Back to Journals » Clinical Ophthalmology » Volume 15
Factors Associated with Visual Outcome after Primary Repair of Open-Globe Injury by Ophthalmology Residents in Training in a Tertiary Eye Center
Authors Tirakunwichcha S, Pongsachareonnont P
Received 6 January 2021
Accepted for publication 3 March 2021
Published 23 March 2021 Volume 2021:15 Pages 1173—1181
DOI https://doi.org/10.2147/OPTH.S300753
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Suppapong Tirakunwichcha,1 Pear Pongsachareonnont2
1Ophthalmic Plastic and Reconstructive Surgery Unit, Department of Ophthalmology, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand; 2Vitreoretinal Research Unit, Department of Ophthalmology, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand
Correspondence: Suppapong Tirakunwichcha Email [email protected]
Purpose: To assess factors associated with visual outcome after open-globe injury (OGI) repair by trainees.
Methods: In this observational study, charts of OGIs repaired by trainees at King Chulalongkorn Memorial Hospital, Chulalongkorn University, Bangkok were retrospectively reviewed. Preoperative, intraoperative, and postoperative outcomes (day 1, month 1, and month 6 postoperation) were analyzed.
Results: A total of 78 OGIs presented in a 10-year period. A biphasic pattern was found among the young and the elderly. Approximately 73.6% of the cases had had surgical repair outside office hours. A majority of cases had been caused by machinery and hammers, and had visual acuity (VA) < 20/200. Three cases were reported as having been unsuccessful intraoperatively for globe repair. A fifth of the cases required evisceration/enucleation within 2 weeks of presentation. Presenting VA worse than hand motion was associated with the risk of evisceration/enucleation (OR 14.5, P=0.013). VA improved at 6 months postoperation to the range of counting fingers and 20/200 (OR 15.6, P< 0.01). High ocular trauma scores (OTSs) was associated with lower risk of evisceration/enucleation, and 12% retinal detachment (RD) was discovered, of which 90% occurred within 1 month after OGI repair.
Conclusion: Most OGIs were efficiently managed by the trainees, seldomly requiring assistance from subspecialists. Poor initial VA was associated with high risk of visual loss, whereas higher OTSs were inversely related to lower risk of evisceration or enucleation. There was a higher percentage of participants with final VA of 20/100– 20/20 than the preoperative period. Precaution and careful evaluation of RD in the early postoperative period is recommended.
Keywords: evisceration, traumatic open-globe injury, residency training, ruptured globe
Introduction
Open-globe injury (OGI) is an ocular emergency encountered by general practitioners and ophthalmologists. According to the Birmingham Eye Trauma Terminology system,1 both rupture (inside-out injury) and laceration (outside-in injury) can lead to severe damage and visual impairment. Mir et al reported an incidence of 4.49 per 100,000 population in the US from 2006 to 2014,2 Batur et al 3.4 per 100,000 in eastern Turkey,3 and a large population-based study in Singapore 3.7 per 100,000 population.4
Factors associated with visual outcome after OGI repair are poor initial visual acuity (VA), retinal detachment (RD), relative afferent pupillary defect (RAPD), prolapsed vitreous, young age, blunt-force eye injury, wound size >6 mm, and no initial light reflex.5–7 On some occasions, enucleation or evisceration is necessary to prevent further ocular infection, sympathetic ophthalmia, or unsuccessful primary globe repair. The incidence of complications ranges from 1.7% to 25%.3,4,8,9 Savar et al retrospectively reviewed enucleations at the Massachusetts Eye and Ear Infirmary post-OGI, and found that there were only 20% from primary enucleation. A vast majority were secondary enucleations due to blindness and painful eye conditions.10 Timing of early wound closure and prophylactic antibiotic treatment also played an important role in the development of endophthalmitis following OGI.11,12
In rural and remote areas, there are not many ophthalmologists who repair OGI. Therefore, many cases are transferred to tertiary eye centers. Early primary repair is crucial and affects visual prognosis. In the US, the Accreditation Council for Graduate Medical Education (ACGME) requires ophthalmology residents to perform four globe repairs, which is believed to be an acceptable minimum to become an ophthalmologist.13 In Thailand, the Royal College of Ophthalmologists of Thailand (RCOPT) requires a minimum of three primary globe repairs in an ophthalmology-training program (www.rcopt.org). Our center is located in the capital city of Thailand and surrounded by five other eye centers. In our hospital, ophthalmology trainees are at the forefront and responsible for the management of all OGI cases under closed supervision.
