Back to Journals » OncoTargets and Therapy » Volume 12
Extracellular Hsp90α clinically correlates with tumor malignancy and promotes migration and invasion in esophageal squamous cell carcinoma
Authors Wang X, An D, Wang X, Liu X, Li B
Received 22 November 2018
Accepted for publication 15 January 2019
Published 11 February 2019 Volume 2019:12 Pages 1119—1128
DOI https://doi.org/10.2147/OTT.S195529
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
This paper has been retracted.
Xintong Wang, 1 Dianzheng An, 1 Xinlei Wang, 2 Xiaomeng Liu, 3 Baosheng Li 1
1Department of Radiation Oncology, Shandong Cancer Hospital Affiliated to Shandong University, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Gastroenterology, Qingdao Hiser Medical Center, Qingdao, Shandong, People’s Republic of China; 3University of Jinan, School of Medicine and Life Sciences, Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China
Purpose: Extracellular Hsp90α (eHsp90α) is known to be involved in tumor invasiveness and metastasis, and its prognostic value in many kinds of tumors has been identified. We aimed to evaluate the clinical and functional role of eHsp90α in esophageal squamous cell carcinoma (ESCC).
Patients and methods: A total of 193 patients with newly diagnosed ESCC were retrospectively evaluated. The relationship between serum Hsp90α levels before treatment and ESCC malignancy of the patients was analyzed. To test the role of eHsp90α in migration and invasion of ESCC cell lines, transwell assay was performed. Western blotting was used to explore the possible mechanism in which eHsp90α promotes ESCC migration and invasion.
Results: We found that the serum Hsp90α level before treatment is positively correlated with ESCC malignancy. Moreover, high serum Hsp90α level before treatment was significantly correlated with positive lymph node (LN) metastasis, which is the main prognostic factor for ESCC patients. Meanwhile, we demonstrated that eHsp90α promoted migration and invasion of ECA109 and ECA9706 in vitro. Further investigations revealed that eHsp90α stabilized MMP-2 and promoted epithelial-to-mesenchymal transition evidenced by downregulation of E-cadherin and upregulation of N-cadherin. On the other hand, Hsp90α neutralizing antibody functionally blocked the secreted Hsp90α and reversed those effects.
Conclusion: Our findings prove the critical role of eHsp90α in promoting ESCC migration and invasion, indicating it can be not only a promising predictor for ESCC LN status, but also an effective target in ESCC therapeutics, especially in preventing LN metastasis.
Keywords: extracellular Hsp90α, esophageal squamous cell carcinoma, lymph node metastasis, migration, invasion, MMP-2
Introduction
Esophageal carcinoma is the eighth most common cancer and the sixth leading cause of cancer death in the world.1 In Asian countries, the predominant histological type is esophageal squamous cell carcinoma (ESCC), which has a high risk of lymph node (LN) metastasis.2 It is well known that LN metastasis is an important prognostic factor for patients with ESCC, and regional LNs have been the most common initial site of ESCC recurrence.3 Therefore, current efforts have been focused on the development of clinically relevant biomarkers that predict LN status in ESCC patients.
