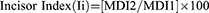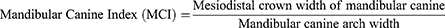Back to Archived Journals » Research and Reports in Forensic Medical Science » Volume 12
Forensic Dentistry as an Analysis Tool for Sex Estimation: A Review of Current Techniques
Authors Heng D , Manica S, Franco A
Received 30 August 2022
Accepted for publication 4 December 2022
Published 16 December 2022 Volume 2022:12 Pages 25—39
DOI https://doi.org/10.2147/RRFMS.S334796
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Henrik Druid
Dennis Heng,1 Scheila Manica,1 Ademir Franco1,2
1Centre for Forensic and Legal Medicine and Dentistry, University of Dundee, Scotland, UK; 2Division of Forensic Dentistry, Faculdade São Leopoldo Mandic, Campinas, Brazil
Correspondence: Dennis Heng, Centre for Forensic and Legal Medicine and Dentistry, University of Dundee, Scotland, UK, Email [email protected]
Abstract: Sex estimation is an integral part of the forensic human identification process. Determining the sex of an unknown deceased victim helps with the dental profiling process, as only missing persons of the same sex need to be considered for identification. Nowadays, methods that are commonly used in dental sex estimation have either low accuracy or reliability or require complex equipment or tools to perform. Forensic tools for dental sex determination can broadly be categorised into metric, non-metric, and biochemical methods. This paper provides an in-depth review into the existing methods for dental sex estimation and presents the strengths and limitations of each method. It will also briefly discuss prospective developments in the field of dental sex estimation.
Keywords: dental sex estimation, sexual dimorphism, human identification, forensic dentistry
Introduction
Sex refers to a set of biological attributes that comprises anatomical and physiological features such as chromosomes, gene expressions, hormone levels and reproductive anatomy.1 It differs from gender, where gender refers to characteristics pertaining to femininity and masculinity and its associated socially constructed roles, behaviours, expressions, and identities.1 Fundamentally, sex and gender are not the same as chromosomal sex is assigned at birth and is categorised into either male (XY) or female (XX) while gender is recognised as a spectrum.
To understand how sexual dimorphism can be observed in teeth and in the human skeleton, we first need to understand how chromosomal sex affects human growth and development. Sex differentiation is divided into the following sequential stages: genetic, gonadal, hormonal, and phenotypic.2 At the genetic stage, chromosomal sex is established after fertilisation whereby XY indicates male and XX indicates female. The presence of the Y-chromosome defines the male sex, while the absence of it defines the female sex. These chromosomal differences then give rise to the development of the different gonads, which are the testes in males or ovaries in females. The presence of the Y-chromosome plays a role in signalling for the production of male gonadal development and testosterone production.3 When the Y-chromosome is absent, the fetus will then develop female sex organs. As the sex organs begin to develop, hormonal and phenotypic differences start to arise throughout the human anatomy and give rise to greater differentiation between the male and female sex. Biochemical methods of dental sex estimation are therefore based on seeking out the Y-chromosome in males or inactive X-chromosome in female cells, for instance.
Previous authors hypothesised that under the influence of the Y-chromosome, mitotic activity in the tooth germs is enhanced, resulting in greater dentine thickness and larger crowns in males compared to females.4,5 A study by Dempsey et al found statistically significant differences of larger permanent tooth crown sizes of maxillary second molars in females of opposite-sex human twin pairs over females of single-sex twin pairs and female singletons,6 suggesting that the diffusion of male sex hormones from male to female co-twins in utero may result in increased tooth sizes. The type of sex hormones does seem to affect the growth of the dental, and Zilberman and Smith found that the greatest difference in dentine thickness between male and female children occurs during puberty.7 Teeth that are fully formed by pubertal age also exhibit sexually dimorphic features (albeit less distinct), and this could be attributed to low levels of sex hormones already present since the earlier years of life.8 As such, the differences in sex and hormones appear to have a considerable effect on dental development that could lead to a measurable difference when performing dental sex estimation. These measurable differences therefore give rise to metric and non-metric methods of dental sex estimation.
Sex estimation is an important part of the dental profiling process in human identification.9 Accurately estimating the sex of an unknown deceased individual can simplify the identification process as only the profiles with the same sex need to be considered for identification.9 Current methods of dental sex estimation can be broadly classified into metric, non-metric, and biochemical methods.9 These tools and methods have their various strengths and shortcomings with varying degrees of accuracy and applicability, and the choice of dental sex estimation method to employ would depend heavily on the type and quality of the post-mortem remains that are found which may often be fragmented, commingled or incomplete. This paper therefore aims to provide a review into these methods and to discuss their strengths and limitations, and briefly evaluate prospective methods that may hopefully overcome limitations of these existing techniques.
Metric Methods
Metric methods of dental sex estimation involve making measurements of dental structures either directly (by measuring the tooth) or indirectly (through imaging, dental radiography, or dental casts). Post-mortem remains with teeth present can undergo metric analysis without sustaining too much harm to the remains. These methods are commonly used in research studies as they are non-invasive, inexpensive, convenient to perform, and are easily repeatable However, metric methods of dental sex estimation do not have the highest accuracy rates,9 and hence might be less frequently utilised in forensic practice.
Metric methods are based on the science and evidence that the differences in sex would lead to measurable and statistically significant differences in the size of teeth in males vs females.7,10 Studies that analysed teeth from both sexes found a general trend where teeth in males are consistently larger than teeth in females.11–14 The canine was also found to consistently be the most dimorphic tooth and was significantly larger in size in males than in females.13,14
Odontometrics
Odontometrics is the measurement and study of tooth size and is a valuable technique for dental sex estimation.15–17 Odontometrics started as one of the earliest methods of dental sex estimation following the study of Hunt Jr and Gleiser in 1955 that estimated age and sex from preadolescent children from bones and teeth.18 Since then, numerous studies have quantified sexually dimorphic differences between males and females through odontometric techniques and demonstrated that males possess larger teeth than females in general.11–14
Measurements made in most studies of sexual dimorphism usually include the mesiodistal and buccolingual crown diameters,15,16 along with alternative measurements such as the mesiodistal and buccolingual cervical dimensions, diagonal diameters of teeth and root lengths.17,19 Multivariate statistical analysis is then performed once the necessary measurements have been obtained, and the accuracy of sex estimation is then calculated as a percentage score.
In terms of the tooth type, canines were found to be the most sexually dimorphic,13,14,21–23 with other teeth such as the molars and premolars showing smaller size differences.20–22 Most studies have demonstrated that teeth in males are larger than teeth in females, but in some studies reverse dimorphism (where the female teeth are larger than male teeth) was found, and the authors attributed that this could be due to geographic variations, environmental, or genetic factors, or theorised that it is a consequence of reduced sexual dimorphism and increasing overlap between the sexes.24,25 Overall, odontometric studies are generally in consensus to confirm that sexual dimorphism is exhibited in the overall crown size with average values being larger in male teeth compared to female teeth.
Recent research in odontometrics is taking a closer look at the dental tissues within the crown.26–28 Since dimorphism in tooth size is the product of varying amounts of enamel, dentine and pulp within the tooth, studies have analysed the various dental layers to find that male teeth have significantly greater quantities of dentine than females while female teeth were found to have greater enamel thickness than male teeth.26–28 Alvesalo observed that the Y-chromosome promotes growth in both enamel and dentine of the dental crown, whereas the effect of the X-chromosome seems to be limited to only enamel formation.29 Authors have also noted that the sexual dimorphism in canines and in the posterior teeth is due to greater amounts of dentine in male teeth, whereas the differences in enamel volumes do not make a large contribution to the overall tooth size dimorphism.12,30 These differences in dental tissues volumes and surface areas could also be useful in estimating sex as they present a high degree of sexual dimorphism.26
While the literature presents with numerous evidence that demonstrate sexual dimorphism through linear measurements of teeth, results were not consistent enough to suggest that odontometrics could be used as a sole indicator for sex.31 To improve upon this, efforts by previous authors have led to the calculation of dental indices, whereby dental proportions were also used to attempt to differentiate the sexes in addition to odontometric analysis.31 Examples of indices include the Incisor index, Crown index and the Mandibular Canine index.