The present study evaluated the success rate and factors associated with visual outcome 6 months after surgical OGI repair by trainees.
Methods
Retrospective charts of all OGIs at King Chulalongkorn Memorial Hospital (KCMH) that had been repaired by ophthalmology trainees during January 2005 to December 2015 were reviewed. Data were searched using ICD10 codes H055, H160, S052, S053, S055, S056, and S059, the operative record listed with the diagnosis of ruptured globe, and penetrating/perforating ocular injuries. Data on sex, age at presentation, cause of injury, time of presentation, day of the week, geographical location where the injury occurred, waiting time for the operation, Snellen VA score at presentation, clinical presentation, operating time, operating complications, surgeon type, and postoperative medications were collected. All OGIs had been repaired by trainees at the tertiary-care hospital under closed supervision of the on-call faculty. Evisceration/enucleation were done by an oculoplastic surgery team if necessary and vitrectomy by a vitreoretinal team. Postoperative visits were scheduled by trainees and on-duty faculty. Clinical results and complications were collected postsurgery at day 1, week 1 (defined as 1–2 weeks postoperation), month 3 (defined as 1–3 months postoperation), and month 6 (defined as 4–6 months postoperation). Successful OGI repair was defined as ocular survival with no need for evisceration and/or enucleation. Secondary outcome was VA at 6-month follow-up. VA was categorized into five groups: 20/20–20/40 (category 0), 20/50–20/100 (category 1), 20/200 to counting fingers (category 2), hand motion to light perception (LP; category 3), and no LP (NLP; category 4).
Patient consent was not required by the institutional review board of the Faculty of Medicine, Chulalongkorn University. No identifying data from patient information was demonstrated, and confidentiality was assured. Medical records were reviewed by two experienced ophthalmologists. The study was registered in the Thai Clinical Trial Registry (TCTR20171025002) and approved by the institutional review board (IRB 059/60). The study was conducted in accordance with the Declaration of Helsinki.
Statistical Analysis
Continuous variables are presented as means ± SD. Categorical variables are presented as numbers and percentages. Fisher’s exact test was used to compare the categorical variables. Mann–Whitney U tests were used to assess continuous variable outcomes. Factors associated with evisceration/enucleation and factors associated with improvement in VA at 6-month follow-up were assessed with binary logistic regression for dichotomous data and multivariate analysis for continuous data. Stata I/C version 11.5 (StataCorp, TX, USA) was used for data analysis. P<0.05 was considered statistically significant.
Results
Medical records of 78 OGIs repaired by trainees under faculty supervision at KCMH from 2005 to 2015 were retrospectively reviewed. Patient age ranged 2–79 years (mean 32.3±15.7). In sum, 68 patients were male (87.2%), 69 patients (93.2%) had no known underlying disease, and the rest had diabetes mellitus or hypertension. Most cases (61, 78.2%) presented outside office hours at the emergency room (ER; Table 1). Most injuries occurred in Bangkok (64%). The number of patients who presented themselves at the ER or were referred to KCMH by primary-care hospitals were comparable. More than half the cases (53.3%) were caused by machinery and hammers, 19.5% had been struck by an object or person, 11.7% had been injured by cutting or piercing, 6.5% by traffic accident, 5.2% by falls, and 3.9% by firearms. Presenting VA of ≤20/200 (category 2–4) was 89.4%, of which 14.7% had NLP. No had bilateral eye injuries, and laterality was nearly equal. Intraocular foreign bodies (IOFBs) were found in 25.6%, and 16.7% of cases had concurrent eyelid lacerations.