Hsp90 is a molecular chaperone that assists the conformational maturation, folding, and refolding of client proteins during stress and protects them from degradation.4 It is exploited by cancer cells to support activated oncoproteins that are essential for oncogenic transformation. A previous study showed that Hsp90 is abundantly expressed in esophageal cancer as well as in esophageal cancer cell lines.5 Hsp90α is one of the isoforms of Hsp90, which can also be secreted to the extracellular space.6 It may be found either in a secreted form or on the cell surface, both forms are detected in diverse tumor types.7 Despite the fact that the exact mechanism of Hsp90α secretion is not completely understood, some parts of the process have been elucidated. PKA-dependent phosphorylation of the Thr-90 residue, along with cleavage of the EEVD motif from the C-terminal tetratricopeptide repeat domain, leads to Hsp90α secretion.8 Nowadays, the importance of eHsp90α for tumor cell migration and invasion has been recognized. Furthermore, Hsp90α can be detected in the blood of cancer patients, and the serum Hsp90α level is positively associated with tumor malignancy, especially regional and distant metastasis.9 Lots of encouraging results in multiple malignant tumors have demonstrated that eHsp90α may be a useful diagnostic and prognostic biomarker to assess disease status and predict outcome.8
eHsp90α interacts with extracellular clients10,11 and surface receptors8,12 to promote cell migration and invasion in cancer. MMP-2 is a key protein involved in cancer invasiveness and metastasis. Several studies demonstrated that Hsp90α secreted by tumor cells interacts with and promotes the activity of MMP-2,11,13–15 thus enhancing tumor cells’ invasiveness and metastasis. In addition, increased migration and invasion is a characteristic of cells that have undergone epithelial-to-mesenchymal transition (EMT). It is one of the most important processes through which tumor cells acquire the ability of migration and invasion. Tumor surface Hsp90α correlates with elevated expression of several key drivers of EMT.16 However, no reports about eHsp90α affecting ESCC migration and invasion through regulating MMP-2 or EMT have come out yet.
Despite its growing importance, the role of eHsp90α in migration and invasion of ESCC remains largely undefined. In this report, we evaluated the relationship between serum Hsp90α level before treatment and ESCC malignancy as well as LN status. We also explored the effect of eHsp90α on migration and invasion in vitro and preliminarily investigated the related mechanism.
Patients and methods
Patients
The medical records of 193 patients with newly diagnosed ESCC who underwent esophagectomy at Shandong Cancer Hospital between May 2015 and February 2018 were retrospectively reviewed in this study. Tumor staging was determined according to the 7th edition of the International Union Against Cancer tumor node metastasis system (TNM) classification. Clinical data of the patients including age, gender, tumor length, tumor location, tumor differentiation, TNM stage, and serum Hsp90α level before treatment were recorded. The cut-off value of Hsp90α was defined as 82.06 ng/mL, according to the 95% CIs of cancer-free Chinese patients. All resected specimens were submitted for pathologic examination. The pathologists examined all slides to evaluate the depth of the primary tumors and node involvement. This study complied with the standards of current ethical guidelines and was approved by the Institutional Ethics Committee of Shandong Cancer Hospital. All subjects included in the study reviewed the study protocol and gave written informed consent to participate in the study.
Cell lines and cell culture
ECA109 and ECA9706 were purchased commercially from American Type Culture Collection (ATCC) (Manassas, VA, USA). Both of the two cell lines were cultured in DMEM (Hyclone, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS, (Hyclone, Thermo Fisher Scientific), 100 U/mL penicillin G, and streptomycin (Invitrogen, Thermo Fisher Scientific) in a 37°C incubator with humidified atmosphere and 5% CO2.
Reagents and antibodies
Recombinant Hsp90α protein (rHsp90α) was purchased from Abnova (P3387; Taipei, Taiwan). Anti-Hsp90α neutralizing antibody (Hsp90α Ab, ADI-SPS-771-F) was purchased from Enzo Life Sciences, New York, NY, USA.
Antibodies used for Western blotting were anti-Hsp90α rabbit monoclonal antibody, anti-MMP-2 rabbit monoclonal antibody, anti-E-Cadherin rabbit monoclonal antibody, anti-N-Cadherin rabbit monoclonal antibody, and anti-β-actin rabbit monoclonal antibody (Cell Signaling Technology, Beverly, MA, USA).
