Back to Journals » Clinical Ophthalmology » Volume 13
Expression of interleukin-6 in ocular surface squamous neoplasia
Authors Eghtedari M , Beigi V, Maalhagh M , Ashraf H
Received 4 July 2019
Accepted for publication 8 August 2019
Published 30 August 2019 Volume 2019:13 Pages 1675—1680
DOI https://doi.org/10.2147/OPTH.S221911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Masoomeh Eghtedari,1,2 Vahid Beigi,2 Mehrnoosh Maalhagh,2 Hossein Ashraf2
1Pathology Department, Shiraz University of Medical Sciences, Shiraz, Iran; 2Ophthalmology Department, Shiraz University of Medical Sciences, Shiraz, Iran
Correspondence: Masoomeh Eghtedari
Poostchi Ophthalmic Research Center, Poostchi Street, Shiraz, Iran
Tel +98 713 230 2830
Fax +98 713 629 1779
Email [email protected]
Purpose: Interleukin-6 (IL-6) is an important cytokine in the cascade of inflammation and cancer progression. The aim of this study was to identify IL-6 expression in ocular surface squamous neoplasia (OSSN) in comparison with non-neoplastic conjunctival tissue.
Methods: Twenty paraffin-embedded tissue sections of conjunctiva from patients with OSSN including conjunctival intraepithelial neoplasia (CIN) in all grades of severity and squamous cell carcinoma (SCC) were assessed by immunohistochemistry staining for IL-6. Twenty non-neoplastic conjunctival sections from age matched patients were selected as the control group. Tissues with more than one focus of inflammatory cell infiltration were excluded from the study. The mean area of positive staining was recorded and the intensity of staining was scored in both groups and compared by statistical methods.
Results: The mean staining area in the dysplasia group was significantly more than non-neoplastic conjunctival tissue (63.5±25.96 and 30±15.98 percent respectively; P-value of <0.0001). Nuclear staining was observed in both groups and the difference was not statistically significant.
Conclusion: IL-6 expressed more in the dysplastic group in compare to non-neoplastic conjunctiva and can therefore be used to diagnose dysplastic state of the conjunctiva; however, in our study, intensity of staining does not correlate with the severity of dysplasia statistically; most probably because of a low sample size in each category. The role of nuclear staining is not clear. Our findings can be an introduction toward targeted treatment of ocular surface neoplasia by the aim of newer anti-IL agents. Further investigation is needed.
Keywords: interleukin-6, squamous cell carcinoma, dysplasia, conjunctival intraepithelial neoplasia
Introduction
Cytokines and chemokines are known for their essential role in inflammation.1 They are also found to contribe to cancer development2 by interfering in apoptosis, cell growth and differentiation as well as angiogenesis. It was previously shown that interleukins (ILs) such as IL-1, IL-6, IL-8, and IL-18 play roles in the different cascades that cause cancer.2–6
IL-6 is a multifunctional cytokine that was first identified for its role in differentiating B lymphocytes from plasma cells. In adjunct with IL-1, IL-6 is responsible for inducing an acute phase of inflammation;5 on the other hand, it has a role in cancer transformation6 and an increased serum level of IL-6 is considered to be a poor prognostic marker in gastric, renal, and ovarian cancers.1,3 It is also a reliable biomarker in determining the recurrence and prognosis in the neoplasms of the head and neck region.3
Ocular Surface Squamous Neoplasia (OSSN) is a term for identifying the spectrum of diseases that involve the corneal or conjunctival epithelium with dysplasia.7 There are several risk factors for OSSN, such as ultraviolet exposure, human papilloma virus types 16 and 18, and smoking.8 To aid the understanding of the pathophysiology of OSSN, there are other researches focusing on limbal stem cell markers; research by Jongkhajornpong et al9 for example, showed that up regulation of ABCB5 (which is an ATP-binding cassette superfamily member) might lead to transformation towards OSSN.