 |
Table 1 Baseline demographics and characteristics of open-globe injuries |
Friday was the commonest presenting day (21.6%) of the week, but there was no statistically significant difference among the days (X2, P=0.5; Table 2). The wound was mostly found at the center of the cornea (28.2%), followed by superior (24.4%), temporal (23.1%), nasal (16.7%), and inferior (7.7%). Of the corneal wounds, 71% had prolapsed intraocular contents. The Seidel test was not performed in 62.8%, due to overt findings. The remaining 37.2% were tested, but only 37.9% of these had a positive result. Ocular findings at first examination were irregular pupils (48.7%), hyphema (48.7%), shallow anterior chamber (AC; 53.9%), and various tissue incarcerations (64%). A total of 37 cases had prolapsed uveal tissue, ten prolapsed vitreous, two prolapsed retinae, and one lens prolapse. All cases underwent imaging investigation: 45% plain skull films, 20.5% ophthalmic ultrasound, 17.9% orbital CT scans, and 1.3% orbital MRI.
 |
Table 2 Distribution of case presentations during the week |
Around 73.6% of cases received surgical repair outside office hours, of which 43% was performed from 4 pm to 12 am and 30.6% between 1 am and 8 am. Mean duration from presentation to the operating room was 6 hours, 18 minutes ± 4 hours, 9 minutes. Most cases (93.6%) were operated on under general anesthesia (GA). Only eight cases (10%) had concurrent pars plana vitrectomy when the globe was repaired, and eleven (14%) had pars plana vitrectomy within 1 week. Mean duration of operation time was 1 hour, 58 minutes (range 30 minutes to 10 hours, 9 minutes). Nearly all cases (96.1%) were operated on by second- and third-year trainees, and only 3.9% by first-year resident under close supervision by experienced faculty after consultation and discussion. None of the cases received primary repair from an ophthalmologist as primary surgeon. Six cases needed surgical assistance: four had assistance from retina subspecialists/fellows and two from cornea subspecialists/fellows. Three of 78 cases were reported intraoperatively to be unsalvageable due to severe injuries: two cases had been punched and the other case hit by a jackhammer. Systemic cefazolin was administered to 66 cases (84%), while systemic gentamicin was used in 40 cases (51%) during administration and oral antibiotics continued. Ten cases (12.8%) developed RD, of which nine cases had happened at 1 month postsurgery and one the first 6 months. None had RD after 6 months.
Fourteen of 70 cases (20%) with NPL on day 1 required evisceration/enucleation within 1 or 2 weeks of presentation. At 6 months postoperation, an additional five cases needed procedures. None required evisceration/enucleation after 6 months postsurgery.
Presenting VA of NPL was found in eleven cases (14.7%) and hand motion (HM)-PL in 33 (44%, Table 3). In addition, another three cases with NPL presented on day 1 postsurgery (total 14 of 70 cases, 20%). Primary evisceration/enucleation was performed on NPL eyes on the presenting day if consent had been obtained, and supervised by the oculoplastic team, of which 53% was done during 12 am to 7:59 am, 24% 4 pm to 11:59 pm, and 2% 8 am to 4 pm the next day. Secondary evisceration/enucleation of another three cases required procedures within 1 or 2 weeks, and an additional five cases at 6 months postoperation. However, there was no statistically significant difference between evisceration/enucleation rates and time of operation (during or outside office hours, P=0.21), cause of injury (P=0.57), age (P=0.22), time from admission to operation (P=0.67), day of presentation (P=0.19), or operation duration (P=0.50).
 |
Table 3 Visual acuity alteration during follow-up of open-globe repairs |
On univariate analysis, baseline VA < HM demonstrated a significant association with evisceration/enucleation (OR 8.8, 95% CI 1.9–41.7; P<0.006). Age at every 10-year increment also showed an association with higher risk of evisceration/enucleation (OR 1.6, 95% CI 1.0–2.6; P=0.037). After age adjustment with multivariate analysis, VA < HM remained associated with risk of evisceration/enucleation (OR 14.5, 95% CI 1.7–120.4; P=0.013), whereas increasing age was no longer associated.
Regarding visual improvement and VA, cases of visual improvement (category 0,1) had increased from eight to 20 (29%) at 1 month, followed by 36.1% at 6 months, according to the residual. The baseline VA of ≤ HM(category 3) exhibited a significant improvement to final VA ≤ 20/200 (category 2) at 6 months (OR 15.6, 95% CI 3.5–68.7; P<0.01).
