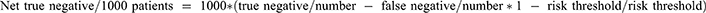Back to Journals » Clinical Interventions in Aging » Volume 18
External Validation of the Revised Cardiac Risk Index and the Geriatric-Sensitive Perioperative Cardiac Risk Index in Oldest Old Patients Following Surgery Under Spinal Anaesthesia; a Retrospective Cross-Sectional Cohort Study
Authors Fayed N , Elkhadry SW, Garling A, Ellerkmann RK
Received 11 March 2023
Accepted for publication 2 May 2023
Published 10 May 2023 Volume 2023:18 Pages 737—753
DOI https://doi.org/10.2147/CIA.S410207
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Nirmeen Fayed,1,2 Sally Waheed Elkhadry,3 Andreas Garling,1 Richard K Ellerkmann1,4
1Anethesia and Critical Care Department, Klinikum Dortmund, Dortmund, Germany; 2Anesthesia and Critical Care Department, National Liver Institute Menoufia University, Shebin-Alkoom, Egypt; 3Epidemiology and Preventive Medicine Institute, National Liver Institute, Menoufia University, Shebin-Alkoom, Egypt; 4Anesthesia and Critical Care Department, Bonn University, Bonn, Germany
Correspondence: Nirmeen Fayed, Anesthesia Department Klinikum Dortmund, Germany, Mollwitzer Straße 4, Dortmund, 44141, Germany, Tel +49 17647154842, Email [email protected]
Background: The Revised Cardiac Risk Index (RCRI) and the Geriatric Sensitive Cardiac Risk Index (GSCRI) estimate the risk of postoperative major adverse cardiac events (MACE) regardless of the type of anesthesia and without specifying the oldest old patients. Since spinal anesthesia (SA) is a preferred technique in geriatrics, we aimed to test the external validity of these indices in patients ≥ 80 years old who underwent surgery under SA and tried to identify other potential risk factors for postoperative MACE.
Methods: The performance of both indices to estimate postoperative in-hospital MACE risk was tested through discrimination, calibration, and clinical utility. We also investigated the correlation between both indices and postoperative ICU admission and length of hospital stay (LOS).
Results: The MACE incidence was 7.5%. Both indices had limited discriminative (AUC for RCRI and GSCRI were 0.69 and 0.68, respectively) and predictive abilities. The regression analysis showed that patients with atrial fibrillation (AF) were 3.77 and those with trauma surgery were 2.03 times more likely to exhibit MACE, and the odds of MACE increased by 9% for each additional year above 80. Introducing these factors into both indices (multivariable models) increased the discriminative ability (AUC reached 0.798 and 0.777 for RCRI and GSCRI, respectively). Bootstrap analysis showed that the predictive ability of the multivariate GSCRI but not the multivariate RCRI improved. Decision curve analysis (DCA) showed that multivariate GSCRI had superior clinical utility when compared with multivariate RCRI. Both indices correlated poorly with postoperative ICU admission and LOS.
Conclusion: Both indices had limited predictive and discriminative ability to estimate postoperative in-hospital MACE risk and correlated poorly with postoperative ICU admission and LOS, following surgery under SA in the oldest-old patients. Updated versions by introducing age, AF, and trauma surgery improved the GSCRI performance but not the RCRI.
Keywords: geriatric, anesthetic techniques, subarachnoid, risk, major adverse cardiac event
Introduction
Worldwide, the proportion of people aged 80 years or older, defined as the “oldest-old persons”1 is growing even faster than that of older people overall and is projected to triple, from 157 million in 2022 to 459 million in 2050.2 In Germany, over 6 million people were at least 80 years old in 2020. This number is expected to reach 10 million in 2050, representing a share of 13%.3
Seventy-two% of Germans above the age of 65 suffer from cardiovascular diseases4 and the cardiovascular diseases per se are the leading cause of death in approximately 40% of the reported mortalities in Germany.5 Meanwhile, the prevalence and severity of cardiovascular diseases increase significantly with age,6 currently with the recent advances in surgery and anesthetic practice, the number of geriatric patients who need to be submitted to non-cardiac surgery is increasing7 so that we are confronted each day with oldest-old high-risk cardiac patients who should undergo non-cardiac surgery. Cardiovascular complications are the most important causes of morbidity and mortality in the first 30 days after non-cardiac surgery. Postoperative cardiac arrest (CA) and myocardial infarction (MI) are associated with a hospital mortality rate of 65%8,9 and 25%10,11 respectively, with more susceptibility in older patients.12
In various aspects, SA seems to be a well-accepted anesthetic technique that is supposed to reduce perioperative complications in geriatric patients, particularly in those older than 90 years with hip fractures12 and those with American Society of Anaesthesiologists class (ASA) III.13 It is also considered as a safe and successful anesthetic modality for elderly patients undergoing particular surgeries14–16 with some evidence of improved postoperative outcome scores including consciousness, respiration, circulation, laboratory tests, and blood loss.17 Thus, accurate preoperative cardiac risk stratification in this age group, and under this preferred anesthetic technique, is essential for perioperative individualized decision-making about surgical feasibility and postoperative management.
