Back to Journals » Clinical Ophthalmology » Volume 10
Feasibility study of sustained-release travoprost punctum plug for intraocular pressure reduction in an Asian population
Authors Perera S , Ting DS, Nongpiur M, Chew P, Aquino MC, Sng C, Ho S, Aung T
Received 9 December 2015
Accepted for publication 2 February 2016
Published 26 April 2016 Volume 2016:10 Pages 757—764
DOI https://doi.org/10.2147/OPTH.S102181
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Shamira A Perera,1–3 Daniel SW Ting,1,2 Monisha E Nongpiur,1–3 Paul T Chew,3 Maria Cecilia D Aquino,3 Chelvin CA Sng,3 Sue-Wei Ho,1,2 Tin Aung1–3
1Singapore Eye Research Institute, Singapore; 2Singapore National Eye Centre, Singapore; 3Department of Ophthalmology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore
Purpose: To investigate the efficacy and safety of a punctum plug-based sustained drug release system for a prostaglandin analog, travoprost (OTX-TP), for intraocular pressure (IOP) reduction in an Asian population.
Methods: This is an initial feasibility, prospective, single-arm study involving 26 eyes and a bioresorbable punctum plug containing OTX-TP. An OTX-TP was placed in the vertical portion of the superior or inferior canaliculus of patients with primary open-angle glaucoma or ocular hypertension. The main outcome measure was the IOP-lowering efficacy of OTX-TP at 3 (8 am) and 10, 20, and 30 days (8 am, 10 am, and 4 pm), compared to baseline.
Results: A total of 26 OTX-TP were inserted for 17 subjects. The mean (standard deviation) age was 57.2 (13.8) years. At 10 days, all plugs were still present, and the IOP reduction from baseline was 6.2 (23%), 5.4 (21%), and 7.5 mmHg (28%) at 8 am, 10 am, and 4 pm, respectively. At 10 days, the mean IOP (standard error of mean) was 21.2 (1.2), 20.4 (0.8), and 19.7 (1.0) at 8 am, 10 am, and 4 pm, respectively, showing no discernible IOP trend during the course of the day. At 30 days, plug retention had declined to 42%, and the overall IOP reduction had decreased to 16%.
Conclusion: The sustained-release OTX-TP is able to reduce IOP by 24% (day 10) and 15.6% (day 30), respectively. It is a potentially well-tolerable ocular hypotensive for glaucoma patients with a history of poor compliance.
Keywords: ocular hypertension, travoprost, bioresorbable, punctum plug, glaucoma, Asian
Introduction
The primary goal of glaucoma treatment is to prevent progressive visual field loss by lowering intraocular pressure (IOP). The success of medical therapy of glaucoma patients thus depends largely on patients’ adherence and persistency to the medication. The noncompliance rate in glaucoma patients was reported to be as high as 59% in a systematic review.1 The reasons for poor compliance to topical medications can be due to difficulty in administering eye drops, complex treatment regimes, poor comprehension on the part of patients, lack of confidence in their ophthalmologist’s advice, and simple forgetfulness.2 Furthermore, studies on persistency, which measure prescription refills and refill times, have shown that less than 50% of glaucoma subjects continue therapy and refill prescriptions as required.3 A pharmacy study has reported persistency to be lower in the Singapore population compared to that previously reported in Caucasians, being 11.5% after 3 years.4 Suboptimal medical management of glaucoma due to poor adherence and persistence can lead to a greater socioeconomic burden of blindness, which has implications for patients and health care systems.5
Travoprost is a prostaglandin analog that can reduce the mean IOP from 25% to 32% and can be sustained throughout the 24-hour cycle.6–8 The travoprost punctum plug (OTX-TP) is a novel sustained-release travoprost delivery system that consists of a rod-shaped, dried polyethylene glycol-based hydrogel punctum plug designed to be placed in the vertical portion of the superior or inferior canaliculus. Embedded in the punctum plug are poly(lactic acid) microspheres which contain encapsulated travoprost, the active pharmaceutical ingredient. As the OTX-TP hydrates in the tear fluid within the canaliculus, it swells in volume to fill the potential space. The microspheres, which are bioresorbable particles, slowly degrade via hydrolysis and gradually release the drug in a controlled fashion over a period of 30 days. The retention and placement of OTX-TP can be identified by the presence of the color additive D&C Violet No 2, which serves as a visualization aid.