Numbers of follow-up patients decreased over time: 47 of 75 cases (62.7%) came to their 6-month follow-up.
Table 4 shows ocular trauma scores (OTSs) and final VA at 6 months. We found that high OTSs were associated with lower risk of evisceration/enucleation (OR 0.94, 95% CI 0.9–1.0; P=0.0018). In addition, high OTSs were associated with lower risk of VA ≤ 20/200 (categories 2–4) at 6-month follow-up (OR 0.95, 95% CI 0.9–1.0; P=0.034).
 |
Table 4 Ocular trauma scores (OTSs) and final VA after globe repair |
Discussion
OGI is a public health problem resulting in devastating visual damage. Eye protection should be a global issue.14 Prevention with protective eyewear can certainly reduce the numbers of eye injuries, particularly in work-related activities. AlMahmoud et al found that workers who wore safety goggles and safety glasses had less significant eye injuries.15 On the contrary, protective eyewear/goggles can result in good or fair visual outcomes after OGI repair.16 The mean age in the current study was 32.3±15.7 (2–79) years, which indicated that eye injuries were common among those of working age. There was an 87.2% male preponderance, consistent with the previous studies2,3,17,18 reporting mean age of 20.7–48 years and 75.3%–91.9% male. Age at which OGI occurs shows a biphasic pattern: the first peak at age 20–30 years, the second peak at age >70 years.4 This study also found a similar bimodal pattern for OGI; the first peak occurred among people aged 20–40 years, the second among people aged >60 years (Figure 1). According to Wong et al, this finding is likely to be a universal pattern.4 Protection from OGI should be targeted in these two populations. In addition, due to occupational hazard, the vast majority of causes of injuries can be prevented. In Thailand, occupational safety and health are compulsory in all factories and regulated under the Factory Act 1992 and Occupational Safety, Health, and Environment Act 2011 mandating eye protection/goggles made of polycarbonate. The National Occupational Safety and Health (OSH) policy, which is now in the second phase of the National OSH Agenda “Decent Safety and Health for Workers” (2017–2021), together with the draft of the second phase of the National Master Plan on OSH and Environment (2017–2026), is ongoing (www.osh.labour.go.th). The cause of 53.3% of injuries in this study was machinery, with hammers being the most common etiology, corroborating the findings of Ahn et al.17 The second-commonest (19.5%) cause of injury was being struck, which is different from the finding from Mir et al,2 in which being struck was the commonest cause. Other causes of injury were cutting or piercing (11.7%), followed by traffic accidents (6.4%) and falls (5.2%). Gunshot injuries were the least common cause of OGI.
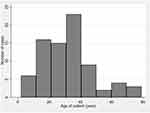 |
Figure 1 Age histogram. |
OGIs often occurred on Friday (21.6%), the beginning of the weekend, which might reflect celebration, parties, alcohol consumption, and traveling. However, Madhusudhan et al reported that 50% of OGIs happened at home and were related to domestic activities. A common age for domestic OGIs was <10 years, while occupational trauma was frequently found at age 16–35 years.19 Children were prone to OGIs at home, and the majority were with a sharp object or a toy gun. Parents should keep an eye on their children at all times to reduce the chance of OGI.6 Occupational and traffic-related injuries were also common in adolescents, which can be prevented with regulations and laws to eliminate unnecessary trauma.
Most patients (78.2%) were injured outside office hours, most on Friday, and promptly received first aid before they were sent to the ER. A retrospective review of OGI at a tertiary referral eye hospital in Australia showed the same pattern.20 However, a 17-year review of OGI in children from Canada found that OGIs were more frequent from Saturday to Monday.21 People tend to have relax and sometimes get careless on Friday nights, and thus are more prone to OGI. For children, they play more or engage in unsafe activities during the weekend, leading to a higher chance of OGI.