The role of IL-6 in the neoplasms of conjunctiva and cornea has not been elucidated completely. In a study by Di Girolamo10 on pterygia, it was disclosed that IL-6 has a role in induction of matrix metalloproteinases after exposure to UV light. Up regulation of IL-6 was also observed in the corneal stromal cells after exposure to UV light.11
In this study, we aim to discover whether there is any increase in expression of this cytokine in the sample tissues with OSSN in comparison to non-neoplastic conjunctiva, which may help to find a possible mechanism involved in dysplasia, and later on to establish new insight into the treatment of dysplastic states of the conjunctiva and cornea by targeting certain cytokines.
Method
The protocol for use of human tissue samples was in accordance with the ethical regulations of the hospital and approved by the Ethics Committee of Shiraz University of Medical Sciences. In order to conduct research activities, written informed consent for use of archived tissue samples were taken from patients when the specimen is received by the laboratory. The age and sex of patients were recorded based on the data available on the pathology report sheets.
Twenty paraffin-embedded tissue blocks of patients with OSSN and twenty tissue blocks of non-neoplastic conjunctival tissue were retrieved from the archive of the ocular pathology laboratory at Khalili Hospital, Shiraz, Iran.
Light microscopic assay
Histologic tissue sections of conjunctival biopsies with the diagnosis of dysplasia were examined by conventional light microscopy. All diagnoses were re-confirmed through slide review. Grading of dysplasia was made based on cellular atypia and the amount of thickness involvement. Conjunctival intra epithelial neoplasia (CIN) was graded as mild, moderate, or severe according to the thickness and degree of cellular atypia; according to the standard protocol.12 In all three grades, cells with atypia are restricted by the basement membrane. Full thickness cellular atypia was considered as carcinoma in situ. If atypical cells invaded the basement membrane layer, the lesion was considered as invasive squamous cell carcinoma (SCC). Non-neoplastic conjunctival tissues (mainly pterygium samples) were included as controls. In order to avoid any confounding effect, tissues with more than one focus of inflammation were excluded from both groups and replaced by new samples.
Immunohistochemistry staining
Staining protocol was driven from the antibody supplier company (https://docs.abcam.com/pdf/protocols/ihc-immunostaining.pdf) with slight modifications.
Five micrometer-thick tissue sections were cut from paraffin-embedded tissue blocks and handled for immunohistochemistry staining. Samples were rehydrated by being passed through descending concentrations of alcohol baths and water (100%, 90%, 75%). Sections were subjected to antigen retrieval by heating. Incubation in 0.05 H2O2 was done to block endogenous peroxidase activity.
Then, sections were washed in PBS and incubated with a blocking antibody (goat serum in Tris buffered saline). A primary antibody was added (anti IL-6 antibody; ab 9324 abcam, Cambridge, UK in 1:250 dilution) and kept overnight at 4 °C. After that, sections were washed and incubated with Horseradish peroxidase (HRP) conjugated with a secondary antibody (1:500 dilution) for 45 minutes. Chromogenic reaction was checked with Diaminobenzidine. Finally, slides were counterstained with hematoxylin and examined under the light microscope.
Microscopic assay of antibody-labeled sections
Sections were examined under the BX41 Olympus light microscope. Images captured from four different fields containing squamous epithelium by the aid of the DP12 camera attached to the microscope and navigated by AnalySIS LS Starter software. Areas that were stained brown with chromogranin, were scored based on the intensity of cytoplasmic or nuclear staining and the percentage of the positive cytoplasmic area from the average of four microscopic fields at a fixed magnification of ×400. Nuclear staining was recorded by averaging the number of positive nuclei in four microscopic fields at a fixed magnification of ×400. Cytoplasmic and nucleus staining was graded based on the intensity (0, +1, +2, +3).