Risk indices have been developed to aid in preoperative cardiac risk assessment. The RCRI,18 which is widely used to estimate perioperative risk, was derived and validated in patients undergoing non-cardiac surgeries without identifying any age group, while the GSCRI19 was validated to estimate the probability of perioperative MI or CA in patients >65 years following non-cardiac surgery. Yet, both indices did not consider the type of anesthesia or specify the oldest-old patients in whom postoperative cardiac complications are more prevalent.
The hemodynamic changes of the cardiovascular aging20–22 and the SA associated hemodynamic effects in healthy23 and cardiac24 elderly patients were extensively investigated and described in details in previous studies.
Our hypothesis was that the interaction between the hemodynamic changes of aging and that of SA could affect the postoperative cardiovascular outcome and subsequently influence the ability of the previously mentioned indices to precisely estimate the risk of postoperative MACE.
Aim of the study: the primary outcome was to test the performance of the RCRI and the GSCRI (through their external validation) in terms of the occurrence of postoperative in-hospital MACE in patients ≥ 80 years old who were submitted to surgery under SA and try to identify other potential risk factors for postoperative in-hospital MACE in this setting. The secondary outcome was to correlate both indices with the perioperative blood pressure and heart rate changes, vasopressor requirements, postoperative ICU admission, and LOS.
Patients and Methods
Inclusion and Exclusion Criteria
This retrospective cross-sectional cohort study was performed at Klinikum Dortmund, a tertiary-care teaching hospital with over 33,000 operations per year. The study protocol was approved by Universitätklinikum Bonn, Germany (29.12.2020) under the following serial number DV 571/2020. Ethical approval was waived and consent to participate is not required as being retrospective study. Patient data confidentiality was completely respected and the information was used only for research purposes. All methods were carried out in accordance with the relevant guidelines and regulations.
The electronic medical files of all patients ≥ 80 years who had undergone surgery under SA from January 2016 to September 2019 were revised.
Exclusion Criteria
Long operations that mandated additional general anesthesia, more than one operation during hospital admission, and files with incomplete data.
Collected Data
Potential preoperative cardiovascular risk factors, including all risk factors for both indices.
Intraoperative blood pressure and heart rate values and required vasopressors.
Post-anesthesia care unit: initial, minimal, and final readings of blood pressure and heart rate.
Postoperative in-hospital MACE (defined as MI, CA, and heart failure (HF)), in-hospital mortality, the need for postoperative intermediate care (IMC)/ ICU admission, and LOS.
Risk Estimation Using the RCRI and the GSCRI
The RCRI risk score was calculated by 1-point assignment for each of the following variables:1) history of ischemic heart disease 2) heart failure 3) stroke or transient ischemic attack 4) insulin-dependent diabetes mellitus 5) serum creatinine level ≥ 2 mg / L and 6) high-risk surgery (intra-thoracic, vascular and intra-peritoneal), for a maximum score of 6.
Patients were categorized into 4 risk classes (I, II, III, and IV) depending on the number of preoperative risk factors according to the RCRI. The GSCRI depends on the following predictors to estimate the risk of postoperative MACE: 1) history of stroke 2) ASA class 3) surgery type 4) functional status 5) serum creatinine level 6) history of HF 7) diabetes mellitus. The predicted risk percentage for each patient was calculated25 and the patients were stratified into 5 risk classes (A, B, C, D, and E), with cut-off values similar to those of the RCRI. Table 1 demonstrates the RCRI and GSCRI risk classes and the corresponding expected postoperative MACE risk percentages according to the number of risk predictors.
 |
Table 1 The Revised Cardiac Risk Index (RCRI) and the Geriatric Sensitive Cardiac Risk Index (GSCRI) Risk Classes with Their Corresponding Expected Risk Percentages |
Statistical Analysis
Descriptive Statistics
Statistical analysis was done using R version 4.1.1. Numeric variables were presented in the median and interquartile ranges, as they were not normally distributed. Wilcoxon rank-sum test was used for non-parametric data. Categorical variables were presented by count and %. Fischer’s exact test and chi-square test were used for categorical data. Statistical significance is set at 0.05.
External Validation of the RCRI and the GSCRI
- Discrimination: C-statistics, the area under the curve (AUC), was used to assess discrimination. This measure assesses whether patients who experience the outcome have a higher predicted risk than patients who do not. AUC of 0.60 means that 60% of all patients who had MACE were classified as high risk according to the indices, AUC < 0.7 is considered poor discrimination, while an AUC of 0.7–0.8 is usually considered good and 0.9 is excellent.
- Calibration determines whether the absolute predicted risks are similar to the observed risks. Both calibration plots and the Chi-square Goodness-of-fit test were used to assess the risk predictive ability of both indices.
- Clinical utility was evaluated using net benefit (NB) (which puts benefits and harms on the same scale and is conveniently expressed in the unit of true positives or true negatives) and decision curve analysis (DCA) (which considers the consequences of using risk prediction models and making decisions based on risk thresholds).