Root Length
Similar to using crown dimensions for sex estimation, some authors have explored the possibility of using root lengths for dental sex estimation as well.17 Zorba et al studied 774 permanent teeth from 102 individuals and found a high degree of sexual dimorphism with males showing numerically higher values in root length than females.32 Similarly, Govindaram et al studied permanent teeth from 1000 panoramic radiographs and similarly found statistically significant differences between the root measurements of males and females, with males showing consistently larger values than females.33 Govindaram et al also concluded that the maxillary canines exhibited the greatest degree of sexual dimorphism from root length analysis, which reinforces the existing literature further.33
The studies mentioned above demonstrate positive trends in using root lengths for sex estimation, but these studies are few in number and the results of these studies may be population specific.33 More evidence in support of this trend is required.
Incisor Index and Mandibular Canine Index
Two of the more commonly used indices involving metric analysis for dental sex estimation are the Incisor Index and the Mandibular Canine Index. Aitchison presented the Incisor Index in 1964,34 where the mesiodistal diameters of both mandibular and maxillary lateral (MDI2) and central (MDI1) incisors are measured to compute scores according to the formula below:
Separate values for the maxillary and mandibular incisors would be obtained and compared against reference values to estimate sex.
In validating the Incisor Index for use as a method of dental sex estimation, Rani tested this index in a sample of 180 dental models of a Nalgonda population and found only statistically significant results in the mandibular right incisor (out of all four incisors tested).35 Prasanna et al investigated the Incisor Index as a method of sex estimation in a sample of 150 dental models from a South Indian population and found no statistically significant results in males compared to females.36 The authors did however note that the maximum mesiodistal dimensions of all incisors in males were higher than in females.36
Studies validating the Incisor Index are sorely lacking, but even the limited literature present is not in consensus to validate the Incisor Index as a reliable method of sex estimation. Perhaps, more research validating the Incisor Index is required before a definite conclusion can be made.
In contrast, the Mandibular Canine Index introduced by Rao et al in 1989 is probably the most common index used in metric analysis for dental sex estimation.37 According to the authors, the mandibular canines were chosen because they were known to be consistently and highly dimorphic and were most likely to survive severe trauma and survive longest in the human dentition.37 Anthropologically, it was hypothesised that males have larger mandibular canines than females in relation to the threat of aggression.
The Mandibular Canine Index (MCI) is derived as a ratio of the mesiodistal crown width of the mandibular canine over the mandibular canine arch width:
The reference points for the measurements are illustrated in Figure 1. The value derived is then compared against a cut-off value, which helps to classify the sex of the sample depending on whether the value is greater or smaller than the cut-off. As such, this method requires measurements of the lower canines and the arch width, but such remains may not always be present or wholly intact.
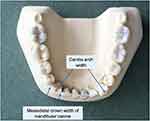 |
Figure 1 Measurements for mandibular canine index according to Rao et al. |
From their study of 766 subjects from an Indian population, Rao et al derived accuracy rates of 84.3% and 87.5% for sex estimation of males and females, respectively. Subsequently, many authors tested the MCI method in their own populations, and some were in support of the MCI as a method of sex estimation. Patel et al studied the MCI in 400 subjects in a West Indian population and found an overall accuracy of 78.8% in sex estimation,38 and other authors reported similarly high accuracy rates of 80% and above as well.39,40
However, when other authors tested the validity and applicability of the MCI in more diverse populations, they found much lower accuracy rates compared to the original authors.41,42 Silva et al found low accuracy classification rates (54.2%) in using the MCI for sex estimation in their study of 120 subjects from a Portuguese sample,41 while Atreya et al also reported lower accuracy rates (57.5–62.5%) of using the MCI for sex estimation in their study of 80 subjects from a Nepalese population.42 Both groups of authors proposed for more population-specific studies and cautioned the application of the MCI in their ethnic populations for sex estimation.41,42
Variations in tooth size and arch width size could vary amongst different populations, which would affect the MCI and render it less accurate for sex estimation. A study by Acharya & Mainali found no statistically significant differences in using the MCI for sex estimation in their study of 117 samples, but only found consistently larger dimensions in male teeth over female teeth.43 The authors hypothesised that since the MCI is a ratio, larger teeth and larger arch widths in males would cancel each other out, making it difficult for the MCI to accurately estimate sex.43 They concluded by concurring that the canines do exhibit consistent and a great degree of sexual dimorphism in the dentition, but caution against using only single metric-type methods or indices for sex estimation.43
In lieu of emerging conflicting data, a systematic review and meta-analysis performed by Dony et al calculated an overall sensitivity of 0.65 and specificity of 0.63 for the MCI method for sex estimation.44 The authors revealed that there was a lack of homogeneity in the data across the numerous studies that utilised and applied the MCI for sex estimation and concluded that the MCI is not an accurate tool for sex estimation.44 A recent systematic review by Rocha et al considered the MCI to be an auxiliary method for sex estimation due to the conflicting data present and cautioned the application of the MCI method across different populations.45
Both systematic reviews were in agreement that canine still showed a high degree of sexual dimorphism,44,45 which could point to weaknesses inherent in the methodology or calculations of results of the MCI method, leading to controversies in its accuracy. Perhaps future studies can analyse variations of the MCI method to improve upon its accuracy for sex estimation,46 given that the canine has been consistently shown to be one of the most sexually dimorphic teeth in the dentition.
Imaging/Radiography
Dental imaging and radiography are increasingly prevalent in clinical practice due to technological advancements. Clinical photographs of the patient’s face and dentition as well as both 2-D and 3-D radiographs are valuable dental records that play a huge role in forensic dentistry as they display crucial and objective dental information on them. Figure 2 shows a sample of a dental panoramic radiograph of a 14-year-old boy.
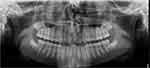 |
Figure 2 Sample of a dental panoramic radiograph of a 14-year-old boy. |
When performing metric analysis on these records, dental measurements are made indirectly on the photographs, radiographs, or 3-D models as a convenient alternative to direct intra-oral measurements. These measurements to be made are easily defined by the operator: they could involve the height, width or length of a tooth; they could be a subset of a tooth such as root length, the amount of dentine or size of the pulp chamber; or even involve bony structures such as the mandible, etc. These odontometrics can be replicated as often as required and be done at the expert’s convenience; it does not require the patient to be physically present and take up their time. Minimum image quality, resolution and size for photographs and x-rays are required to allow accurate measurements to be made, and benchmarks scales (such as the ABFO (American Board for Forensic Odontology) No. 2 Scale) should be included to provide a frame of reference for these measurements.