Statistical analysis
Data were analyzed with SPSS version 23. The expression of IL-6 in OSSN and non-neoplastic tissues was compared using Fisher’s Exact Test. The Mann-Whitney U-Test was used to compare the percentage area of cytoplasm stained in the groups under the study (calculation was made separately for each set of staining intensity). P-values of less than 0.05 were considered to be statistically significant.
Results
The age range of selected patients was 35- to 68-years-old in the disease group and control samples were selected from the same age range. The male to female ratio was 1.77 in the disease group and 1.65 in the control group.
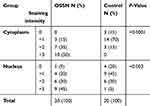
Overall, the mean of epithelial cytoplasmic staining area for IL-6 in the control and OSSN groups were 30±15.98 and 63.5±25.96percent and therefore, was considerably more in OSSN (P-value<0.0001). Nuclear staining was also significantly more in OSSN compared to the controls (P-value of <0.003). The cytoplasmic staining grade in OSSN group was mainly +3 while it was 0 and +1 in most cases of the control group. Table 1 shows the intensity of cytoplasm and nuclear staining among OSSN and control groups.
Considering the subgroups in OSSN (CIN I, II, III and SCC), even though the cytoplasm and nucleus stained more intensely in higher grades of dysplasia in comparison with lower grades, the difference was not statistically significant (P-value: 0.766 for nuclear staining and and 0.079 for cytoplasm). The related data is presented in Table 2.
 |
Table 2 Staining distribution in the nucleus and cytoplasm in different grades of dysplasia |
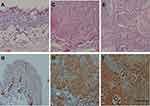
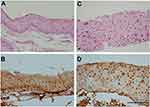
Figure 1A–F show that the cytoplasmic staining intensity for interleukin-6 in control samples is less than the OSSN group. Intense nuclear staining is observed in dysplasia cases as well as in one case among the control group (Figure 2A–D).
Discussion
In this study, we have shown that the expression of IL-6 is higher in the dysplastic tissue of the ocular surface compared to non-neoplastic samples At the time of writing, many different characteristics of IL-6 have been described in cancer. For example, a high serum level of IL-6 was observed in patients with lymphatic, bladder, and renal cancer.13
The function of IL-6 is highly dependent on the cell type; since it goes through different signaling pathways based on the cell characteristics;14 for example, it has an accelerating effect on cell growth in multiple myeloma, while it seems to have inhibitory effect on cell growth in breast and lung cancer.15
Levels of IL-6 rise in some chronic inflammatory conditions as well, which ultimately might lead to unexpected cell growth and progression to cancer.16 It has also been revealed that the IL-6 level is significantly higher in a cancerous bladder rather than cystitis and therefore, can be used as a differentiating marker.17,18
The function of this protein in tumors is assumed to be conducted by interfering with apoptosis pathway. IL-6 is secreted from cancerous cells, which may induce or inhibit apoptosis via paracrine or autocrine mechanisms.19 On the other hand, IL-6 and its target molecule STAT3, play an essential role in promoting angiogenesis in some cancerous tissue, especially in cervical cancer.20 The role of IL-6 has been identified in many other epithelial tumors, such as ovarian cancer21 and some head and neck tumors;3,14 however, to the best of our knowledge, there is no published data on its significance in ocular surface neoplasms and our study revealed a possible role for this cytokine. In the study by Di Girolamo10 on pterygia, superficial epithelial cells at the head of pterygium showed moderate expression of IL-6 but the expression was minimal or absent at basal epithelium. Even though Di Girolamo did not include dysplastic cases, the study reported that invading squamous cells showed intense staining,10 which is in accordance with our findings. Induction of angiogenesis, proliferation of epithelium and anti-apoptotic properties of cytokines are the proposed mechanisms involved.22–24
Traditionally, planning for the treatment of OSSN is based on the size of lesion and stage of the tumor. Surgical excision is the conventional treatment for localized lesions;25 however, large or diffused lesions cannot be excised due to complications, such as limbal stem cell deficiency.1 Primary topical chemotherapy with interferon alpha or mitomycin C is considered for large or diffuse lesions.1,25 Sometimes, a combination of surgery and chemotherapy is needed. Nevertheless, recurrence rate with any modality of treatment is still high1,26 and targeted immunotherapy like blocking cytokines can be an area for further investigation.