Model Update
This aims to adjust the model to better fit the external validation cohort by adding more predictors (according to the regression analysis). Because a prediction model is expected to perform better in the original derivation sample than in new but similar samples, a bootstrap analysis was performed.
Statistical Procedures
The data set was divided into 2 parts to assess the model performance, 80% to train the model and 20% to test the model and assess the prediction ability of the model.
Four models have been created: the univariate models of the RCRI and the GSCRI and the multivariate models (by introducing age, trauma surgery, and AF).
Bootstrap Analysis
Resampling was performed 100 times and calibration curves that relate the predicted probability to the observed number of events were produced.
NB: the number of net true positives and negatives per 1000 patients was calculated by using this equation: 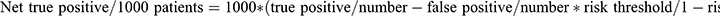
The study’s secondary outcome was assessed by performing Spearman correlation.
This study was written following the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) Statement,26 and the World Medical Association Declaration of Helsinki.27
Results
The total number of candidates was 1042 patients and only 836 patients were included in the study, as shown in Figure 1.
 |
Figure 1 Study flowchart. |
The median age was 85 (82–89) years and 52.4% of subjects were female. From the surgical point of view, 54.1% of the patients underwent non-elective surgeries, 7.2% of all surgeries were high-risk, and the majority of the patients (65.3%) and most of those who had experienced in-hospital MACE (82.5%) underwent trauma surgery. The distribution of the patients across RCRI risk classes showed that most of the patients were classified as risk class I (45.6%). However, the majority of the patients and most of those who experienced in-hospital MACE were classified as risk class C according to the GSCRI. Some factors that were not considered in the design of both indices, such as pre-existing AF, pulmonary hypertension, and aortic stenosis, showed high statistically significant prevalence in patients with in-hospital MACE. Prevalence of preoperative cerebrovascular events, coronary artery disease, and HF was not statistically significantly different when comparing those with and without in-hospital MACE. The preoperative patient characteristics and cardiovascular-related risk factors distributed across MACE status are presented in Table 2.
 |
Table 2 Patients’ Preoperative Characteristics and Relevant Clinical Data Across MACE Status |
Intraoperative and immediate postoperative systolic and diastolic blood pressure and heart rate changes showed that patients with in-hospital MACE had significantly lower blood pressure and higher heart rate than those without in-hospital MACE (Table 3).
Concerning other intraoperative data, patients who had experienced in-hospital MACE, had a statistically significant longer duration of surgery, required more blood and fluid transfusion, more vasopressor support, and should have been invasively monitored more frequently compared to those without in-hospital MACE as shown in Table 4.
The postoperative outcome, including MACE, was presented in Table 5. Sixty-three patients had at least one MACE (7.5%) with a total record of 81 major adverse cardiac events and a mortality rate of 60.3% (38/63). A statistically significantly higher incidence of pulmonary artery embolism, postoperative acute kidney injury, and ICU admissions also accompanied the occurrence of MACE.
The regression analysis demonstrated that increased age, AF, trauma surgery, aortic stenosis, pulmonary hypertension, renal failure, and insulin-dependent diabetes mellitus (IDDM) were statistically significantly associated with the poor postoperative cardiovascular outcome while the association between cerebrovascular events; coronary artery disease, and HF did not reach statistical significance (Table 6).
External Validation
Discrimination: the C-statistics showed that both indices had limited discriminative ability. The AUC for RCRI was 0.69 (95% CI: 0.70, 0.83) and for GSCRI was 0.68 (95% CI: 0.83, 0.93) (Figure 2).
Regarding calibration, The Chi-square Goodness-of-Fit test showed that the RCRI underestimated the MACE risk in all risk classes while the GSCRI underestimated the risk in the B, C, and D classes and overestimated the risk in class E. (Table 7) The calibration plots (Figure 3) also confirmed this miscalibration.
Update of the Models
The regression analysis (Table 6) showed that patients with AF were 3.77 and those with trauma surgery were 2.03 times more likely to exhibit MACE, and the odds of MACE increased by 9% for each additional year above 80. Introducing these risk factors into the models improved their discriminative ability as the AUC reached 0.798 (95% CI: 0.73, 0.85) for the multivariable RCRI and 0.777 (95% CI: 0.702, 0.83) for the multivariable GSCRI (Figure 2).
Calibration curves of the multivariate (after introducing age, trauma surgery, and atrial fibrillation) RCRI and GSCRI with a bootstrap resampling validation indicated that the predictive ability of the multivariable GSCRI has improved as there are few differences between the ideal and the apparent lines up to 40% risk. The multivariable RCRI underestimates the risk between a risk index of 10–20% and overestimates the risk when the incidence is >20% and matches only when the risk is lower than 10% as evidenced by the huge difference between the ideal and the apparent lines (Figure 4).
The multivariable RCRI showed a slightly better clinical utility, while the multivariable GSCRI showed superior clinical utility at ≥10% expected risk, as shown by the DCA (Figure 5).