The purpose of this study was to evaluate the safety and efficacy of the OTX-TP in patients with primary open-angle glaucoma and ocular hypertension (OHT) over 30 days.
Methods
This was a prospective, single-arm feasibility study conducted at two centers in Singapore (the Singapore National Eye Center and National University Hospital, Singapore). The study was approved by the respective institutional review boards and conducted in accordance with the Declaration of Helsinki. The study also had the approval of the Clinical Trials Section of the Health Sciences Authority of Singapore. Written informed consent was obtained from all subjects.
To assess for eligibility, participants underwent a standardized interview and eye examination that included visual acuity measurement using a logarithm of minimum angle of resolution chart (LogMAR; Lighthouse, Inc., Long Island, NY, USA), slit-lamp examination (Model BQ 900; HAAG-STREIT, Bern, Switzerland), stereoscopic optic disc examination with a 78-diopter lens (Volk Optical, Inc, Mentor, OH, USA), and gonioscopy, performed in the dark using a Goldmann 2-mirror lens (HAAG-STREIT) at high magnification (X16).
The inclusion and exclusion criteria of our study are listed in Table 1. Primary open-angle glaucoma was defined as glaucomatous optic neuropathy (highlighted by a cup:disc ratio of >0.7, and/or the presence of neuroretinal rim notching) with a compatible visual field defect and open angles on gonioscopy, while OHT patients had normal optic discs and visual fields, and open angles. A threshold examination of the central 24° of visual field (SITA 24-2 program; Carl Zeiss Meditec, Dublin, CA, USA), showing a glaucoma hemifield test “outside normal limits”, and a cluster of three contiguous points on the pattern deviation plot depressed at P<5% level of occurring in age-matched normal subjects and not crossing the horizontal meridian, were considered compatible with glaucoma. Test reliability was determined if the following criteria were satisfied: fixation loss <20%; false-positive <33%; and/or false-negative <33%.9
All patients who met the inclusion and exclusion criteria were recruited at the screening visit (Visit 1). Patients on previous treatment with topical antiglaucoma drops were required to complete a minimum washout period, before OTX-TP insertion at the subsequent visit (Visit 2, baseline visit), as follows: 4 weeks for β-adrenergic blockers and prostaglandin analogs, 3 weeks for α-adrenergic blockers, and 7 days for pilocarpine or carbonic anhydrase inhibitors.
At the baseline visit, IOP was recorded at 8 am, 10 am, and 4 pm; and the OTX-TP was inserted for eligible patients after the 4 pm IOP assessment (between 4 and 4:30 pm). The OTX-TP was inserted into either the superior or inferior vertical canaliculus of each subject’s eligible eye(s), depending on ease of insertion. The patients were subsequently followed up on day-3 (IOP check at 8 am), day-10 (IOP check at 8 am, 10 am, and 4 pm), day-20 (IOP check at 8 am, 10 am, and 4 pm), and day-30 (IOP check at 8 am, 10 am, and 4 pm). A day-34 visit was conducted at 8 am as a postremoval safety check before the patient exited the study.
All IOP measurements were performed using Goldmann applanation tonometry (GAT; HAAG-STREIT) by two qualified ophthalmologists. This process was masked in that one observer would measure the IOP and the second person would read the IOP off the tension knob of the tonometer. Two measurements were performed in each eye at each time point, and the mean of these measurements was used in the analyses. In addition, at each study visit, the participants underwent an evaluation that included measurement of visual acuity, systemic blood pressure and pulse rate, and a slit-lamp examination to assess for hyperemia and for retention of the OTX-TP. Hyperemia was graded from Grades 1 to 3 by comparison with standardized photographs.10
For an eligible subject with both eyes that met the eligibility criteria, the OTX-TP was inserted in both eyes, but only the eye with the higher baseline IOP was included in the analysis. If a subject had bilateral glaucoma or OHT, but only one eye met the criteria, the eligible eye was treated with the OTX-TP, while the fellow eye was continued on the previous topical antiglaucoma medication.