Most cases presented with initial VA ≤20/200 (89.4%, category 2–4), and 14.7% had calamities with NLP. Only 37.2% had Seidel tests performed, which contributed to overt ocular findings with flat AC (53.9%) and prolapsed intraocular tissue (71%). All cases were promptly scanned for IOFBs using various imaging methods, with 25.6% positive findings. The orbital CT scan has now become the most valuable technique in ocular trauma, and MRI should be avoided in eyes suspected of having metallic FBs.22 IOFBs can affect final VA, since the nature of injury, toxicity, and risk of endophthalmitis are often from lethal weapons. In this study, trainees performed primary wound closure and AC IOFB removal, except for the posterior segment IOFB case, which was later managed by retinal subspecialists. We found a nine-case (14.3%) increase in NPL cases from day 1 to 1 month after presentation. The trainees were able to successfully repair 85.7% of OGIs without evisceration/enucleation. Guven showed that higher OTS (category 4,5) score can better predict final VA than lower categories (1–3).23 We also found that high OTS was associated with lower risk of evisceration/enucleation and VA worse than 20/200. However, the limitations of OTSs were lack of initial baseline VA and RAPD, because these measures were difficult to obtain during an acute-trauma situation.12,21
OG repair should be performed within 12 hours of injury to prevent the risk of endophthalmitis (OR 1.013, 95% CI 1.002–1.024; P=0.02).24 Treatment given as early as possible is extremely important. Elapsed time of >12 hours and prolapsed intraocular tissue are risk factors of visual loss.25 In this study, 73.6% of cases were operated on between 4 pm and 8 am, since 93.6% of cases needed general anesthesia, and operating rooms or anesthesiologists were occupied. The mean waiting time from presentation to globe repair in the current study was 6 hours, 18 minutes ± 4 hours, 9 minutes, which was short and in line with Guven’s study.23 This represented all processes of presentation, examination, investigation, operating room preparation, admission, and operation being within the “golden” period. There was no endophthalmitis found. Blanch et al26 reported mean time from OGI to surgery of 24.2 hours (range 5 hours to 7 days) and du Toit et al 80.14 hours (range 5 hours to 51 days),27 especially in rural areas. Fu et al, found that OGI patients in rural areas had a longer time from injury to presentation and higher risk of IOFBs when referred to tertiary-care hospitals.28 Prophylactic antibiotic treatment for 48 hours helps to reduce posttraumatic endophthalmitis.12
Most cases (96.1%) were repaired by second- and third-year trainees after consultation and under the close supervision of experienced on-duty faculty. Third-year trainees mostly performed as primary surgeons and second year trainees as assistants. The training program for first-year residents included helping within patients’ eye examinations, preparing for operations, and observing surgeries. In contrast, Rodrigues et al showed that 42.5% of trainees in the UK had never repaired corneal laceration and 60% had never repaired a ruptured eyeball.29 In the US, residents are required to have done four globe repairs by the ACGME.13 Average eyeball repairs from 2009 to 2015 numbered 7.2–7.6 cases per year.30 A well-prepared training program that increases the trainee’s competence in performing surgery and the result of surgery being dependent on the trainee’s performance is important. Zafar et al stated that preoperative case preparation with faculty discussion led to higher levels of self-perceived preparedness and competence.31 According to a recommendation of the RCOPT, each trainee should perform a minimum of three cases of globe repair as primary surgeon. Our center showed an average of eight cases that needed amendment per year, whereas there were ten trainees each year, which number was lower to mandatory. However, this particular number represented just one center, and did not reflect all residents performing in Thailand, in contrast to other studies. Another reason is our institution is located in the center of the capital, and there are another five large university hospitals and more than ten ophthalmology services nearby. Moreover, the population in the city are highly educated, white-collar workers with fewer occupational trauma incidents than those in rural or remote areas, where factories are based. However, there is an elective program in affiliated hospitals to enhance surgical skills for our trainees each year.
