposition of human analogues. Int J Legal Med. 2015;129(3):641–650. doi:10.1007/s00414-014-1024-y
43. Brasseur C, Dekeirsschieter J, Schotsmans EMJ, et al. Comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry for the forensic study of cadaveric volatile organic compounds released in soil by buried decaying pig carcasses. J Chromatogr A. 2012;1255:163–170. doi:10.1016/j.chroma.2012.03.048
44. Agapiou A, Zorba E, Mikedi K, McGregor L, Spiliopoulou C, Statheropoulos M. Analysis of volatile organic compounds released from the decay of surrogate human models simulating victims of collapsed buildings by thermal desorption–comprehensive two-dimensional gas chromatography–time of flight mass spectrometry. Anal Chim Acta. 2015;883:99–108. doi:10.1016/j.aca.2015.04.024
45. Forbes SL, Perrault KA, Stefanuto PH, Nizio KD, Focant JF. Comparison of the decomposition VOC profile during winter and summer in a moist, mid-latitude (CFB) climate. PLoS One. 2014;9(11):e113681. doi:10.1371/journal.pone.0113681
46. Perrault KA, Nizio KD, Forbes SL. A comparison of one-dimensional and comprehensive two-dimensional gas chromatography for decomposition odour profiling using inter-year replicate field trials. Chromatographia. 2015;78(15–16):1057–1070. doi:10.1007/s10337-015-2916-9
47. Perrault KA, Rai T, Stuart BH, Forbes SL. Seasonal comparison of carrion volatiles in decomposition soil using comprehensive two-dimensional gas chromatography – time of flight mass spectrometry. Anal Methods. 2015;7(2):690–698. doi:10.1039/C4AY02321H
48. Perrault KA, Stefanuto PH, Stuart BH, Rai T, Focant JF, Forbes SL. Reducing variation in decomposition odour profiling using comprehensive two-dimensional gas chromatography: gas Chromatography. J Sep Sci. 2015;38(1):73–80. doi:10.1002/jssc.201400935
49. Perrault KA, Stefanuto PH, Stuart BH, Rai T, Focant JF, Forbes SL. Detection of decomposition volatile organic compounds in soil following removal of remains from a surface deposition site. Forensic Sci Med Pathol. 2015;11(3):376–387. doi:10.1007/s12024-015-9693-5
50. Perrault K, Stuart B, Forbes S, Longitudinal A. Study of decomposition odour in soil using sorbent tubes and solid phase microextraction. Chromatography. 2014;1(3):120–140. doi:10.3390/chromatography1030120
51. Metcalf JL. Estimating the postmortem interval using microbes: knowledge gaps and a path to technology adoption. Forensic Sci Int Genet. 2019;38:211–218. doi:10.1016/j.fsigen.2018.11.004
52. Cernosek T, Eckert KE, Carter DO, Perrault KA. Volatile organic compound profiling from postmortem microbes using gas chromatography–mass spectrometry. J Forensic Sci. 2020;65(1):134–143. doi:10.1111/1556-4029.14173
53. Raymer J, Rojas-Guevara JU, Prada-Tiedemann PA. Decomposition residual odor volatiles in soil from a west Texas environment. Revista Criminalidad. 2020;62(3):24.
54. Blanar K, Prada-Tiedemann PA. Characterization of the volatile odor profile from larval masses in a field decomposition setting. Forensic Chem. 2020;21:100288. doi:10.1016/j.forc.2020.100288
55. Mona S, Jawad M, Noreen S, Ali S, Rakha A. Forensic entomology: a comprehensive review. Adv Life Sci. 2019;6(2):48–59.
56. Zhu GH, Yu XJ, Xie LX, et al. Time of death revealed by hydrocarbons of empty puparia of Chrysomya megacephala (Fabricius) (Diptera: calliphoridae). Field Experiment A, Wicker-Thomas C ed. PLoS One. 2013;8(9):e73043. doi:10.1371/journal.pone.0073043
57. Frere B, Suchaud F, Bernier G, et al. GC-MS analysis of cuticular lipids in recent and older scavenger insect puparia. An approach to estimate the postmortem interval (PMI). Anal Bioanal Chem. 2014;406(4):1081–1088. doi:10.1007/s00216-013-7184-7
58. Zhu GH, Jia ZJ, Yu XJ, et al. Predictable weathering of puparial hydrocarbons of necrophagous flies for determining the postmortem interval: a field experiment using Chrysomya rufifacies. Int J Legal Med. 2017;131(3):885–894. doi:10.1007/s00414-016-1507-0
59. Paula MC, Michelutti KB, Eulalio ADMM, Piva RC, Cardoso CAL, Antonialli-Junior WF. New method for estimating the post-mortem interval using the chemical composition of different generations of empty puparia: indoor cases. Ginzel MDed. PLoS One. 2018;13(12):e0209776. doi:10.1371/journal.pone.0209776
60. Ye G, Li K, Zhu J, Zhu G, Hu C. Cuticular hydrocarbon composition in pupal exuviae for taxonomic differentiation of six necrophagous flies. J Med Entomol. 2007;44(3):450–456. doi:10.1093/jmedent/44.3.450
61. Lunas BM, Paula MC, Michelutti KB, Lima‐Junior SE, Antonialli‐Junior WF, Cardoso CAL. Hydrocarbon and fatty acid composition from blowfly eggs represents a potential complementary taxonomic tool of forensic importance. J Forensic Sci. 2019;64(6):1720–1725. doi:10.1111/1556-4029.14119
62. Stadler S, Stefanuto PH, Byer JD, Brokl M, Forbes S, Focant JF. Analysis of synthetic canine training aids by comprehensive two-dimensional gas chromatography–time of flight mass spectrometry. J Chromatogr A. 2012;1255:202–206. doi:10.1016/j.chroma.2012.04.001
63. Tipple CA, Caldwell PT, Kile BM, et al. Comprehensive characterization of commercially available canine training aids. Forensic Sci Int. 2014;242:242–254. doi:10.1016/j.forsciint.2014.06.033
64. Nizio KD, Ueland M, Stuart BH, Forbes SL. The analysis of textiles associated with decomposing remains as a natural training aid for cadaver-detection dogs. Forensic Chem. 2017;5:33–45. doi:10.1016/j.forc.2017.06.002
65. Alexander MB, Hodges TK, Wescott DJ, Aitkenhead-Peterson JA. The effects of soil texture on the ability of human remains detection dogs to detect buried human remains. J Forensic Sci. 2016;61(3):649–655. doi:10.1111/1556-4029.13084
66. Alexander MB, Hodges TK, Bytheway J, Aitkenhead-Peterson JA. Application of soil in Forensic Science: residual odor and HRD dogs. Forensic Sci Int. 2015;249:304–313. doi:10.1016/j.forsciint.2015.01.025
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