Cell migration and invasion assays
Cell migration assay was performed by transwell chambers using 24-well plates with 8 μm pores (Corning Incorporated, Corning, NY, USA). ECA109 and ECA9706 cells were starved in the serum-free DMEM for 12 hours, 1×105 cells were seeded in the upper chamber after being resuspended in serum-free medium. The lower chambers were filled with DMEM plus 10% FBS, either rHsp90α (0 μg/mL, 5 μg/mL, 10 μg/mL), or control IgG (10 μg/mL), Hsp90α Ab (5 μg/mL, 10 μg/mL) was added in. After 12 hours of incubation at 37°C, the cells that had migrated through the insert were fixed with 100% methanol and stained with 0.1% crystal violet. Ten random fields for each membrane were counted. The experimental procedures of invasion assay were similar to the migration assay except that the membrane was coated with Matrigel (Corning Incorporated), 2×105 cells were seeded in the upper chamber and the time of incubation extended to 24 hours.
Protein extraction and Western blotting
ECA109 and ECA9706 cells were plated in 6 cm dishes. When the cells grew to 80% confluence, the media were replaced with fresh medium without FBS. The conditioned media (CM) from serum-free cultures were collected and concentrated 10-fold through a Millipore Amicon Ultra-15 (30K) column and analyzed for protein concentration. Cells treated with rHsp90α (10 μg/mL), control IgG (10 μg/mL) or Hsp90α Ab (10 μg/mL) were lysed with cold RIPA buffer. Total cell lysate (TCL) were centrifuged at 12,000× g for 15 minutes at 4°C, and total protein concentration was determined using the BCA Protein Assay Kit (Beyotime, Shanghai, People’s Republic of China). CM and cell extract samples were electrophoresed through 10% SDS polyacrylamide gels under denaturing conditions and transferred to PVDF membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked in 5% non-fat milk that was dissolved with 1× TBST, and incubated with corresponding primary antibodies at 4°C overnight. Membranes were subsequently washed in 1× TBST and were incubated with secondary antibodies for 1 hour at room temperature. Specific antigen-antibody interactions were detected with enhanced chemiluminescence.
Statistical analysis
Statistical Package for Social Sciences software (SPSS Version 22.0) was used for all statistical analysis. Categorical variables were compared using chi-squared or Fisher’s exact tests, continuous variables were compared using independent sample Student’s t-test. Logistic regression analysis was used to evaluate the association between clinical variables and LN status. For the experiments related to cells, the data were shown as mean ± SD and three individual experiments were performed in triplicate. Statistical significance was assessed by Student’s t-test for two-group comparison. Significance was defined as P<0.05.
Results
Serum Hsp90α level before treatment was positively correlated with ESCC malignancy
LN metastasis is an important prognostic factor for patients with ESCC. Therefore, the clinicians are paying more and more attention to evaluating the status of LNs in ESCC patients. The preoperative serum Hsp90α levels of ESCC patients were analyzed in the study. Levels above the cut-off value (82.06 ng/mL) were defined as high, while those below the value were defined as low. There were significant differences in T stage (P=0.021), N stage (P=0.011), and clinical stage (P=0.016) between the two groups (Table 1). The proportion of patients with positive LN metastasis in high and low Hsp90α groups was 53.7% and 36.9%, respectively. The low Hsp90α group tended to have earlier T stage and N stage compared with high Hsp90α group.
  | Table 1 Clinical characteristics of the ESCC patients according to baseline serum Hsp90α level |
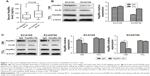
By dividing the patients into LN negative group and LN positive group, the relationship between LN status and clinicopathological characteristics as well as serum Hsp90α level was shown in Table 2. As shown in the table, tumor length (P=0.012), T stage (P=0.004), and serum Hsp90α level (P=0.021) were significantly different between the two groups. The serum Hsp90α levels before treatment of ESCC patients with positive LN metastasis were significantly higher than that of LN negative group patients (Figure 1A). Furthermore, by logistic regression analysis (Table 3), positive LN metastasis was significantly associated with T stage (P=0.037, OR =0.859, 95% CI for OR =0.311–2.374) and the preoperative serum Hsp90α level (P=0.027, OR =0.276, 95% CI for OR =0.091–0.833). Thus, ESCC patients with advanced T stage and higher serum Hsp90α level were more likely to have LN involvement. These results demonstrated that high serum Hsp90α level before treatment was significantly correlated with positive LN metastasis in ESCC and may serve as an independent predictor for ESCC LN status.