Mean operation time by trainees was 1 hour, 58 minutes (range 30 minutes to 10 hours, 9 minutes) which was acceptable. Evisceration/enucleation was performed in 14 cases (20%) in badly damaged and irreparable eyes compared to 20% in Savar et al,10 of which 77% were operated on before office hours next day. At 1-month follow-up, 20 of 69 cases (29%) were blind, 19 had received evisceration/enucleation, and one denied. However, Soni et al reported that in 23% NLP cases, VA recovered to LP-20/100.32 The current study found 12.8% RDs after OGI repair, of which 90% occurred within the first month. Stryjewski et al created the RD-OGI score to monitor RD after OGI repair. The RD-OGI score is based on three clinical findings: baseline VA, zone of injury, and vitreous hemorrhage. High RD-OGI scores are associated with RD by 30 days after surgery.33,34 Early diagnosis of RD after OGI repair can be diagnosed with B-scan ultrasonography, and we recommended this on the first day after the operation. Prognosis and risk of RD should be discussed in depth with the OGI patients, and B-scan ultrasound should be done as early as possible, especially within 1 month for patients who could not get fundus-examination results.
Initial VA was one of the important predictive factors of final VA outcome. Lee et al found that the initial VA < LP and history of golf-ball injury were critical risk factors associated with visual loss.25 Rahman et al reported that initial VA < 6/60, blunt-force injury, presence of RAPD, and lid laceration were significant risk factors associated with enucleation.8 The current study found that presenting VA < HM was significantly associated with evisceration/enucleation (OR 8.8, 95% CI 1.9–41.7; P<0.006). With age adjustment in the multivariate analysis, it showed fourteenfold the likelihood of evisceration/enucleation (OR 14.5, 95% CI 1.7–120.4; P=0.013); however, the trainees successfully repaired 85.7% of the OGIs without eyeball evacuation. On the contrary, high OTSs indicated a lower risk of evisceration/enucleation (OR 0.94, 95% CI 0.9–1.0; P=0.0018).
Blanch et al showed that final VA at 6–12 months was related to initial VA, OTS, and elapsed time from injury to surgery.26 Agrawal et al revealed that age, mode of injury, and time lag before primary repair had statistically significant outcomes in final VA.35 Fujikawa et al also stated that poor initial VA, ruptured eyeball, RD, VH, zone 3 injury, history of penetrating keratoplasty, and lens dislocation had poor prognosis.36 Gupta et al found that presence of RAPD, worse initial VA, ocular adnexal injury, IOFBs, and VH were associated with poor final VA on multivariate analysis.18 According to the CART (classification and regression tree) model, final visual outcome is dependent on the presence of RAPD, initial VA (20/20-HM), lid laceration, and wound location.37 The predicted final VA logMAR reduced by 0.37 for every 24 hours of delayed amendment.26
Regarding visual improvement and VA category, visual improvement (category 0,1) increased from 10.7% (eight of 75 cases) at preoperation to 29% (20 of 69 cases) at 1 month, followed by 36.1% at 6 months. Baseline VA ≤ HM category (category 3), which was associated with evisceration/enucleation, nevertheless exhibited a significant improvement to final VA ≤20/200 (category 2) at 6 months (OR 15.6, 95% CI 3.5–68.7; P<0.01). Follow-up cases being reduced to 60% might have been caused by back-referral, traveling problems, daily life expenses in the city, and acceptance of final VA by patients who did not need many visits in uncomplicated cases. Limitation of the present study is its retrospective design, the fact that it was conducted at one referral tertiary eye-care center, and data gathering being definitely not perfect. Further studies should be considered in collaboration with all referral tertiary eye centers in all subspecialty training.
Conclusion
All OGI cases were treated by ophthalmology trainees at KCMH tertiary hospital. Elapsed time prior to surgery was comparable to other studies. Most cases were efficiently managed by the trainees with good results, seldom needing assistance from the subspecialists. Poor initial VA < HM showed a high risk of visual loss. High OTS was negatively correlated with evisceration or enucleation. The final visual outcome for VA category 20/100-20/20 tended to be high compared to the preoperative period. Insufficient OGI cases should reflect planning of training programs in different areas to establish a qualified ophthalmologist in order to serve the community. Special health policies should target the young and the elderly to prevent OGIs due to their bimodal pattern in terms of age.
Data Sharing Statement
The data sets collected, generated, and/or analyzed during the current study are not publicly available, but data are available from the corresponding author on reasonable request.
Disclosure
Both authors report no conflicts of interest for this work.