For the purposes of research in forensic dentistry, photographs and radiographs can also act as input data and can easily be anonymised and blinded to observers to ensure minimal bias when conducting studies. Especially for population studies, collecting huge amounts of metric data by clinically examining patients is too time-consuming and tedious. Instead, analysing panoramic radiographs or 3-D imaging of hundreds or thousands of patients is much more manageable. Pilot studies involving dental radiography and automated machine learning for forensic purposes have also been published in the literature. Ortiz et al explored the usefulness of machine learning and automation by matching pairs of panoramic radiographs for personal identification and found an approximate accuracy rate of 85% by the automated process.47 Esmaeilyfard et al used measurements made from cone beam computed tomography (CBCT) images of the first mandibular molar and evaluated them for sexual differences and found that the predictive model was able to predict sex from linear measurements of the first mandibular molar from CBCT images to over 90% accuracy.48
The increasing use of imaging and radiography around the world could shift how we practice forensic dentistry, by incorporating more elements of technology and automated machine learning to assist us with disaster victim identification or age and sex estimation. The advantages of imaging include clear documentation of the patient’s condition with easy storage, comparison, and portability of the records.49 Importantly, the use of radiography should be prudent, and patients should not be unnecessarily exposed to radiation for the purposes of research.
Non-Metric Methods
Non-metric methods for sex estimation are attributed to the presence or absence of morphological traits from hard and soft tissues. Hard tissue analysis involves analysing dental and skeletal morphology, while soft tissue analysis comprises cheiloscopy and rugoscopy analysis. Compared to metric methods that involve measurements that can be reliably repeated, non-metric analysis may be more prone to interpreter subjectivity.
In general, dental morphological traits have not been found to be as sexually dimorphic as tooth size variables, and studies that attempted to estimate sex from such traits were found to favour either the male or female sex with a lack of unanimous agreement. According to Scott et al, the canine distal accessory ridge is the only morphological trait in the human dentition that exhibits sexual dimorphism.50 Figure 3 illustrates the region on the canine that exhibits the distal accessory ridge feature. An earlier study by Scott found variation in the canine distal accessory ridge in terms of frequency and degree of expression, and that males in his study showed more frequent and pronounced expression of the trait than females.51 However, the author discussed that the appearance of this morphological trait might be influenced by ancestry or population variance.51
 |
Figure 3 Upper right canine with distal accessory ridge (marked out by the blue shaded region). |
In contrast, the skull is one of the most sexually dimorphic bones in the human body52 and analysing its morphology for sex estimation might be out of the scope of forensic odontology. If so, the forensic odontologist should work with a forensic anthropologist to estimate sex from the skull, as the former might be unfamiliar with the relevant techniques of skull sex estimation. However, this is constrained to the quality and type of remains present, as well as the availability of the necessary expertise in the local context – but this may be easily overcome with increasing digital communication with technological advancements.
Dental Morphology and Anomalies
Many studies have evaluated various morphological traits to determine if any of them could be sufficiently sexually dimorphic to allow for a reliable method of sex estimation. Presently, dental morphology plays a critical role in forensic ancestry estimation instead of sex estimation, such as a prevalence of the Carabelli’s cusp in the Caucasoid race, or shovel-shaped incisors in the Mongoloid race.53 Identifying the possible ancestry of an unknown deceased individual can also contribute towards human identification, but presently there is a lack of association between most dental morphological features and sex estimation.50 Furthermore, increasing rates of human migration and interancestral marriages around the world are diluting these distinctive inter-population differences, but the presence of these features may help to narrow down the subject pool when establishing a profile.
Similarly, dental anomalies may present as inconsistencies or variations from the normal number, shape, size, or degree of development of the tooth.54 The etiology of these dental anomalies is complex, owing to the influence of genetic, epigenetic, or environmental factors.54 The incidence of dental anomalies occurring is low, and their uniqueness can contribute towards human identification too. However, the occurrence of dental anomalies was found to be independent from the sex of an individual,55 which demonstrates the inability of dental anomalies to be a viable sex estimation method.
The Arizona State University Dental Anthropology System (ASUDAS) is a reference system created by Turner, Nichol, and Scott in 1991 to document human tooth morphology and its variations. Amongst the set of 42 current dental variants, Scott et al have found that the canine distal accessory ridge is the only morphological trait in the human dentition that exhibits sexual dimorphism.50 Future studies investigating the association of dental morphology and anomalies with sex estimation is perhaps required.
Cheiloscopy
Cheiloscopy is the field of study in forensic dentistry that relates to lip prints and patterns. Lip prints were found to be unique amongst individuals and the analysis of lip prints and patterns is a technique that could facilitate forensic human identification.56 In a systematic review and meta-analysis done by Franco et al, it was found that the most prevalent classification used in cheiloscopy studies is the Suzuki and Tsuchihashi (1970) classification,57 which classifies lip patterns into five distinct categories.
Previous studies have investigated cheiloscopy as a possible method of sex estimation, and these studies were in agreement to have found potential for cheiloscopy for use in sex estimation.58 However, the trends found in the studies to suggest either sex were conflicting and not unanimous59–61 – this renders it more difficult for a specific lip pattern to suggest the definite sex. Furthermore, the studies have limited sample sizes (understandably because these studies were difficult to conduct),59–61 and Franco et al found that most of the studies on cheiloscopy were highly heterogenous, generally observational and cross-sectional with a lack of standardisation in methodology, which could have incorporated greater error into the findings.57 The pooled accuracy was 76.8% and the authors concluded that cheiloscopy is not a reliable tool in forensic practice for sex estimation.57
In forensic practice, cheiloscopy involves obtaining a lip print from the individual and then comparing the print to a known source. One major disadvantage of using lip prints for sex estimation in dental profiling is that the usefulness of cheiloscopy heavily depends on the lips being intact at the time of post-mortem examination. Decomposition begins almost immediately after death, and mechanical trauma or thermal injury to the lips will cause greater distortion and disfiguration, rendering cheiloscopy ineffective. In crime scenes, cheiloscopy may be more effective in identifying perpetrators as it can be captured and retrieved from objects, surfaces or cigarettes.61 The use of cheiloscopy for sex estimation would first require a standardised procedure in obtaining the lip prints, and more validation from further studies is needed before it can gain greater acceptance as a method of sex estimation.
Rugoscopy
Rugoscopy, or palatoscopy, is a field in forensic dentistry that relates to the study of palatal rugae. The palatal rugae are the keratinised ridges or mucosal elevations found on the anterior part of the palatal mucosa, and a sample picture of palatal rugae is seen in Figure 4. Once formed, they remain stable for life and are unique to each individual, which facilitates forensic human identification.62 Rugoscopy has its value in human identification over other conventional methods, especially in deceased victims who are missing teeth or are completely edentulous.63,64 Available maxillary removable prostheses that sufficiently capture the palatal rugae can also act as ante-mortem records to facilitate identification.64
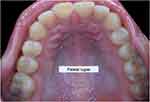 |
Figure 4 Sample picture of palatal rugae (Adapted from Shaimaa Abdellatif, CC BY-SA 4.0, via Wikimedia Commons; available from: https://commons.wikimedia.org/wiki/File:Rugae_area.jpg#file). |
The technique of rugoscopy is similar to cheiloscopy whereby prints are obtained from the individual and compared to known samples. Rugoscopy is advantageous over cheiloscopy because the palatal rugae are comprised of dense connective tissue that is more resistant to decomposition, thermal or traumatic insults than the external soft tissues like the lips or skin. Some studies have also postulated rugoscopy as a possible method of sex estimation but have found limited success due to a lack of unifying consensus from the available evidence.65,66 For instance, Smriti et al found a higher incidence of circular and backward-directed rugae in females, and more forward-directed rugae in males in their study of a South Indian population,65 whereas Gadicherla et al found a predominantly wavy pattern of rugae in males and predominantly curvy rugae in females.66 An exploratory study by Gautam et al found that straight rugae patterns were the most common patterns in both sexes and found that males exhibited longer and increased numbers of straight rugae than females.67
Most studies found potential for rugoscopy to be a possible sex estimation method and concluded that palatal rugae can be used as a complementary technique for sex estimation. A systematic review and meta-analysis by Andrade et al showed high sensitivity and specificity rates (89% and 83% respectively) of rugoscopy for sex estimation, but also revealed strong heterogeneity and methodological limitations of the studies included in the review.68 The authors therefore proposed that future studies in rugoscopy should first establish protocols for standardised best practices before further validating rugoscopy as a method for sex estimation, along with further validation from studies of different populations.68
Non-Dental Structures
Teeth are useful tools for post-mortem analysis as they are one of the most resilient structures in the body and can survive most conditions of nature. Adjacent to the teeth, many structures that make up the skull have also been found to be sexually dimorphic and are capable of accurate and reliable sex estimation as well. Two of these structures that may be of interest to the forensic odontologist are the mandible and the maxillary sinus. Studies that analysed these structures have employed both metric and non-metric methods to estimate sex from them and found varying degrees of success and accuracy.