Secondary Outcome
Correlations of both indices with blood pressure and heart rate changes, vasopressors need, ICU admission, and LOS are shown in Table 8. Our results showed statistically significant evidence of negligible correlations between both indices and the previously mentioned clinical parameters, with correlation coefficients of less than 0.3 in all cases.
There was no reported data on post-SA complications, including convulsions, total spinal block, epidural hematoma, or postoperative neurological defects.
Discussion
This retrospective study reported a limited ability of RCRI and GSCRI to predict postoperative in-hospital MACE and a poor correlation of both indices with postoperative ICU admission and LOS following surgery under SA in the oldest-old patients. Implementing other potential risk factors such as increasing age, AF, and trauma surgery into both indices has improved the performance of the GSCRI, while RCRI was still of limited predictive ability and clinical utility.
We selected these two indices, in particular, following the European Society of Cardiology/European Society of Anesthesiology (ESC/ESA) guidelines,28 which have recommended using two risk scales for preoperative estimation of postoperative MACE: the Surgery Risk Calculator of the American College of Surgeons-National Surgical Quality Improvement Program (NSQIP) and the Revised Cardiac Risk Index. Of the NSQIP scales, we have selected the GSCRI as being specific for geriatric patients.
In the RCRI and GSCRI derivation studies, the two indices showed good discriminative ability when applied to detect postoperative MACE with an AUC of 0.76 for both. Whereas applying the RCRI to the same GSCRI dataset yielded an AUC of 0.63 (13% reduction, P < 0.001).19
A careful revision of the GSCRI derivation cohort according to age subgroups as shown in the published supplemental material of Alrezk et al study19 showed a lower discriminative ability of both indices when applied to estimate postoperative MACE in the patients > 85 years, with an AUC of 0.55 and 0.66 for the RCRI and the GSCRI results, respectively, which comes in agreement with our results. The poor discriminative ability of RCRI was also previously reported when externally validated to detect postoperative MACE risk following vascular29 and renal transplant30 surgeries.
Many differences between the current study and the original validating studies of both indices may explain their poor performance when externally validated in our patients.
The mean age was 66 and 74 years in the RCRI and GSCRI cohorts, respectively, which is lower than the mean age in the current study (85.9 years). Our results showed that those with MACE had a statistically significantly higher age compared to those without MACE (median age, 87 vs 85 years, p = 0.0001). The regression analysis showed that MACE incidence increased progressively with age. Many other studies have also proved the role of age as a risk factor for postoperative MACE.31–34
The urgency and types of surgeries were different. While Lee et al in the RCRI validating study18 have only included elective surgeries and Alrezk et al in the GSCRI validating study19 have excluded emergency surgeries, we have included elective and non-elective surgeries including trauma surgery and emergency vascular surgeries.
Both indices have considered surgery as a predictive risk factor from different points of view. RCRI has classified surgeries based on their risk (low, moderate, high) while GSCRI has stratified surgeries anatomically; yet, the study spectrum of both indices did not include trauma surgery.
Many other research teams have proved that urgent and emergency surgeries increased the incidence of MACE by OR ranging from 1.6 to 2.8.32,35–37 Trauma surgery, in particular, carries two hazardous aspects: being non-elective surgery and being associated with an imbalanced systemic inflammation that causes various perioperative complications38,39 which leads to worse cardiovascular outcomes.40 This supports our results that identified trauma surgery as a risk predictor, increasing the odds of postoperative in-hospital MACE by 203%.
AF as a predictive risk factor was not considered in the design of both indices, where it was statistically significantly prevalent in those with MACE in the current study. The regression analysis has also shown that AF increases the odds of in-hospital postoperative MACE by 377%. AF is one of the most common perioperative cardiac arrhythmias encountered during orthopedic surgery41,42 and has been extensively studied as a cause of perioperative morbidity and worse outcomes.43–45
In a retrospective orthopedic study46 including 906 total knee arthroplasty under epidural anesthesia, patients with AF were 2.6, 3.3, and 2.6 times more likely to have postoperative acute MI, ventricular tachycardia, and HF, respectively.
The role of AF as a risk factor for postoperative MACE was also supported by Puelacher et al results37 that confirmed its significant association with postoperative MI after non-cardiac surgery.
By introducing age, trauma surgery, and AF as risk predictors into both indices (multivariate RCRI and multivariate GSCRI), we have noticed that the discriminative ability of the RCRI and the GSCRI has increased by 14.80% and 8.06%, respectively. The bootstrap analysis of the multivariate GSCRI confirmed an improved predictive ability up to a 40% MACE risk. This result seems to be satisfactory, as the highest recorded risk of postoperative MACE following non-cardiac surgery was 19.4%.33
However, the bootstrap analysis of the multivariate RCRI has proven its limited predictive ability even after the implementation of the previously mentioned risk factors. Compared to RCRI, GSCRI considers the ASA class and the functional status of patients as predicting factors and is specific to patients over 65 years old. This may explain why multivariate GSCRI has better predictive ability than multivariate RCRI. The influence of the clinical condition and the frailty status of the patients on the postoperative MACE incidence was shown in a recently published study47 where the risk of 30-day mortality, MI, and CA was 0.35% in the low frailty risk group and 2.12% in the high-risk frailty group in the patients with RCRI class I.