References
1. Kuhn F, Morris R, Witherspoon CD. Birmingham Eye Trauma Terminology (BETT): terminology and classification of mechanical eye injuries. Ophthalmol Clin North Am. 2002;15(2):139–143. doi:10.1016/S0896-1549(02)00004-4
2. Mir TA, Canner JK, Zafar S, et al. Characteristics of open globe injuries in the United States From 2006 to 2014. JAMA Ophthalmol. 2020;138(3):268–275. doi:10.1001/jamaophthalmol.2019.5823
3. Batur M, Seven E, Esmer O, et al. Epidemiology of adult open globe injury. J Craniofac Surg. 2016;27(7):1636–1641. doi:10.1097/SCS.0000000000003001
4. Wong TY, Tielsch JM. A population-based study on the incidence of severe ocular trauma in Singapore. Am J Ophthalmol. 1999;128(3):345–351. doi:10.1016/S0002-9394(99)00167-1
5. Tök OY, Tok L, Eraslan E, et al. Prognostic factors influencing final visual acuity in open globe injuries. J Trauma. 2011;71(6):1794–1800. doi:10.1097/TA.0b013e31822b46af
6. Gupta A, Rahman I, Leatherbarrow B. Open globe injuries in children: factors predictive of a poor final visual acuity. Eye (Lond). 2009;23(3):621–625. doi:10.1038/eye.2008.32
7. Acar U, Yildiz EH, Acar DE, et al. Posttraumatic intraocular pressure elevation and associated factors in patients with zone I open globe injuries. Ulus Travma Acil Cerrahi Derg. 2013;19(2):115–118. doi:10.5505/tjtes.2013.51437
8. Rahman I, Maino A, Devadason D, et al. Open globe injuries: factors predictive of poor outcome. Eye (Lond). 2006;20(12):1336–1341. doi:10.1038/sj.eye.6702099
9. Knyazer B, Bilenko N, Levy J, et al. Open globe eye injury characteristics and prognostic factors in southern Israel: a retrospective epidemiologic review of 10 years experience. Isr Med Assoc J. 2013;15(3):158–162.
10. Savar A, Andreoli MT, Kloek CE, et al. Enucleation for open globe injury. Am J Ophthalmol. 2009;147(4):595–600.e1. doi:10.1016/j.ajo.2008.10.017
11. Ahmed Y, Schimel AM, Pathengay A, et al. Endophthalmitis following open-globe injuries. Eye (Lond). 2012;26(2):212–217. doi:10.1038/eye.2011.313
12. Andreoli CM, Andreoli MT, Kloek CE, et al. Low rate of endophthalmitis in a large series of open globe injuries. Am J Ophthalmol. 2009;147(4):601–608.e2. doi:10.1016/j.ajo.2008.10.023
13. ACGME. Ophthalmology Case Log Information. 2021.
14. Colyer MH. Open-globe injuries: a global issue of protection. Clin Exp Ophthalmol. 2019;47(4):437–438. doi:10.1111/ceo.13562
15. AlMahmoud T, Elkonaisi I, Grivna M, et al. Eye injuries and related risk factors among workers in small-scale industrial enterprises. Ophthalmic Epidemiol. 2020;27(6):453–459. doi:10.1080/09286586.2020.1770302