Mandible
The mandible (or the lower jawbone) is known to be one of the most sexually dimorphic bones in the human skeleton.69,70 It is very strong and highly durable due to its shape and composition and is often recovered in an intact fashion from post-mortem remains and can be easily used for sex estimation.69 In general, males present with larger and more robust mandibles with prominent muscular attachment sites than female mandibles.71 Prominent male features also include gonial flaring, a broad ascending ramus, high symphysis, and a small mental eminence.71 Males were also found to have everted gonial angles and a squared chin, compared to inverted gonial angles and a pointed chin in females.72
Both metric and non-metric analyses of the mandible have been performed to determine its value in sex estimation. Vinay et al performed metric analyses on 250 mandibles from a South Indian population in their study and found that the male mandibles had consistently larger bigonial breadths, larger bicondylar breadths and longer mandibular lengths over female mandibles.71 Kumar et al performed similar metric analyses in their study of 156 mandibles and found consistently larger values of the bigonial breadth, bicondylar breadth and mandibular length in males over females as well.73 Both studies produced results that were in consensus with other studies done in various populations, demonstrating the similarly dimorphic nature of the mandible amongst different racial groups.69,74,75
In contrast, the use of morphological features of the mandible for sex estimation is not as strong in consensus than metric analysis. Khan & Sharieff studied 200 dry human mandibles in their study and found that the shape of the lingula, shape of the coronoid process (triangular shape was found more in male mandibles whereas hook and rounded type were more prevalent in female mandibles), and presence of the median pit (found more commonly in female mandibles) may be used for sex estimation.76 Nagaraj et al studied 90 mandibles and found that the following features: the contour of the inferior border of the mandible, the shape of the chin and shape of the coronoid process bilaterally allowed for sex differentiation,77 while Nirmale et al conclude that the type of lingula and coronoid process present can be used for sex estimation from a study of 84 mandibles.78
It is important to note at this point that the degree of sexual dimorphism in mandibles may be population specific due to influences from genetic and environmental factors, hence more studies involving different population groups could be valuable to further reinforce both metric and non-metric analysis of the mandible as valid methods of sex estimation.
Maxillary Sinus
The maxillary sinus is one of four paranasal sinuses found in the skull. It is located just above the roots of the maxillary posterior teeth and is often well captured in panoramic radiographs that are commonly used in clinical and forensic dentistry nowadays.
Various authors have examined the maxillary sinuses to determine if it is sufficiently sexually dimorphic to aid in sex estimation. A study by Tambawala et al showed that males had statistically significantly higher values for both maxillary sinuses in terms of height, width, and length dimensions, with the sinus height being the best predictor of sex.79 Paknahad et al found similar findings in their study of 100 subjects and reported an overall accuracy rate of 76% in sex estimation from the maxillary sinuses, as well as sinus height being the best predictor of sex.80 Farias Gomes at al subsequently developed a formula for sex estimation through measurements of the maxillary sinus from CBCT scans in a Brazilian population, and the formula gave an overall accuracy of 84% with promising applications.81
Leao de Queiroz et al performed a study with 64 subjects to evaluate dimensions of maxillary sinuses on panoramic radiographs for the purposes of sex estimation.82 The authors concluded that the height and width of the maxillary sinuses as captured on panoramic radiographs can be used to estimate the gender of adult human subjects.82 Though the authors calibrated the sample before performing their measurements, it is understood that panoramic radiography is less accurate and more prone to distortion than 3-D CBCT imaging due to the way panoramic radiographs are captured. Additionally, the volume of the maxillary sinus cannot be captured or precisely calculated from panoramic radiographs – this represents a huge shortcoming of panoramic radiography. Nevertheless, the studies are in consensus in their findings.79,80,82
In a systematic review by Nunes Rocha et al that examined studies that estimated sex with the maxillary sinus using computed tomography, it is worthy to note that many of these studies have small sample sizes and were also recently published within the last 10 years.83 While the authors concluded their review on a positive note, they cautioned that the application of this method across different populations may result in varying efficacies. CBCT imaging is a new and emerging technology and is slowly becoming more widespread and ubiquitous. Over time, more diverse population studies with larger sample sizes should emerge to test the strength of using the maxillary sinus for sex estimation and hopefully reinforce the association.
Biochemical Methods
In natural disasters, human remains may sometimes be lost, shattered, or burnt, and most soft tissues and sometimes teeth may be severely damaged and not be viable for metric or non-metric analysis. Biochemical methods of sex estimation are useful when post-mortem remains are incomplete or damaged since only a small sample is required for analysis, or when poor or missing ante-mortem data results in an inability to identify the unknown victim via comparative analysis. Biochemical methods started to develop in the 1990s and possess the highest accuracy and reliability in sex estimation.9 The dental pulp is often the target sample for biochemical testing, as it is encased and well protected by the dental hard tissues from trauma or high temperatures.84 Through PCR amplification, only a small sample is required for confirmatory testing – but this may prove even difficult at times depending on the condition of the remains, which may have been subjected to prolonged decomposition or thermal degradation.
Currently, popular biochemical methods for dental sex estimation include testing for the presence of Barr bodies, the sex-determining region Y protein (SRY) analysis or amelogenin protein analysis. Amelogenin and SRY analysis are considered as reliable indicators for the purposes of sex determination by DNA tracing.85 The use of a particular indicator, or use of multiple indicators may improve the accuracy of the result, but the real-life application of these techniques would also depend on the condition and type of remains available. Additionally, though these processes are highly accurate and reliable, biochemical methods of sex estimation require more time, cost, and equipment to conduct that may not be available in medicolegal units around the world.
Barr Bodies
Barr bodies are inactive X chromosomes found in a cell with more than one X chromosome present, and that is usually in the female sex. A typical female has one Barr body per cell nucleus, while a typical male has none. Barr bodies were first discovered by Barr and Bertram in 1949, and have since become a useful tool in sex determination.86
Barr bodies are typically visible under microscopy where they are found adjacent to the nuclear membrane. To view the Barr bodies, samples can be taken from intact dental pulps, bodily fluids, or mucosal epithelium, which are then preserved with formalin.86 The samples are then sectioned into thin slices and stained with nuclear dyes (such as Hematoxylin and Eosin (H&E)) and then viewed under the microscope. Compared to other biochemical methods, Barr bodies are one of the simpler biochemical methods for sex estimation as it can be readily viewed under a microscope upon completed slide preparation.86
Barr bodies are usually obtained from the dental pulp over other sources of soft tissues as the pulp is well protected within the tooth. Its usefulness in sex determination depends on the viability of the sample – Das et al found that sex can still be determined accurately from the study of XY chromosomes from mucosal epithelium up to a period of 4 weeks after death,86 while Khorate et al found that sex determination from the human pulp was possible up to 7 weeks.87 Reddy et al found that the dental pulp from female subjects showed the presence of Barr bodies up to a maximum of 400 degrees Celsius.88 Galdames et al found that teeth subjected beyond 400 degrees Celsius did not provide any viable tissues for analysis86 – demonstrating that even though the dental pulp is well-protected by the resilient dental hard tissues, it would not be able to survive all destructive conditions.