Of interest in this study is that the prevalence of some established preoperative cardiovascular risk factors, such as prior cerebrovascular events, coronary artery disease, and HF, which are supposed to increase the incidence of MACE according to both indices, was not statistically significantly different when comparing patients with and without in-hospital MACE. Moreover, the regression analysis has shown that the association of the previously mentioned preoperative cardiovascular risk factors with in-hospital postoperative MACE does not reach statistical significance.
Our observation regarding the cerebrovascular events being of non-predictive value in the current study may explain why the GSCRI has overestimated the MACE incidence in risk class E. By revising the data of the GSCRI risk class E, we have found that the total number of patients was 69, and 91.3% of them (63/69) had a prior history of cerebrovascular events, which is considered a risk factor in the GSCRI but not in the current study. Similarly, another recent study33 could not identify cerebrovascular events as an independent risk factor for perioperative cardiac complications in patients over 80 years with coronary artery disease undergoing non-cardiac operations. The prevalence of preoperative cerebrovascular events in that study was not significantly different in those with and without postoperative in-hospital MACE (18.8% vs 18.9%, OR: 1.003, p = 0.99).
Besides the role of the recent advances in coronary artery disease management and the extensive use of percutaneous interventions, which help in the reduction of short-term mortality, cardiac death, and MI,48,49 the nature of the operations in this study (trauma, vascular, and urological surgeries), which required only T10 sensory level block, could reduce the hypotensive effect of SA and subsequently limit the coronary hypoperfusion. This may explain the statistically non-significant prevalence of coronary artery disease between those with and without MACE and the regression analysis results.
SA may be a good choice for patients with HF because of the less reduction in blood pressure, the avoidance of tachycardia, and the preservation of myocardial contractility when compared to general anesthesia. This may explain the non-significant difference in the prevalence of HF between those with and without postoperative MACE and the regression analysis results. However, the lack of data concerning the phenotype, duration, and severity of HF limits the interpretation.
Aortic stenosis and pulmonary hypertension are expected to increase the incidence of postoperative adverse cardiovascular outcomes, especially under SA, which is contraindicated in severe cases. In agreement with this came our results, which indicated that the prevalence of aortic stenosis and pulmonary hypertension was statistically significantly higher in those with postoperative MACE than those without, and the regression analysis has shown that they are statistically significantly associated with postoperative MACE. The current study included 46 (5.5%) patients with aortic stenosis, of whom 11 were diagnosed with a severe degree, and 16 (1.9%) patients with pulmonary hypertension. The plausible explanation for providing SA to patients with severe aortic stenosis might be due to their submission to emergency operations. This did not allow for complete preoperative cardiac assessment, and severe aortic stenosis was most probably first diagnosed after the occurrence of complications.
Another difference between our study and the derivation studies of both indices is the restriction to one type of anesthesia (SA) in the current study. Neuraxial anesthesia was found to improve postoperative cardiac outcome (reduced postoperative MI, pulmonary embolism, and mortality by about one-third)50 or at least was not associated with altered odds of adverse cardiovascular outcomes in high-risk patients undergoing non-cardiac surgeries.51
Although the POISE trial52 concluded that the neuraxial block was associated with an increased risk of adverse cardiovascular outcomes, SA as a sole anesthetic technique was not associated with a high incidence of MACE (OR 0.89) or MI (OR 0.74) when compared with general anesthesia in that trial. This discrepancy is most likely attributed to including patients who had combined general anesthesia and thoracic epidural block under the category of a “neuraxial block”, which led to high odds of MACE in the neuraxial group when compared to patients who had only general anesthesia.
The in-hospital postoperative MACE incidence was 7.5% in the current study. Sazgary et al53 have reported a higher postoperative MACE incidence of 15.2% in high-risk cardiac patients. The anesthetic technique (general and spinal versus only spinal), the study period (30 days versus the in-hospital outcome), and the screening for detection of non-symptomatic MACE compared to the only clinically based diagnosis in our study may explain the higher incidence of MACE in the compared study.
In another study, which included patients over 80 years old with coronary artery disease, the incidence of postoperative MACE was 19.4%.33 The differences between that study and ours are the longer study period of 30 days and the use of general and neuraxial anesthesia.
Sabaté et al,54 on the other hand, have reported a lower MACE incidence of 4.3%, which may be attributed to the lower age (mean 67 compared to 85.9 years), a better ASA score (55.4% ASA II against 85.1% ASA III and IV), and the small percentage of emergency (7.1 versus 54.1%) and high-risk (5.2% versus 7.17%) surgeries in the compared and current study, respectively. Besides that, the surgery spectrum in the Sabaté et al study did not include trauma surgeries.