For the assessment of OTX-TP presence in the canaliculus, a handheld transilluminator torch was used to find any rod-shaped shadow deep in the canal. Slit-lamp biomicroscopy was also used to assess for the presence of the more superficially placed OTX-TP through direct visualization down the punctum.
All subjects were actively monitored for comfort, ocular complaints, and postinsertion adverse ocular events throughout the study period. An adverse event was defined as any undesirable event occurring in a subject regardless of whether it was considered related to the investigational drug. A serious adverse event was defined as an event that was potentially fatal, life threatening, permanently disabling, requiring hospitalization, or requiring intervention to prevent permanent impairment or damage.
Statistical analyses
The analyses for the outcome measures used all available data for each study visit on an intent-to-treat (ITT) basis. Predetermined time points for the study included results at days 3, 10, 20, and 30. To adjust for the possible effect of missing data on the study results, the analysis of mean changes for IOP was performed using the single imputation methods of last-value carried forward.
The primary outcome measure of our study was IOP-lowering efficacy (defined as the mean IOP reduction at 8 am, 10 am, and 4 pm on day 30 post-OTX-TP insertion), while the secondary outcome measure was the retention rate and tolerability of OTX-TP at day 30. Data were described in terms of mean ± standard deviation for continuous variables and as frequencies and percentages for categorical variables. Wilcoxon signed ranks paired test was used to assess the mean changes in IOP from baseline at each individual time point. An appropriate Bonferroni correction (α/5) was applied to correct for the number of time points evaluated, resulting in a P-value threshold of 0.01 to be considered statistically significant. If both eyes were eligible for analysis, the eye with the higher baseline IOP reading was used. As this was a feasibility study, no formal sample size estimation was performed. Statistical analysis was performed using the statistical package SPSS Version 17 (SPSS, Chicago, IL, USA).
Results
A total of 17 subjects were recruited and included in the ITT analysis. The mean (standard deviation) age was 57.2 (13.8) years and the majority of the subjects were male (76%) and Chinese (65%) (Table 2). Figure 1 shows the subject disposition of our study. For the 17 subjects who were recruited for the trial, 26 OTX-TP were inserted. However, the higher IOP eye was selected for the IOP analysis, and so seven right eyes and ten left eyes were finally considered for the study.
  | Table 2 Demographics of the study population |
  | Figure 1 Subject disposition. |
In our study, more than three-quarters of OTX-TP (77%, n=20) were deemed to be “easy to insert” by the operator. Of the 26 OTX-TP, most patients tolerated them well, except for one patient who developed bilateral severe epiphora, requiring removal and reinstitution of topical ocular hypotensives on day 1. Subsequently, glaucoma medication was also restarted in two other subjects due to plug extrusion (day 10, IOP 26 mmHg) and inadequate IOP control (day 20, IOP 29 mmHg). The last observed IOP readings prior to the institution of therapy were carried forward in the ITT analysis.
Table 3 shows the mean IOP at 8 am, 10 am, and 4 pm at baseline visit and at days 3, 10, 20, and 30; Figure 2 illustrates the mean IOP at each study visit and time point. Following OTX-TP insertion, there was a statistically significant reduction in mean IOP at all study visits (P-value <0.01 for all), with the greatest drop in IOP observed at day 10 (Table 4 and Figure 3). The mean reduction in IOP was 5.4, 6.4, 4.7, and 4.3 mmHg at days 3, 10, 20, and 30, respectively. This corresponded to a percentage drop of 20.3%, 24%, 17%, and 15.6%, respectively (Table 4 and Figure 3), which appeared to show a trend corresponding to plug retention, although no statistically significant differences in IOP measurements were noted. The comparable IOP reduction at days 10, 20, and 30 (P>0.05 for all pairwise comparisons, data not shown) at each visit suggested a sustained effect of the drug. Table 5 shows the percentage of eyes achieving target IOP at various follow-up visits.