Amelogenins
Amelogenins are the principal protein component found in the enamel matrix. They are a type of extracellular matrix protein and are involved in the development of tooth enamel. In humans, the amelogenin gene is found on both the X and Y-chromosomes, and the size difference between these two chromosomes provides the basis for differentiation in males from females.89 To determine the sex of an unknown sample, a DNA sample is first obtained from the tooth enamel. Sex of the sample can then be tested by either running a known primer against the sample, or by putting the sample through mass spectrometry to identify the ratio of X to Y peptides.
Amelogenins have been used for many years as a tool for sex determination in the fields of archaeology, anthropology, meat production and sample identification. The efficacy of the amelogenin marker subsequently came into question when cases of false identification of males as females started surfacing.90,91 It was hypothesised that this could be due to mutation in the primer-binding region, or deletion of the AMELY gene in males that led to possibly erroneous conclusions, particularly since deletions in the amelogenin region of the Y chromosome were far more frequent than in the X chromosome.91 The failure rate of amelogenin for sex estimation varies amongst populations around the world, but the maximum failure rate was observed in the Indian population, as demonstrated by the studies by Kashyap et al and Thangaraj et al.92,93
Though these failure rates may be low, a low percentage in the discrepancy still matters as it may lead to a false conclusion, wrongful identification, or an erroneous judgement. Hence, to overcome these errors, alternatives in the genotyping kits or different sets of primers should be used in testing for the AMELY gene.94 Alternatively, different markers or techniques such as the SRY analysis were developed to overcome this limitation in using amelogenin as a sex determinant.90,92,93,95
Y-Chromosome Analysis
The genetic difference between females and males is defined by the presence or absence of the Y-chromosome. The presence of the Y-chromosome triggers male development, while its absence will allow female gonadal development to proceed during human embryogenesis. As such, the sex-determining region of the “Y” chromosome (SRY) can be used as a sex marker in forensic studies.
George et al identified sex by amplification of SRY gene using real-time PCR from isolated epithelial cells of the removable partial denture.96 They concluded that saliva-stained acrylic dentures can act as a source of forensic DNA and co-amplification of SRY gene with other routine sex-typing markers will give unambiguous sex identification. Reddy et al studied the epithelial cells adherent to a toothbrush as a source of DNA for sex determination using real-time PCR.90 All male samples in their study showed positive results and out of the 15 female samples, four were wrongly identified as males. A study by Khan et al found that dental pulps from teeth exposed to different environmental conditions for 60 days were still able to have the sex correctly identified with 100% accuracy through SRY analysis – demonstrating how well-protected the pulp is within the tooth to give sufficient sample material for accurate testing.97
Given that amelogenin analysis has been found to be faulty in males who showed a deletion of the AMELY gene, many authors have turned to SRY analysis for confirmation in their studies or proposed additional Y-chromosome markers for unambiguous sex determination.92,93,95 However, certain genetic syndromes such as the Klinefelter syndrome (46, XXY), Turner syndrome (46, X0), mutations in the SRY gene, or conditions such as microchimerism can give rise to unreliable or false results, affecting the reliability of Y-chromosome analysis too. As such, the use of an alternative technique or indicator, or in combination with the SRY analysis, may be required to estimate sex reliably.
Future Methods
In recent times, the use of artificial intelligence and computer-aided resources has benefitted forensic sciences by automating processes that previously required manual involvement. Not only did this advancement save valuable time and labour, but it also reduced human error and bias to improve upon the accuracy of the outcomes. For instance, KMD Plassdata DVI (KMD A/S, Ballerup, Denmark) was developed for use in mass disasters to allow computer-aided disaster victim identification and simplifies the process of matching countless possible ante-mortem records to available post-mortem data, which might otherwise be subject to accidental omissions or erroneous matching under stress. Moreover, a user-friendly interface with language translation available facilitates greater international cooperation and a quicker match rate boosts morale in times of need.
Emerging studies from the current literature have utilised machine-learning approaches in sex estimation using cranial measurements from computerised tomography images of the cranium or pelvis.52,98 Results of high accuracy were unsurprisingly achieved, as in forensic anthropology it is well known that the cranium and pelvis are the most sexually dimorphic skeletal bones. Conversely, results achieved by studies examining only the mandible were lowered in accuracy99 – reflecting the less dimorphic nature of the mandible. However, the results from these emerging studies demonstrate that machine-learning approaches can be a promising alternative for sex estimation.
On this note, the authors of this review are conducting a preliminary study that aimed to test the ability of Convolutional Neural Networks (CNNs) in sex estimation through analysing teeth from a series of annotated panoramic radiographs. CNNs were chosen as they are currently amongst the best machine-learning algorithms with great ability and performance for segmenting and classifying images. In this study, each panoramic radiograph was labelled with its known sex and age, and then annotated to highlight only the dental regions and run through the CNN to test its accuracy in sex estimation. The sample was stratified by sex and age, and accuracy amongst all age and sex groups remain generally high (above 80%), demonstrating a promising application of the use of CNNs in estimating sex from teeth from panoramic radiographs. More validation is first required; but this preliminary study enabled a greater understanding of which dental anatomical features are sexually dimorphic. This can then be further tested in future laboratory or machine-learning studies to explore what these dimorphic differences are.
Conclusion
Sex estimation is an essential part of the human identification process.9 Many sex estimation methods have been developed over time, but these methods have variable accuracy and reliability. Biochemical analysis of teeth remains the most accurate dental sex estimation method but has limitations in forensic practice.9 On the other hand, metric and non-metric methods are easy to perform, non-invasive, repeatable but may fall short in their accuracy and reliability compared to biochemical methods.
An ideal method of dental sex estimation should be easy and convenient to perform, be repeatable, non-invasive with a high degree of accuracy and reliability. While no such method currently exists, the search for one continues while forensic dentists continue to rely upon those that are available to us. More importantly, in determining which method(s) of dental sex estimation to employ, it would depend on the type and quality of the remains that are present, as well as the time, cost, and facilities available to perform the required analyses. Multiple methods may be employed to improve upon the accuracy of dental sex estimation with consensus from different parameters. It is the duty of the forensic odontologist to be familiar with current sex estimation techniques, and to employ the one(s) that are most appropriate to the case at hand.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Heidari S, Babor TF, De Castro P, Tort S, Curno M. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev. 2016;1:2. doi:10.1186/s41073-016-0007-6
2. Makiyan Z. Studies of gonadal sex differentiation. Organogenesis. 2016;12(1):42–51. doi:10.1080/15476278.2016.1145318
3. Karkanaki A, Praras N, Katsikis I, Kita M, Panidis D. Is the Y chromosome all that is required for sex determination? Hippokratia. 2007;11(3):120–123.