Our results showed statistically significant evidence of a negligible correlation between the two indices and the postoperative ICU admission, LOS, intraoperative and immediate postoperative blood pressure and heart rate changes, and vasopressor requirements. This result was expected because both indices underestimated the likelihood of postoperative MACE that is always associated with ICU admission, prolonged LOS, and hemodynamic instability with subsequent use of vasopressors. In contrast to our results, Ackland et al55 have found that a RCRI score ≥3 was associated with a significant increase in postoperative LOS and ICU admission. This may be explained by their elective ICU admission based on the preoperative cardiac risk severity according to the RCRI, while we have almost depended on the perioperative clinical evaluation of the patients for postoperative ICU admissions.
Our study has the following limitations: first, the retrospective and single-center design. Second, the number of events was smaller than the recommended 100,56 yet we have recorded 81 events which is more than those recorded in the original validation set of the RCRI (36 events), and other validating studies.29,57 This number of events is also accepted as a required sample size for external validation of risk models for binary outcomes, according to a recently published study.58 Third, this study, being retrospective, is also limited by the short-term (in-hospital) outcome.
Furthermore, we could not find details about the perioperative management of anticoagulant therapy in patients with AF, but there was no single recorded epidural hematoma among our patients. Also, some important laboratory parameters such as serum lactate level were not measured.
Although the age restriction (≥80 years) and anesthetic approach (only SA) can limit the generalizability of our findings, we consider this a powerful aspect because we targeted a clinically relevant subgroup in which precise preoperative cardiovascular risk stratification is very essential. To our knowledge, this is the first study to address the type of anesthesia and to include only the oldest-old patients in the external validation of postoperative cardiovascular risk indices. Conclusion: Both RCRI and GSCRI had limited ability to predict postoperative in-hospital MACE and correlated poorly with postoperative ICU admission and LOS following surgery under SA in the oldest-old patients. Updated versions by introducing age, AF, and trauma surgery had improved the predictive ability and the clinical utility of the multivariable GSCRI, while the multivariable RCRI was still of limited predictive and clinical utility.
Acknowledgment
We would like to thank Dr.Nouran Hamza for her enormous support in the statistical analysis.
Funding
There are no funding sources.
Disclosure
The authors declare that they have no competing interests.
References
1. Encyclopedia.com. Oldest old. Availiable from: https://www.encyclopedia.com/social-sciences/encyclopedias-almanacs-transcripts-and-maps/oldest-old.
2. United Nations Department of Economic and Social Affairs, Population Division. World population prospects 2022: summary of results. UN DESA/POP/2022/TR/NO. 3; 2022.
3. Eisenmenger M, Pötzsch O, Sommer B. Germany’s population by 2050. In: Results of the 11th Coordinated Population Projection” Statistisches Bundesamt. Wiesbaden: Federal Statistical Office; 2006.
4. Statista. Share of adults diagnosed with cardiovascular conditions* in Germany in 2018, by age; 2018. Availablefrom: Statistahttps://www.statista.com/statistics/975997/germanycardiovascular-conditions-by-age/.
5. RKI. CardiovascularDisease. Availablefrom: https://www.rki.de/EN/Content/Health_Monitoring/Main_Topics/Chronic_Disease/Cardiovascular_Disease/cardiovascular_disease_node.html#:~:text=Cardiovascular%20diseases%20are%20the%20leading,approximately%2040%25%20of%20all%20deaths.
6. Nanayakkara S, Marwick TH, Kaye DM. The ageing heart: the systemic and coronary circulation. Heart. 2018;104(5):370–376. doi:10.1136/heartjnl-2017-312114
7. Jeong O, Park YK, Jung MR, Ryu SY. Compliance with guidelines of enhanced recovery after surgery in elderly patients undergoing gastrectomy. World J Surg. 2017;41(4):1040–1046. doi:10.1007/s00268-016-3845-y
8. Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J. 2005;173:627–634. doi:10.1503/cmaj.050011
9. Sprung J, Warner ME, Contreras MG, et al. Predictors of survival following cardiac arrest in patients undergoing noncardiac surgery: a study of 518,294 patients at a tertiary referral center. Anesthesiology. 2003;99(2):259–269. doi:10.1097/00000542-200308000-00006
10. Chow WB, Rosenthal RA, Merkow RP, Ko CY, Esnaola NF. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American college of surgeons national surgical quality improvement program and the American Geriatrics Society. J Am Coll Surg. 2012;215:453–466. doi:10.1016/j.jamcollsurg.2012.06.017
11. Roger VL`, Go AS, Lloyd-Jones DM, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi:10.1161/CIR.0b013e3182009701
12. Matsuo M, Yamagami T, Higuchi A. Impact of age on postoperative complication rates among elderly patients with Hip fracture: a retrospective matched study. J Anesth. 2018;32(3):452–456. doi:10.1007/s00540-018-2494-8
13. Messina A, Frassanito L, Colombo D, et al. Hemodynamic changes associated with spinal and general anesthesia for Hip fracture surgery in severe ASA III elderly population: a pilot trial. Minerva Anestesiol. 2013;79(9):1021–1029.