  | Table 2 Correlation between clinicopathological characteristics as well as serum Hsp90α level and lymph node status |
  | Table 3 Multivariate analysis of the clinicopathological factors related to lymph node status |
ESCC cells secreted Hsp90α and the secreted Hsp90α could be functionally blocked by Hsp90α Ab
Recent studies indicated that the secretion of Hsp90α was elevated in malignant tumor cells.17 What is more, secreted Hsp90α has been identified as a widespread regulator of cancer cell motility, invasion, and metastasis.7 To confirm the function of eHsp90α in ESCC cell lines, the secretion of Hsp90α from ECA109 and ECA9706 was examined first. Without any stimulation, we found that both of the two cell lines secreted a certain level of Hsp90α (Figure 1B), and the cytosolic Hsp90α levels of the two cell lines were almost the same. The result confirmed that Hsp90α was being secreted in ECA109 and ECA9706 cells, therefore validating the utility of the two cell models. Subsequently, the amount of Hsp90α in CM and TCL from ECA109 and ECA9706 was analyzed when treated with IgG control or Hsp90α Ab. As shown in Figure 1C, the amount of Hsp90α in CM from the two cell lines was remarkably decreased (P<0.001). However, Hsp90α Ab had little effect on the expression of intracellular Hsp90α.
rHsp90α promoted the migration and invasion of ESCC cells and blockage of secreted Hsp90α inhibited this function
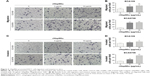
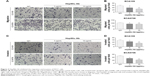
Tumor cells have managed to constitutively secrete Hsp90α during invasion and metastasis.18 In this study, transwell migration and invasion assays were used to detect the biological function of rHsp90α and Hsp90α Ab in ESCC cells. We set two concentrations of rHsp90α and Hsp90α Ab representatively. The numbers of migrated cells (Figure 2A and B) and invaded cells (Figure 2C and D) treated with rHsp90α (5 μg/mL or 10 μg/mL) were significantly higher than those of the negative control (P<0.05, P<0.01). Consequently, we used Hsp90α Ab to functionally block eHsp90α, and found that the numbers of migrated and invaded cells of the Hsp90α Ab treated group were significantly smaller than IgG control group (Figure 3, P<0.01, P<0.001). All the data demonstrated that eHsp90α promoted migration and invasion in ESCC cell lines, and the blockage of secreted Hsp90α can efficiently suppress this function.
eHsp90α stabilized MMP-2 and induced molecular changes consistent with EMT in ESCC cells
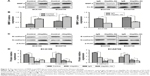
MMP-2 is one of the extracellular client proteins of eHsp90α. Hsp90α secreted by tumor cells can interact with and facilitate the activation of MMP-2, thus promoting tumor invasiveness.19 We asked whether secreted Hsp90α could play the same role in ESCC cells. To address this tissue, MMP-2 expression in the CM of ECA109 and ECA9706 treated with 10 μg/mL rHsp90α, control IgG or Hsp90α Ab was analyzed. As expected, the amount of MMP-2 was increased upon treatment with rHsp90α. However, Hsp90α Ab reversed the effect. The amount of MMP-2 in CM of the two ESCC cell lines decreased (Figure 4A and B). Our results indicated that the secretion of Hsp90α can stabilize MMP-2, which subsequently promoted ESCC cells’ migration and invasion.