4. Alvesalo L, Tammisalo E, Townsend G. Upper central incisor and canine tooth crown size in 47, XXY males. J Dent Res. 1991;70(7):1057–1060. doi:10.1177/00220345910700070801
5. Alvesalo L. Sex chromosomes and human growth. A dental approach. Hum Genet. 1997;101(1):1–5. doi:10.1007/s004390050575
6. Dempsey PJ, Townsend GC, Richards LC. Increased tooth crown size in females with twin brothers: evidence for hormonal diffusion between human twins in utero. Am J Hum Biol. 1999;11(5):577–586. doi:10.1002/(SICI)1520-6300(199909/10)11:5<577::AID-AJHB1>3.0.CO;2-Y
7. Zilberman U, Smith P. Sex- and age-related differences in primary and secondary dentin formation. Adv Dent Res. 2001;15:42–45. doi:10.1177/08959374010150011101
8. Kuiri-Hänninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm Res Paediatr. 2014;82(2):73–80. doi:10.1159/000362414
9. Capitaneanu C, Willems G, Thevissen P. A systematic review of odontological sex estimation methods. J Forensic Odontostomatol. 2017;35(2):1–19. doi:10.1007/s00784-011-0537-8
10. Guatelli-Steinberg D, Sciulli PW, Betsinger TK. Dental crown size and sex hormone concentrations: another look at the development of sexual dimorphism. Am J Phys Anthropol. 2008;137(3):324–333. doi:10.1002/ajpa.20878
11. Adeyemi TA, Isiekwe MC. Comparing permanent tooth sizes (mesio-distal) of males and females in a Nigerian population. West Afr J Med. 2003;22(3):219–221. doi:10.4314/wajm.v22i3.27953
12. Schwartz GT, Dean MC. Sexual dimorphism in modern human permanent teeth. Am J Phys Anthropol. 2005;128(2):312–317. doi:10.1002/ajpa.20211
13. Ayoub F, Shamseddine L, Rifai M, et al. Mandibular canine dimorphism in establishing sex identity in the Lebanese population. Int J Dent. 2014;2014:235204. doi:10.1155/2014/235204
14. Bakkannavar SM, Monteiro FN, Arun M, Pradeep Kumar G. Mesiodistal width of canines: a tool for sex determination. Med Sci Law. 2012;52(1):22–26. doi:10.1258/msl.2011.010152
15. Acharya AB, Mainali S. Sex discrimination potential of buccolingual and mesiodistal tooth dimensions. J Forensic Sci. 2008;53(4):790–792. doi:10.1111/j.1556-4029.2008.00778.x
16. Garn SM, Lewis AB, Swindler DR, Kerewsky RS. Genetic control of sexual dimorphism in tooth size. J Dent Res. 1967;46(5):963–972. doi:10.1177/00220345670460055801
17. Garn SM, Cole PE, Van Alstine WL. Sex discriminatory effectiveness using combinations of root lengths and crown diameters. Am J Phys Anthropol. 1978;50(1):115–118. doi:10.1002/ajpa.1330500111
18. Hunt EE Jr, Gleiser I. The estimation of age and sex of preadolescent children from bones and teeth. Am J Phys Anthropol. 1955;13(3):479–487. doi:10.1002/ajpa.1330130308
19. Manchanda AS, Narang RS, Kahlon SS, Singh B. Diagonal tooth measurements in sex assessment: a study on North Indian population. J Forensic Dent Sci. 2015;7(2):126–131. doi:10.4103/0975-1475.146371
20. Soundarya N, Jain VK, Shetty S, Akshatha BK. Sexual dimorphism using permanent maxillary and mandibular incisors, canines and molars: an odontometric analysis. J Oral Maxillofac Pathol. 2021;25(1):183–188. doi:10.4103/jomfp.jomfp_400_20
21. Prabhu S, Acharya AB. Odontometric sex assessment in Indians [published correction appears in Forensic Sci Int. 2011 Mar 20; 206 (1–3): 218.e1–2]. Forensic Sci Int. 2009;192(1–3):129.e1–129.e1295. doi:10.1016/j.forsciint.2009.08.008
22. Zorba E, Moraitis K, Manolis SK. Sexual dimorphism in permanent teeth of modern Greeks. Forensic Sci Int. 2011;210(1–3):74–81. doi:10.1016/j.forsciint.2011.02.001
23. Angadi PV, Hemani S, Prabhu S, Acharya AB. Analyses of odontometric sexual dimorphism and sex assessment accuracy on a large sample. J Forensic Leg Med. 2013;20(6):673–677. doi:10.1016/j.jflm.2013.03.040
24. Dash KC, Panda A, Behura SS, Ramachandra S, Bhuyan L, Bandopadhyay A. Employing dimensional disparity of teeth to establish the gender in Odisha population: a dimorphic study. J Int Soc Prev Community Dent. 2018;8(2):174–178. doi:10.4103/jispcd.JISPCD_42_18
25. Abaid S, Zafar S, Kruger E, Tennant M. Mesiodistal dimensions and sexual dimorphism of teeth of contemporary Western Australian adolescents. J Oral Sci. 2021;63(3):247–251. doi:10.2334/josnusd.20-0596
26. García-Campos C, Martinón-Torres M, Martínez de Pinillos M, et al. Modern humans sex estimation through dental tissue patterns of maxillary canines. Am J Phys Anthropol. 2018;167(4):914–923. doi:10.1002/ajpa.23715
27. Saunders SR, Chan AH, Kahlon B, Kluge HF, FitzGerald CM. Sexual dimorphism of the dental tissues in human permanent mandibular canines and third premolars. Am J Phys Anthropol. 2007;133(1):735–740. doi:10.1002/ajpa.20553
28. Sorenti M, Martinón-Torres M, Martín-Francés L, Perea-Pérez B. Sexual dimorphism of dental tissues in modern human mandibular molars. Am J Phys Anthropol. 2019;169(2):332–340. doi:10.1002/ajpa.23822
29. Alvesalo L. Human sex chromosomes in oral and craniofacial growth. Arch Oral Biol. 2009;54(Suppl 1):S18–S24. doi:10.1016/j.archoralbio.2008.06.004
30. García-Campos C, Martinón-Torres M, Martín-Francés L, et al. Contribution of dental tissues to sex determination in modern human populations. Am J Phys Anthropol. 2018;166(2):459–472. doi:10.1002/ajpa.23447
31. Anna J, Harish K. How reliable is sex differentiation from teeth measurements. J Oral Maxillofac Pathol. 2013;4:289–292.
32. Zorba E, Vanna V, Moraitis K. Sexual dimorphism of root length on a Greek population sample. Homo. 2014;65(2):143–154. doi:10.1016/j.jchb.2013.09.005
33. Govindaram D, Bharanidharan R, Ramya R, Rameshkumar A, Priyadharsini N, Rajkumar K. Root length: as a determinant tool of sexual dimorphism in an ethnic tamil population. J Forensic Dent Sci. 2018;10(2):96–100. doi:10.4103/jfo.jfds_10_18