14. Kilinc LT, Sivrikaya GU, Eksioglu B, Hanci A, Dobrucali H. Comparison of unilateral spinal and continous spinal anesthesia for Hip surgery in elderly patients. Saudi J Anaesth. 2013;7:404–409. doi:10.4103/1658-354X.121054
15. Lessing NL, Edwards CC, Lin C, Brown CH. Complex lumbar spine fusion for an elderly patient under spinal anesthesia. Orthopedics. 2017;40(5):e915–e917. doi:10.3928/01477447-20170602-02
16. Lessing NL, Edwards CC, Ledford EC, Dean CL. Spinal anesthesia in elderly patients undergoing lumbar spine surgery. Orthopedics. 2017;40(2):317–322.
17. Jw U, Andres FJ, Eggert E. The role of anaesthesia in geriatric patients with Hip fractures: a prospective study. Eur J Anaesthesiol. 1993;10:380.
18. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049. doi:10.1161/01.CIR.100.10.1043
19. Alrezk R, Jackson N, Al Rezk M, et al. Derivation and validation of a geriatric-sensitive perioperative cardiac risk index. J Am Heart Assoc. 2017;6(11):e006648. doi:10.1161/JAHA.117.006648
20. Lakatta EG. Cardiovascular aging research: the next horizons. JAm Geriatr Soc. 1999;47:613–625. doi:10.1111/j.1532-5415.1999.tb02579.x
21. Lakatta EG. Changes in cardiovascular function with aging. Eur Heart J. 1990;11(suppl_C):22–29. doi:10.1093/eurheartj/11.suppl_C.22
22. Priebe HJ. The aged cardiovascular risk patient. Br J Anaesth. 2000;85(5):763–778. doi:10.1093/bja/85.5.763
23. Lairez O, Ferré F, Portet N, et al. Cardiovascular effects of low-dose spinal anaesthesia as a function of age: an observational study using echocardiography. Anaesth Crit Care Pain Med. 2015;34(5):271–276. doi:10.1016/j.accpm.2015.02.007
24. Rooke GA, Freund PR, Jacobson AF. Hemodynamic response and change in organ blood volume during spinal anesthesia in elderly men with cardiac disease. Anesth Analg. 1997;85(1):99–105. doi:10.1097/00000539-199707000-00018
25. Qx MD; GSCRI. Geriatric-sensitive perioperative cardiac risk index. Available from: https://qxmd.com/calculate/calculator_448/geriatric-sensitive-perioperative-cardiac-risk-index-gscri.
26. Collins GS, Reitsma JB, Altman DG, Moons KGM. Trans- parent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. doi:10.1136/bmj.g7594
27. WorldMedical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
28. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–2431. doi:10.1093/eurheartj/ehu282
29. Fronczek J, Polok K, Devereaux PJ, et al. External validation of the revised cardiac risk index and national surgical quality improvement program myocardial infarction and cardiac arrest calculator in noncardiac vascular surgery. Br J Anaesth. 2019;123(4):421–429. doi:10.1016/j.bja.2019.05.029
30. Olsen VDR, Borges FK, Goldraich LA, et al. Limited predictive role of the revised cardiac risk index in kidney transplant: single center evaluation and comparison with international literature. Curr Probl Cardiol. 2021;46(9):100908. doi:10.1016/j.cpcardiol.2021.100908
31. Banco D, Dodson JA, Berger JS, Smilowitz NR. Perioperative cardiovascular outcomes among older adults undergoing in-hospital noncardiac surgery. J Am Geriatr Soc. 2021;69(10):2821–2830. doi:10.1111/jgs.17320
32. Botto F, Alonso-Coello P, Chan MT, et al.; Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Writing Group, on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564–578.
33. Liu Z, Xu G, Xu L, Zhang Y, Huang Y. Perioperative cardiac complications in patients over 80 years of age with coronary artery disease undergoing noncardiac surgery: the incidence and risk factors. Clin Interv Aging. 2020;15:1181–1191. doi:10.2147/CIA.S252160
34. Hansen PW, Gislason GH, Jørgensen ME, et al. Influence of age on perioperative major adverse cardiovascular events and mortality risks in elective non-cardiac surgery. Eur J Intern Med. 2016;35:55–59. doi:10.1016/j.ejim.2016.05.028
35. Kumar R, McKinney WP, Raj G, et al. Adverse cardiac events after surgery: assessing risk in a veteran population. J Gen Intern Med. 2001;16(8):507–518. doi:10.1046/j.1525-1497.2001.016008507.x
36. Mullen MG, Michaels AD, Mehaffey JH, et al. Risk associated with complications and mortality after urgent surgery vs elective and emergency surgery: implications for defining “quality” and reporting outcomes for urgent surgery. JAMA Surg. 2017;152(8):768–774. doi:10.1001/jamasurg.2017.0918
37. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221–1232. PMID: 29203498. doi:10.1161/CIRCULATIONAHA.117.030114
38. Rj G, te Boekhorst TP, Nuytinck JK, Gimbrère JS. Multiple-organ failure. Generalized autodestructive inflammation. Arch Surg. 1985;120(10):1109–1115. doi:10.1001/archsurg.1985.01390340007001
39. Nuytinck HK, Offermans XJ, Kubat K, Goris JA. Whole-body inflammation in trauma patients. An autopsy study. Arch Surg. 1988;123(12):1519–1524. doi:10.1001/archsurg.1988.01400360089016
40. Amar D, Zhang H, Park B, Heerdt PM, Fleisher M, Thaler HT. Inflammation and outcome after general thoracic surgery. Eur J Cardiothorac Surg. 2007;32((3)):431–434. doi:10.1016/j.ejcts.2007.06.017
41. Hollenberg SM, Dellinger RP. Noncardiac surgery: postoperative arrhythmias. Crit Care Med. 2000;28(10 Suppl):N145–150. doi:10.1097/00003246-200010001-00006
42. Kahn RL, Hargett MJ, Urquhart B, Sharrock NE, Peterson MG. Supraventricular tachyarrhythmias during total joint arthroplasty. Incidence and risk. Clin Orthop Relat Res. 1993;265–269.