  | Figure 2 Mean IOP of the study patients (N=17) at different time points for each study visit. |
  | Figure 3 Mean IOP and percentage reduction in IOP in the study patients (N=17) at different study visits. |
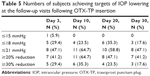  | Table 5 Numbers of subjects achieving targets of IOP lowering at the follow-up visits following OTX-TP insertion |
At day 20, (21/24) 87.5% OTX-TP were visible, and this dropped to (11/24), 41.7% at day 30 (Table 6). The peak ocular complaint rate occurred at 3 days; these were foreign body sensation (38.5%, n=10), itchiness (15.4%, n=4), epiphora (3.8%, n=1), and ocular pain (3.8%, n=1). As a consequence of severe intolerance secondary to epiphora, two OTX-TP from a single patient necessitated removal early in enrollment. The tolerability of the OTX-TP improved subsequently, with only one patient experiencing epiphora and two patients experiencing eye itchiness (Table 7).
  | Table 7 Significant ocular complaints or considered outside normal limits by time point |
Discussion
This study found a sustained IOP-lowering effect due to the OTX-TP over 30 days, combined with a reasonable safety profile and low nonresponder rate. The peak effect (24%) was observed at day 10 while 100% of the plugs were still present. The effect appeared to tail off by day 30 as the plug retention rate declined. The mean IOP at the 3, 10, 20, and 30-day time points was 21.4, 20.4, 22.1, and 22.5 mmHg, respectively, and these reduced from a baseline of 26.8 mmHg. Only two subjects required commencement of topical IOP-lowering therapy, one due to inadequate IOP control and the other as the OTX-TP had extruded on day 10.
After insertion, all plugs could be visualized directly, with the majority not even needing slit-lamp magnification to see the OTX-TP. Retention of the plug (by visualization) was 100% through 10 days, then retention declined to day 30, with 42% of the punctum plugs deemed still visible. This may be an underestimate; the violet colorant dissipated over time and the inability of transillumination to detect a small remnant of the OTX-TP may be one of the potential reasons for such a low retention by visualization. As there was still significant IOP lowering observed in the majority of patients, one cannot determine whether the residual IOP-lowering effect was from undetected remnants of OTX-TP or from residual travoprost after the OTX-TP had been excreted.
Only one subject required removal of the OTX-TP on day 1 due to epiphora. This gave a nontolerance rate of only 1/17, meanwhile the low hyperemia scores (0.1 out of 3 for severity, Table 8) are better than the 33% of hyperemia noted on meta-analysis of travoprost clinical trials.11 However, this was a pilot study with relatively small sample size and short duration, and hence, a larger long-term study may need to be conducted to evaluate the patient acceptability, considering that other local side effects such as lash growth and skin discoloration and dry eye are more common after prolonged use of travoprost. The ocular complaints tended to peak at 3 days and disappeared at day 30, at which point only two subjects complained of itching and one had tearing.
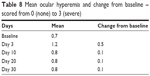  | Table 8 Mean ocular hyperemia and change from baseline – scored from 0 (none) to 3 (severe) |
From previous Phase III studies in the literature, travoprost monotherapy used once at night has been found to lower IOP by 6.5–9.0 mmHg from baseline, with an IOP-lowering effect of 19%–29% at 6 months.11 It is noteworthy that the OTX-TP demonstrated a comparable IOP-lowering effect (24%) at 10 days while all the plugs were still present, with slightly lower IOP reduction at 30 days, most probably related to retention issues. One might expect that diurnal IOP variation from the peaks and troughs seen with topical administration may be smoothed out by the sustained-release pharmacokinetics. At 10 days, the mean IOP (standard error of mean) was 21.2 (1.2), 20.4 (0.8), and 19.7 (1.0) at 8 am, 10 am, and 4 pm, respectively, showing no discernible IOP trend during the course of the day. This study did not reveal any diurnal trend at any time point, showing a similarly narrow diurnal variation throughout the 30 days (Figure 2).
Apart from the sustained IOP-lowering effect, the strengths of the OTX-TP include its atraumatic ease of insertion and paucity of adverse events. However, the uptake and acceptability of this device by patients will depend on physician and patient attitudes toward receiving sustained-release medications in this format, compared to the established method of topical drops. Some patients may have issues with the retention or the placement of punctual plugs for the treatment of dry eye, and so will not be considered as candidates for the use of OTX-TP as a sustained-release device, limiting the applicable population for this plug-based drug delivery system.