34. Aitchison J. Sex differences in teeth, jaws and skulls. Dent Pract. 1964;14:52–57.
35. Rani ST. Applicability of odontometric dimensions and indices in sexual dimorphism among Nalgonda population. J Forensic Dent Sci. 2017;9(3):175. doi:10.4103/jfo.jfds_42_16
36. Prasanna S, Makesh Raj LS, Sai Krishna P, Jai Santhosh Manikandan V, Srikant N. Evaluation of incisor index as a forensic tool in gendural dimorphism – a study in south Indian population. Indian J Forensic Med Toxicol. 2021;15(4):2319–2323. doi:10.37506/ijfmt.v15i4.17053
37. Rao NG, Rao NN, Pai ML, Kotian MS. Mandibular canine index--a clue for establishing sex identity. Forensic Sci Int. 1989;42(3):249–254. doi:10.1016/0379-0738(89
38. Patel RA, Chaudhary AR, Dudhia BB, Macwan ZS, Patel PS, Jani YV. Mandibular canine index: a study for gender determination in Gandhinagar population. J Forensic Dent Sci. 2017;9(3):135–143. doi:10.4103/jfo.jfds_64_16
39. Sreedhar G, Sumalatha MN, Ramesh G, Nagarajappa R, Murari A, Agrawal A. Dimorphic mandibular canines in gender determination in Moradabad population of Western Uttar Pradesh. J Forensic Dent Sci. 2015;7(1):32–36. doi:10.4103/0975-1475.150302
40. Singh SK, Gupta A, Padmavathi BN, Kumar S, Roy S, Kumar A. Mandibular canine index: a reliable predictor for gender identification using study cast in Indian population. Indian J Dent Res. 2015;26(4):396–399. doi:10.4103/0970-9290.167632
41. Silva AM, Pereira ML, Gouveia S, Tavares JN, Azevedo Á, Caldas IM. A new approach to sex estimation using the mandibular canine index. Med Sci Law. 2016;56(1):7–12. doi:10.1177/0025802415575415
42. Atreya A, Shrestha R, Tuladhar LR, Nepal S, Shrestha R, Sah SK. Sex predictability by using mandibular canine index. J Nepal Health Res Counc. 2020;17(4):501–505. doi:10.33314/jnhrc.v17i4.2187
43. Acharya AB, Mainali S. Limitations of the mandibular canine index in sex assessment. J Forensic Leg Med. 2009;16(2):67–69. doi:10.1016/j.jflm.2008.08.005
44. Dony E, Reddy M, Kakodkar P. Mandibular Canine Index (MCI) not an accurate tool for gender identification: results from a systematic review and meta-analysis. Indian J Public Health Res Dev. 2018;9(7):61–69. doi:10.5958/0976-5506.2018.00614.9
45. Rocha MFN, Pinto PHV, Franco A, da Silva RHA. Applicability of the mandibular canine index for sex estimation: a systematic review. Egypt J Forensic Sci. 2022;12(14):1–18. doi:10.1186/s41935-022-00270-w
46. Azevedo Á, Pereira ML, Gouveia S, Tavares JN, Caldas IM. Sex estimation using the mandibular canine index components. Forensic Sci Med Pathol. 2019;15(2):191–197. doi:10.1007/s12024-018-0051-2
47. Ortiz AG, Soares GH, da Rosa GC, Biazevic MGH, Michel-Crosato E. A pilot study of an automated personal identification process: applying machine learning to panoramic radiographs. Imaging Sci Dent. 2021;51(2):187–193. doi:10.5624/isd.20200324
48. Esmaeilyfard R, Paknahad M, Dokohaki S. Sex classification of first molar teeth in cone beam computed tomography images using data mining. Forensic Sci Int. 2021;318:110633. doi:10.1016/j.forsciint.2020.110633
49. Issrani R, Prabhu N, Sghaireen MG, et al. Cone-beam computed tomography: a new tool on the horizon for forensic dentistry. Int J Environ Res Public Health. 2022;19(9):5352. doi:10.3390/ijerph19095352
50. Scott G, Maier C, Heim K. Identifying and recording key morphological (nonmetric) crown and root traits. In: Irish JD, Scott GR, editors. A Companion to Dental Anthropology. Chichester, England: Wiley Blackwell; 2015:247–264. doi:10.1002/9781118845486.ch17
51. Scott GR. Classification, sex dimorphism, association, and population variation of the canine distal accessory ridge. Hum Biol. 1977;49(3):453–469.
52. Toneva D, Nikolova S, Agre G, Zlatareva D, Hadjidekov V, Lazarov N. Machine learning approaches for sex estimation using cranial measurements. Int J Legal Med. 2021;135(3):951–966. doi:10.1007/s00414-020-02460-4
53. Kirthiga M, Manju M, Praveen R, Umesh W. Ethnic association of cusp of Carabelli trait and shoveling trait in an Indian population. J Clin Diagn Res. 2016;10(3):ZC78–ZC81. doi:10.7860/JCDR/2016/17463.7504
54. Puri P, Shukla SK, Haque I. Developmental dental anomalies and their potential role in establishing identity in post-mortem cases: a review. Med Leg J. 2019;87(1):13–18. doi:10.1177/0025817218808714
55. Sella tunis T, Sarne O, Hershkovitz I, et al. Dental anomalies’ characteristics. Diagnostics. 2021;11(7):1161. doi:10.3390/diagnostics11071161
56. Venkatesh R, David MP. Cheiloscopy: an aid for personal identification. J Forensic Dent Sci. 2011;3(2):67–70. doi:10.4103/0975-1475.92147
57. Franco A, Lima LKG, de Oliveira MN, et al. The weak evidence of lip print analysis for sexual dimorphism in forensic dentistry: a systematic literature review and meta-analysis. Sci Rep. 2021;11(1):24192. doi:10.1038/s41598-021-03680-3
58. Ramakrishnan P, Bahirwani S, Valambath S. Assessment of cheiloscopy in sex determination using lysochrome - a preliminary study. J Forensic Dent Sci. 2015;7(3):195–200. doi:10.4103/0975-1475.172434
59. Malik R, Goel S, Kailasam S. Cheiloscopy: a deterministic aid for forensic sex determination. J Indian Acad Oral Med Radiol. 2011;23:17–19. doi:10.5005/jp-journals-10011-1082
60. Sharma V, Ingle NA, Kaur N, Yadav P. Identification of sex using lip prints: a clinical study. J Int Soc Prev Community Dent. 2014;4(Suppl 3):S173–S177. doi:10.4103/2231-0762.149030
61. Nagalaxmi V, Ugrappa S, Ch L, Maloth KN, Kodangal S. Cheiloscopy, palatoscopy and odontometrics in sex prediction and dis-crimination - a comparative study. Open Dent J. 2015;8:269–279. doi:10.2174/1874210601408010269
62. Smitha T, Vaswani V, Deepak V, Sheethal HS, Hema KN, Jain VK. Reliability of palatal rugae patterns in individual identification. J Oral Maxillofac Pathol. 2021;25(3):555. doi:10.4103/jomfp.jomfp_269_21
63. Poojya R, Shruthi CS, Rajashekar VM, Kaimal A. Palatal rugae patterns in edentulous cases, are they a reliable forensic marker? Int J Biomed Sci. 2015;11(3):109–112.
64. de Castro-Espicalsky TL, Freitas P, Ribeiro Tinoco RL, Calmon M, Júnior ED, Rossi AC. Human identification by the analysis of palatal rugae printed in complete dentures. J Forensic Odontostomatol. 2020;38(2):57–62.