To our knowledge, EMT status can be assessed by monitoring molecular markers such as a loss of E-cadherin expression and increased expression of N-cadherin.20 E-cadherin acts as a gatekeeper in suppressing EMT events. Loss of E-cadherin function is a fundamental feature associated with early EMT events.21 To explore the expression of these key proteins involved in EMT events, Western blotting was performed on the extracts of ECA109 and ECA9706 cells, following 24-hour exposure to either 10 μg/mL control IgG or rHsp90α, or Hsp90α Ab. We found that treatment of cells with rHsp90α induced reduction of E-cadherin and increased the expression of N-cadherin compared to control in both cell lines. Conversely, Hsp90α Ab caused an increase in the expression of E-cadherin and decreased the level of N-cadherin compared with control IgG treatment group (Figure 4C and D). The results indicated that treatment of ECA109 and ECA9706 cells with rHsp90α consistently elicited EMT-like events, thereby promoting the migration and invasion of ESCC cells.
Discussion
Nowadays, the role of eHsp90α as a widespread regulator of cancer cell motility, invasion, and metastasis has been recognized. The results of our study demonstrated the potent ability of eHsp90α as a reliable clinically relevant biomarker to predict LN status in ESCC. Meanwhile, eHsp90α promoted migration and invasion in ESCC in vitro. All the data indicate the potential of eHsp90α as a drug target to inhibit ESCC progression, especially LN metastasis.
The presence of LN metastasis is common and has a critical impact on the prognosis of patients with ESCC.2 An interesting feature of our study is the existence of a statistically significant (P=0.027) correlation between high serum Hsp90α level and positive LN metastasis. The serum Hsp90α levels before treatment of ESCC patients with positive LN metastasis were significantly higher (P=0.021) than that of LN negative group. The finding is consistent with a previous study, the serum Hsp90α levels in liver or breast tumor patients with metastasis were much higher than that of patients without metastasis.9 Thus the ESCC patients with elevated serum Hsp90α level before treatment were more likely to have LN involvement. Of note, serum eHsp90α levels were significantly higher in patients with tumor burden, and positively correlated with tumor malignancy and metastasis.9 Shi et al found that elevated serum Hsp90α levels in lung cancer patients were significantly correlated with more advanced disease stage as well as disease progression.22 In this report, we also found that serum Hsp90α level before treatment was positively correlated with ESCC malignancy. The results of these studies indicate that serum Hsp90α level may be a useful diagnostic and prognostic biomarker to assess disease status and predict outcome. Therefore, eHsp90α may have potential as a clinical biomarker because of its preferential secretion in cancer cells and the ability to noninvasively assay eHsp90α levels performed on serum derived from routine blood draws. To further determine the value of eHsp90α in improving early cancer detection and disease progression, testing the association of serum Hsp90α level with clinicopathological characteristics in additional cancer types including ESCC will be an important strategy.
Accumulating evidence indicates that eHsp90α plays an important role in the regulation of tumor invasiveness and metastasis, central processes associated with cancer progression.19,23,24 The secretion of Hsp90α is essential for its invasiveness function and blocking its secretion results in significant inhibition of tumor metastasis.9 In the present study, we confirmed that both ECA109 and ECA9706 cells could secrete a certain level of Hsp90α, and the secretion of Hsp90α was inhibited upon treatment with Hsp90α Ab. Interestingly, the expression of intracellular Hsp90α had little change. Therefore, the drugs that selectively target eHsp90α would not interfere with the important intracellular functions of Hsp90α. This selectivity may, in turn, enable treatment regimens with reduced target-related toxicity. Moreover, the migration and invasion of ECA109 and ECA9706 were increased upon treatment with rHsp90α, while Hsp90α Ab showed an obvious inhibitory effect. This is consistent with the opinion that eHsp90α plays a critical role in activating precursor proteins that contribute to cellular migration and invasion.15 Accordingly, inhibition of eHsp90α could simultaneously disrupt multiple signaling pathways that are responsible for increasing tumor cell movement also making eHsp90α an attractive target for drug therapy to limit ESCC cells’ migration and invasion. Therefore, further investigation can perhaps be extended to the design of delivery systems providing eHsp90α-specific targeting and delivery.