43. Aggarwal VK, Tischler EH, Post ZD, Kane I, Orozco FR, Ong A. Patients with atrial fibrillation undergoing total joint arthroplasty increase hospital burden. J Bone Joint Surg Am. 2013;95(17):1606–1611. doi:10.2106/JBJS.L.00882
44. Kurtz SM, Lau EC, Ong KL, Adler EM, Kolisek FR, Manley MT. Which hospital and clinical factors drive 30- and 90-day readmission after TKA? J Arthroplasty. 2016;31(10):2099–2107. doi:10.1016/j.arth.2016.03.045
45. Wang CT, Chuang E, Yen DJ, Chuang TY, Muo CH, Kao CH. First-ever stroke following hip replacement surgeries: a large population-based survey. Eur J Clin Invest. 2016;46(11):931–939. doi:10.1111/eci.12678
46. Long G, Suqin S, Li G, Weihong Y, Zhenhu W. Impact of atrial fibrillation on postoperative outcomes after total knee arthroplasty-A retrospective study. J Orthop Sci. 2016;21(5):652–657. doi:10.1016/j.jos.2016.05.002
47. Gouda P, Wang X, Youngson E, McGillion M, Mamas MA, Graham MM. Beyond the revised cardiac risk index: validation of the hospital frailty risk score in non-cardiac surgery. PLoS One. 2022;17(1):e0262322. doi:10.1371/journal.pone.0262322
48. Ahmad M, Mehta P, Reddivari AKR, Mungee S. Percutaneous coronary intervention. In: StatPearls. Treasure Island (FL): Stat Pearls Publishing; 2022.
49. Chacko L, Howard P, Rajkumar C, et al. Effects of percutaneous coronary intervention on death and myocardial infarction stratified by stable and unstable coronary artery disease: a meta-analysis of randomized controlled trials. Circ Cardiovasc Qual Outcomes. 2020;13(2):e006363. doi:10.1161/CIRCOUTCOMES.119.006363
50. Leslie K, McIlroy D, Kasza J, et al. Neuraxial block and postoperative epidural analgesia: effects on outcomes in the POISE-2 trial†. Br J Anaesth. 2016;116(1):100–112. doi:10.1093/bja/aev255
51. Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321(7275):1493. doi:10.1136/bmj.321.7275.1493
52. Leslie K, Myles P, Devereaux P, et al. Neuraxial block, death and serious cardiovascular morbidity in the POISE trial. Br J Anaesth. 2013;111(3):382–890. doi:10.1093/bja/aet120
53. Sazgary L, Puelacher C, Lurati Buse G, et al. Incidence of major adverse cardiac events following non-cardiac surgery. Eur Heart J Acute Cardiovasc Care. 2020;10(5):550–558. doi:10.1093/ehjacc/zuaa008
54. Sabaté S, Mases A, Guilera N, et al. Incidence and predictors of major perioperative adverse cardiac and cerebrovascular events in non-cardiac surgery. Br J Anaesth. 2011;107(6):879–890. doi:10.1093/bja/aer268
55. Ackland GL, Harris S, Ziabari Y, Grocott M, Mythen M; SOuRCe Investigators. Revised cardiac risk index and postoperative morbidity after elective orthopaedic surgery: a prospective cohort study. Br J Anaesth. 2010;105(6):744–752. doi:10.1093/bja/aeq245
56. Collins GS, Ogundimu EO, Altman DG. Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat Med. 2016;35:214–226. doi:10.1002/sim.6787
57. Vergouwe Y, Steyerberg EW, Eijkemans MJ, Habbema JD. Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J Clin Epidemiol. 2005;58(5):475–483. doi:10.1016/j.jclinepi.2004.06.017
58. Pavlou M, Qu C, Omar RZ, Seaman SR, Steyerberg EW. Estimation of required sample size for external validation of risk models for binary outcomes. Stat Methods Med Res. 2021;30(10):2187–2206. doi:10.1177/09622802211007522
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.