65. Smriti K, Gupta R, Pentapati KC, et al. Sex assessment by morphological analysis of palatal rugae patterns in a South Indian adult population. Clin Cosmet Investig Dent. 2021;13:77–81. doi:10.2147/CCIDE.S304599
66. Gadicherla P, Saini D, Bhaskar M. Palatal rugae pattern: an aid for sex identification. J Forensic Dent Sci. 2017;9(1):48. doi:10.4103/jfo.jfds_108_15
67. Gautam N, Patil SG, Krishna RG, Agastya H, Mushtaq L, Kumar KV. Association of palatal rugae pattern in gender identification: an exploratory study. J Contemp Dent Pract. 2017;18(6):470–473. doi:10.5005/jp-journals-10024-2067
68. Andrade RNM, Vieira WA, Bernardino ÍM, Franco A, Paranhos LR. Reliability of palatal rugoscopy for sexual dimorphism in forensic dentistry: a systematic literature review and meta-analysis. Arch Oral Biol. 2019;97:25–34. doi:10.1016/j.archoralbio.2018.10.009
69. Gamba Tde O, Alves MC, Haiter-Neto F. Mandibular sexual dimorphism analysis in CBCT scans. J Forensic Leg Med. 2016;38:106–110. doi:10.1016/j.jflm.2015.11.024
70. Tunis TS, Sarig R, Cohen H, Medlej B, Peled N, May H. Sex estimation using computed tomography of the mandible. Int J Legal Med. 2017;131(6):1691–1700. doi:10.1007/s00414-017-1554-1
71. Vinay G, Mangala Gowri SR, Anbalagan J. Sex determination of human mandible using metrical parameters. J Clin Diagn Res. 2013;7(12):2671–2673. doi:10.7860/JCDR/2013/7621.3728
72. Alias A, Ibrahim A, Abu Bakar SN, et al. Anthropometric analysis of mandible: an important step for sex determination. Clin Ter. 2018;169(5):e217–e223. doi:10.7417/CT.2018.2082
73. Kumar A, Akhtar MJ, Kumar B, Sinha RR, Kumar A, Kumar S. Sex determination of human mandible in population of bihar by using metrical parameters. Int J Med Res Prof. 2018;4(6):173–177. doi:10.21276/ijmrp.2018.4.6.036
74. Kujur B, Wakode NS, Gaikwad MR, Wakode S. Most reliable parameter of the mandible used for sex determination. Int J Anat Res. 2017;5:4611–4615. doi:10.16965/ijar.2017.419
75. Ongkana N, Sudwan P. Gender difference in Thai mandibles using metric analysis. Chiang Mai Med J. 2009;48(2):43–48.
76. Khan HS, Sharieff JH. Observation on morphological features of human mandibles in 200 South Indian subjects. Anatomica Karnataka Int J. 2011;5:44–49.
77. Nagaraj T, Veerabasvaiah BT, James L, Goswami RD, Narayanan S, Keerthi I. Use of non-metric characteristics of mandible in sex determination. J Med Radiol Pathol Surg. 2016;2(4):1–4. doi:10.15713/ins.jmrps.60
78. Nirmale VK, Mane UW, Sukre SB, Diwan CV. Morphological features of human mandible. Int J Recent Trends Sci Technol. 2012;3(2):38–43.
79. Tambawala SS, Karjodkar FR, Sansare K, Prakash N, Dora AC. Sexual dimorphism of foramen magnum using cone beam computed tomography. J Forensic Leg Med. 2016;44:29–34. doi:10.1016/j.jflm.2016.08.005
80. Paknahad M, Shahidi S, Zarei Z. Sexual dimorphism of maxillary sinus dimensions using cone-beam computed tomography. J Forensic Sci. 2017;62(2):395–398. doi:10.1111/1556-4029.13272
81. Farias Gomes A, de Oliveira Gamba T, Yamasaki MC, Groppo FC, Haiter Neto F, Possobon RF. Development and validation of a formula based on maxillary sinus measurements as a tool for sex estimation: a cone beam computed tomography study. Int J Legal Med. 2019;133(4):1241–1249. doi:10.1007/s00414-018-1869-6
82. Leao de Queiroz C, Terada AS, Dezem TU, et al. Sex determination of adult human maxillary sinuses on panoramic radiographs. Acta Stomatol Croat. 2016;50(3):215–221. doi:10.15644/asc50/3/3
83. Nunes Rocha MF, Dietrichkeit Pereira JG, Alves da Silva RH. Sex estimation by maxillary sinus using computed tomography: a systematic review. J Forensic Odontostomatol. 2021;1(39):35–44.
84. Veeraraghavan G, Lingappa A, Shankara SP, Mamatha GP, Sebastian BT, Mujib A. Determination of sex from tooth pulp tissue. Libyan J Med. 2010;5. doi:10.3402/ljm.v5i0.5084
85. Maulani C, Auerkari EI. Molecular analysis for sex determination in forensic dentistry: a systematic review. Egypt J Forensic Sci. 2020;10(1):36. doi:10.1186/s41935-020-00210-6
86. Priyadharscini RA, Sabarinath T. Barr bodies in sex determination. J Forensic Dent Sci. 2013;5(1):64–67.
87. Khorate MM, Dhupar A, Ahmed J, Dinkar AD. Gender determination from pulpal tissue. J Forensic Dent Sci. 2014;6(2):107–112. doi:10.4103/0975-1475.132540
88. Reddy AVS, Prakash AR, Killampalli LK, Rajinikanth M, Sreenath G, Sabiha PB. Gender determination using barr bodies from teeth exposed to high temperatures. J Forensic Dent Sci. 2017;9(1):44. doi:10.4103/0975-1475.206494
89. Haas-Rochholz H, Weiler G. Additional primer sets for an amelogenin gene PCR-based DNA-sex test. Int J Legal Med. 1997;110(6):312–315. doi:10.1007/s004140050094
90. Reddy VS, Sriram G, Saraswathi T, Sivapathasundharam B. Isolation of epithelial cells from tooth brush and gender identification by amplification of SRY gene. J Forensic Dent Sci. 2011;3(1):27–32. doi:10.4103/0975-1475.85293
91. Tozzo P, Giuliodori A, Corato S, Ponzano E, Rodriguez D, Caenazzo L. Deletion of amelogenin Y-locus in forensics: literature revision and description of a novel method for sex confirmation. J Forensic Leg Med. 2013;20(5):387–391. doi:10.1016/j.jflm.2013.03.012
92. Kashyap VK, Sahoo S, Sitalaximi T, Trivedi R. Deletions in the Y-derived amelogenin gene fragment in the Indian population. BMC Med Genet. 2006;7:37. doi:10.1186/1471-2350-7-37
93. Thangaraj K, Reddy AG, Singh L. Is the amelogenin gene reliable for gender identification in forensic casework and prenatal diagnosis? Int J Legal Med. 2002;116(2):121–123. doi:10.1007/s00414-001-0262-y
94. Dash HR, Rawat N, Das S. Alternatives to amelogenin markers for sex determination in humans and their forensic relevance. Mol Biol Rep. 2020;47(3):2347–2360. doi:10.1007/s11033-020-05268-y
95. Kastelic V, Budowle B, Drobnic K. Validation of SRY marker for forensic casework analysis. J Forensic Sci. 2009;54(3):551–555. doi:10.1111/j.1556-4029.2009.01007.x
96. George R, Sriram G, Saraswathi T, Sivapathasundharam B. Isolation of epithelial cells from acrylic removable dentures and gender identification by amplification of SRY gene using real time PCR. J Forensic Dent Sci. 2010;2(1):32–36. doi:10.4103/0974-2948.71055
97. Khan R, Tejasvi MLA, Paramkusam G. Comparison of Gender determination from dental pulp and dentin after exposure to various environmental conditions: a polymerase chain reaction-based SRY gene study. Contemp Clin Dent. 2019;10(2):256–262. doi:10.4103/ccd.ccd_472_18
98. Toy S, Secgin Y, Oner Z, Turan MK, Oner S, Senol D. A study on sex estimation by using machine learning algorithms with parameters obtained from computerized tomography images of the cranium. Sci Rep. 2022;12(1):4278. doi:10.1038/s41598-022-07415-w
99. Ortiz AG, Costa C, Silva RHA, Biazevic MGH, Michel-Crosato E. Sex estimation: anatomical references on panoramic radiographs using machine learning. Forensic Imaging. 2020;20:200356. doi:10.1016/j.fri.2020.200356
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.