16. Vasu U, Vasnaik A, Battu RR, et al. Occupational open globe injuries. Indian J Ophthalmol. 2001;49(1):43–47.
17. Ahn JY, Ryoo HW, Park JB, et al. Epidemiologic characteristics of work-related eye injuries and risk factors associated with severe eye injuries: a registry-based multicentre study. Ophthalmic Epidemiol. 2020;27(2):105–114. doi:10.1080/09286586.2019.1683868
18. Gupta R, Gupta S, Chauhan L. Predicting visual outcome after open globe injury using classification and regression tree model: the Moradabad ocular trauma study. Can J Ophthalmol. 2019;54(4):473–478. doi:10.1016/j.jcjo.2018.08.004
19. Madhusudhan ALP, Evelyn-Tai L, Zamri N, et al. Open globe injury in Hospital Universiti Sains Malaysia - a 10-year review. Int J Ophthalmol. 2014;7(3):486–490. doi:10.3980/j.issn.2222-3959.2014.03.18
20. Beshay N, Keay L, Dunn H, et al. The epidemiology of open globe injuries presenting to a tertiary referral eye hospital in Australia. Injury. 2017;48(7):1348–1354. doi:10.1016/j.injury.2017.04.035
21. Bunting H, Stephens D, Mireskandari K. Prediction of visual outcomes after open globe injury in children: a 17-year Canadian experience. J AAPOS. 2013;17(1):43–48. doi:10.1016/j.jaapos.2012.10.012
22. Parke DW
23. Guven S. Verification of ocular trauma score for intraocular foreign bodies in lethal-weapon-related ocular injuries. Mil Med. 2020;185(7–8):e1101–e1105. doi:10.1093/milmed/usaa042
24. Essex RW, Yi Q, Charles PG, et al. Post-traumatic endophthalmitis. Ophthalmology. 2004;111(11):2015–2022. doi:10.1016/j.ophtha.2003.09.041
25. Lee SH, Ahn JK. Emergent risk factors associated with eyeball loss and ambulatory vision loss after globe injuries. J Trauma. 2010;69(1):195–198. doi:10.1097/TA.0b013e3181bbd23b
26. Blanch RJ, Jonathan B, Javidi H, et al. Effect of time to primary repair on final visual outcome after open globe injury. Br J Ophthalmol. 2019;103(10):1491–1494. doi:10.1136/bjophthalmol-2017-311559
27. Du Toit N, Motala MI, Richards J, et al. The risk of sympathetic ophthalmia following evisceration for penetrating eye injuries at Groote Schuur Hospital. Br J Ophthalmol. 2008;92(1):61–63. doi:10.1136/bjo.2007.120600
28. Fu R, Kancherla S, Eller AW, et al. Characteristics and outcomes of open globe trauma in the urban versus rural population: a single center retrospective review. Semin Ophthalmol. 2018;33(4):566–570. doi:10.1080/08820538.2017.1340488
29. Rodrigues IA, Symes RJ, Stephen T, et al. Ophthalmic surgical training following modernising medical careers: regional variation in experience across the UK. BMJ Open. 2013;3(5):e002578. doi:10.1136/bmjopen-2013-002578
30. Chadha N, Liu J, Maslin JS, et al. Trends in ophthalmology resident surgical experience from 2009 to 2015. Clin Ophthalmol. 2016;10:1205–1208. doi:10.2147/OPTH.S106293
31. Zafar S, Chen X, Woreta F, et al. Self-perceived preparedness and competence among ophthalmology residents for open globe repair. Clin Ophthalmol. 2019;13:1273–1278. doi:10.2147/OPTH.S211144
32. Soni NG, Bauza AM, Son JH, et al. Open globe ocular trauma: functional outcome of eyes with no light perception at initial presentation. Retina. 2013;33(2):380–386. doi:10.1097/IAE.0b013e318263cefb
33. Stryjewski TP, Andreoli CM, Eliott D. Retinal detachment after open globe injury. Ophthalmology. 2014;121(1):327–333. doi:10.1016/j.ophtha.2013.06.045
34. Brodowska K, Stryjewski TP, Papavasileiou E, et al. Validation of the retinal detachment after open globe injury (RD-OGI) score as an effective tool for predicting retinal detachment. Ophthalmology. 2017;124(5):674–678. doi:10.1016/j.ophtha.2016.12.032
35. Agrawal R, Rao G, Naigaonkar R, et al. Prognostic factors for vision outcome after surgical repair of open globe injuries. Indian J Ophthalmol. 2011;59(6):465–470. doi:10.4103/0301-4738.86314
36. Fujikawa A, Mohamed YH, Kinoshita H, et al. Visual outcomes and prognostic factors in open-globe injuries. BMC Ophthalmol. 2018;18(1):138. doi:10.1186/s12886-018-0804-4
37. Schmidt GW, Broman AT, Hindman HB, et al. Vision survival after open globe injury predicted by classification and regression tree analysis. Ophthalmology. 2008;115(1):202–209. doi:10.1016/j.ophtha.2007.04.008
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