The transillumination method to determine plug retention of violet-colored plugs proved to be difficult and probably underestimated plug retention, based on the lack of corresponding IOP increase. Labeling the plug with a more obvious fluorescent dye that persists throughout the treatment period will vastly improve visualization in situ. With this, patients themselves might be able to self-assess the OTX-TP presence. Future work will include increasing the maximum period of drug delivery. Longer durations will require greater drug loading to deliver a comparable elution rate of drug over the extended time course. Retention rate improvement efforts should concentrate on optimization of the OTX-TP dimensions and swelling mechanism. Attention should also be paid to assessing the attitudes and acceptance of such a device among patients with glaucoma.
Our study had several limitations. First, the small sample size and the absence of a control group (eg, topical travoprost nocte) preclude more robust conclusions; however, as a pilot feasibility study, the results were promising. Second, the results may not be generalizable to populations where additional topical therapy such as lubricants or other ocular hypotensives are being used alongside the OTX-TP, due to the potential for altered pharmacokinetics from dilution. This is particularly relevant, as studies have shown that almost 40% of patients require additional therapies to control IOP.10 Finally, the results of this study performed on an Asian population may not be extrapolated across other ethnicities, as there may be differences in eyelid anatomy for insertion and retention or in patients’ responses. The 30-day period of investigation may be too short to elicit potential longer term concerns about harboring a prostaglandin analog continuously within the punctum or even problems relating to multiple punctal insertions.
In summary, this initial feasibility trial of the OTX-TP demonstrated significant IOP reduction of up to 24% from baseline over a 1-month period, combined with good retention and low adverse events. It is a potentially well-tolerable ocular hypotensive for glaucoma patients, especially for those with a history of poor compliance.
Disclosure
The study was supported by Ocular Therapeutix, Bedford, MA, USA. Dr Aung has been a Consultant to Alcon, Allergan, Bausch & Lomb, MSD, and Quark; Dr Paul Chew has received research support from Alcon, Allergan, Aquesys, Carl Zeiss Meditec and Ellex; and travel support/honoraria from Alcon, Allergan, Ellex, MSD, Pfizer, and Santen. Dr Perera has received honoraria from Pfizer and Allergan. The authors report no other conflicts of interest in this work.
References
Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(Suppl 1):S57–S68. | ||
Mansouri K, Iliev ME, Rohrer K, Shaarawy T. “Compliance and knowledge about glaucoma in patients at tertiary glaucoma units.” Int Ophthalmol. 2011;31:369–376. | ||
Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the glaucoma and adherence and persistency study (GAPS). Invest Ophthalmol Vis Sci. 2007;48:5052–5057. | ||
Quek DT, Ong GT, Perera SA, Lamoureux EL, Aung T. Persistence of patients receiving topical glaucoma monotherapy in an Asian population. Arch Ophthalmol. 2011;129:643–648. | ||
Fiscella RG, Lee J, Davis EJ, Walt J. Cost of illness of glaucoma: a critical and systematic review. Pharmacoeconomics. 2009;27:189–198. | ||
Riva I, Katsanos A, Floriani I, et al. Long-term 24-hour intraocular pressure control with travoprost monotherapy in patients with primary open-angle glaucoma. J Glaucoma. 2014;23(8):535–540. | ||
Quaranta L, Riva I, Katsanos A, Floriani I, Centofanti M, Konstas AG. Safety and efficacy of travoprost solution for the treatment of elevated intraocular pressure. Clin Ophthalmol. 2015;9:633–643. | ||
Denis P, Covert D, Realini A. Travoprost in the management of open-angle glaucoma and ocular hypertension. Clin Ophthalmol. 2007;1:11–24. | ||
Anderson DR, Patella VM. Automated Static Perimetry. 2nd ed. Vol 363. St Louis, MO: Mosby; 1999. | ||
Honrubia F, García-Sánchez J, Polo V, de la Casa JM, Soto J. Conjunctival hyperaemia with the use of latanoprost versus other prostaglandin analogs in patients with ocular hypertension or glaucoma: a meta-analysis of randomized clinical trials. Br J Ophthalmol. 2009;93:316–321. | ||
Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