Besides, eHsp90α is involved in the activation of several proteases known to modify the extracellular matrix (ECM).10,11,15 MMP-2 is a key protease involved in ECM degradation and is critical for cancer cells invading from primary sites to adjacent tissues and could break down the tissue barriers to invasion and metastasis.25 Here we showed that eHsp90α promoted the migration and invasion of ESCC cells along with increasing the expression of MMP-2. This observation is consistent with a previous study which showed that Hsp90α secreted by fibrosarcoma tumor cells could promote tumor invasiveness by interacting with MMP-2 and then facilitated the maturation of MMP-2.19 Song et al also revealed that eHsp90α stabilized MMP-2 and protected it from degradation in vitro and in vivo. Noticeably, this stabilization effect is isoform-specific.14 It is interesting to hypothesize that eHsp90α could activate a series of proteins. These proteins would act in concert to break down and remodel the ECM and permit cancer cells to invade its microenvironment. Therefore, eHsp90α inhibition may provide an approach for the simultaneous targeting of these proteins thereby significantly inhibiting ESCC progression.
It is well known that EMT correlates with increased metastatic potential and poor prognosis.26 Our data showed that eHsp90 suppressed E-cadherin, while it increased the mesenchymal protein N-cadherin. The molecular and morphological changes are consistent with an EMT,27 thus promoting ESCC cell migration, invasion, and metastasis. Hance et al demonstrated that elevated eHsp90 expression was associated with an increased level of MMP-2 and initiated EMT events in prostate cancer.16 Meanwhile, eHsp90 signaling also supports an EMT phenotype via ERK signaling by inducing expression of EZH2 and reducing expression of the epithelial marker E-cadherin.28 In light of these reports, the ability of eHsp90α to impair E-cadherin function and initiate EMT may have clinical utility in blocking or delaying cancer progression. Although more mechanistic details need to be explored, to some extent, our data prove eHsp90α as a novel and potential factor of ESCC cell EMT plasticity.
Conclusion
Our studies have shown that high serum Hsp90α level before treatment was significantly correlated with positive LN metastasis in ESCC patients. Meanwhile, eHsp90α significantly promoted the migration and invasion of ESCC cells in vitro, probably through stabilizing MMP-2 or initiating EMT events. Along with the discovery of additional eHsp90α client proteins, more functions of eHsp90α will be revealed. Therefore, inhibition of eHsp90α may have clinical utility in preventing LN metastasis to improve ESCC control and patient outcome.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81874224) and a grant from the Key Scientific and Technological Innovation Project of Shandong province, China (no. 2017CXZC1206).
Disclosure
The authors report no conflicts of interest in this work.
References
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Zhu Z, Chen H, Yu W, et al. Number of negative lymph nodes is associated with survival in thoracic esophageal squamous cell carcinoma patients undergoing three-field lymphadenectomy. Ann Surg Oncol. 2014;21(9):2857–2863. | ||
Wu SG, Li FY, Zhou J, et al. Prognostic value of different lymph node staging methods in esophageal squamous cell carcinoma after esophagectomy. Ann Thorac Surg. 2015;99(1):284–290. | ||
Schopf FH, Biebl MM, Buchner J. The Hsp90 chaperone machinery. Nat Rev Mol Cell Biol. 2017;18(6):345–360. | ||
Wu X, Wanders A, Wardega P, et al. Hsp90 is expressed and represents a therapeutic target in human oesophageal cancer using the inhibitor 17-allylamino-17-demethoxygeldanamycin. Br J Cancer. 2009;100(2):334–343. | ||
Ghosh S, Shinogle HE, Garg G, et al. Hsp90 C-terminal inhibitors exhibit antimigratory activity by disrupting the Hsp90α/Aha1 complex in PC3-MM2 cells. ACS Chem Biol. 2015;10(2):577–590. | ||
Hance MW, Nolan KD, Isaacs JS. The double-edged sword: conserved functions of extracellular Hsp90 in wound healing and cancer. Cancers. 2014;6(2):1065–1097. | ||
Wong DS, Jay DG. Emerging roles of extracellular Hsp90 in cancer. Adv Cancer Res. 2016;129:141–163. | ||
Wang X, Song X, Zhuo W, et al. The regulatory mechanism of Hsp90alpha secretion and its function in tumor malignancy. Proc Natl Acad Sci U S A. 2009;106(50):21288–21293. | ||
McCready J, Wong DS, Burlison JA, Ying W, Jay DG. An impermeant Ganetespib analog inhibits extracellular Hsp90-mediated cancer cell migration that involves lysyl oxidase 2-like protein. Cancers. 2014;6(2):1031–1046. | ||
Stellas D, El Hamidieh A, Patsavoudi E. Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by disrupting their interaction with extracellular Hsp90 and inhibits formation of metastatic breast cancer cell deposits. BMC Cell Biol. 2010;11:51–59. | ||
Thuringer D, Hammann A, Benikhlef N, et al. Transactivation of the epidermal growth factor receptor by heat shock protein 90 via Toll-like receptor 4 contributes to the migration of glioblastoma cells. J Biol Chem. 2011;286(5):3418–3428. | ||
Sims JD, McCready J, Jay DG. Extracellular heat shock protein (Hsp)70 and Hsp90α assist in matrix metalloproteinase-2 activation and breast cancer cell migration and invasion. PLoS One. 2011;6(4):e18848. | ||
Song X, Wang X, Zhuo W, et al. The regulatory mechanism of extracellular Hsp90{alpha} on matrix metalloproteinase-2 processing and tumor angiogenesis. J Biol Chem. 2010;285(51):40039–40049. | ||
McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular Hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294–303. | ||
Hance MW, Dole K, Gopal U, et al. Secreted Hsp90 is a novel regulator of the epithelial to mesenchymal transition (EMT) in prostate cancer. J Biol Chem. 2012;287(45):37732–37744. | ||
Zou M, Bhatia A, Dong H, et al. Evolutionarily conserved dual lysine motif determines the non-chaperone function of secreted hsp90alpha in tumour progression. Oncogene. 2017;36(15):2160–2171. | ||
Tsen F, Bhatia A, O’Brien K, et al. Extracellular heat shock protein 90 signals through subdomain II and the NPVY motif of LRP-1 receptor to Akt1 and Akt2: a circuit essential for promoting skin cell migration in vitro and wound healing in vivo. Mol Cell Biol. 2013;33(24):4947–4959. | ||
Eustace BK, Sakurai T, Stewart JK, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6(6):507–514. | ||
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. | ||
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. | ||
Shi Y, Liu X, Lou J, et al. Plasma levels of heat shock protein 90 alpha associated with lung cancer development and treatment responses. Clin Cancer Res. 2014;20(23):6016–6022. | ||
Song X, Luo Y. The regulatory mechanism of Hsp90alpha secretion from endothelial cells and its role in angiogenesis during wound healing. Biochem Biophys Res Commun. 2010;398(1):111–117. | ||
Tsutsumi S, Neckers L. Extracellular heat shock protein 90: a role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007;98(10):1536–1539. | ||
Fares RC, Gomes JA, Garzoni LR, et al. Matrix metalloproteinases 2 and 9 are differentially expressed in patients with indeterminate and cardiac clinical forms of Chagas disease. Infect Immun. 2013;81(10):3600–3608. | ||
Santamaria PG, Moreno-Bueno G, Portillo F, Cano A. EMT: present and future in clinical oncology. Mol Oncol. 2017;11(7):718–738. | ||
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. | ||
Nolan KD, Franco OE, Hance MW, Hayward SW, Isaacs JS. Tumor-secreted Hsp90 subverts polycomb function to drive prostate tumor growth and invasion. J Biol Chem. 2015;290(13):8271–8282. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.