IL expression also may be used to determine prognosis; as has been suggested in head and neck tumors.3
According to our study, cytoplasmic staining can be a marker to differentiate between dysplasia and epithelial hyperplasia. Significance of nuclear staining is not clear since we observed it in non-neoplastic conjunctiva as well, especially in basal epithelial cells. The mechanisms of signaling may include direct action of IL-6 on nuclear structures rather than the previously explained IL-6/STAT3 signaling and myoferlin.27,28
In conclusion, cytoplasmic IL-6 staining can merely identify the invasive lesions from the non-neoplastic state; however, the intensity of staining in our study failed to correlate with the severity of dysplasia. The inability to show the association between the intensity of cytoplasmic staining with the severity of dysplasia in our study might be due to the small sample size in each category. Larger sample sizes are needed to show the correlation, if any.
Our findings may also be helpful in terms of finding new targeted treatments in OSSN, especially in diffuse and recurrent lesions that do not responsd to the available chemotherapeutic agents.
Abbreviations
IL, Interleukin; OSSN, Ocular surface squamous neoplasia; CIN, Conjunctival intra epithelial neoplasia.
Acknowledgment
We would like to thank Dr. Negar Azarpira at the Pathology Department and Ms. Hakimzadeh for their help, and Ms Tayyebe Shabanian for technical support in slide preparation and interpretation. Also, the authors wish to thank Mr. H. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript. The present article is extracted from the thesis written by Vahid Beigi, MD under the supervision of Dr. Masoomeh Eghtedari which was funded by a grant from Shiraz University of Medical Sciences [No. 8977].
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Heikkilä K, Ebrahim S, Lawlor DA. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer. 2008;44(7):937–945. doi:10.1016/j.ejca.2008.02.047
2. Simbiri KO, Jha HC, Dzeng RD, Massaro-Giordano G, Robertson ES. Cytokine and chemokine expression profiles in HIV-1 infected patients with ocular surface squamous neoplasia from Botswana. Cancer Clin Oncol. 2012;1(2):10. doi:10.5539/cco.v1n2p10
3. Duffy SA, Taylor JM, Terrell JE, et al. Interleukin‐6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113(4):750–757. doi:10.1002/cncr.23615
4. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610–621. doi:10.1056/NEJMra052723
5. Barton BE. IL-6: insights into novel biological activities. Clin Immunol Immunopathol. 1997;85(1):16–20.
6. Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–2512. doi:10.1016/j.ejca.2005.08.016
7. Rudkin AK, Dempster L, Muecke JS. Management of diffuse ocular surface squamous neoplasia: efficacy and complications of topical chemotherapy. Clin Experiment Ophthalmol. 2015;43(1):20–25. doi:10.1111/ceo.12377
8. Besley J, Pappalardo J, Lee GA, Hirst LW, Vincent SJ. Risk factors for ocular surface squamous neoplasia recurrence after treatment with topical mitomycin C and interferon alpha-2b. Am J Ophthalmol. 2014;157(2):287–293. e282. doi:10.1016/j.ajo.2013.10.012
9. Jongkhajornpong P, Nakamura T, Sotozono C, Nagata M, Inatomi T, Kinoshita S. Elevated expression of ABCB5 in ocular surface squamous neoplasia. Sci Rep. 2016;6:20541. doi:10.1038/srep20541
10. Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D. UVB-mediated induction of interleukin-6 and −8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2002;43(11):3430–3437.
11. Kennedy M, Kim KH, Harten B, et al. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cell. Invest Ophthalmol Vis Sci. 1997;38(2483):2491.
12. Ma IH, Hu FR, Wang IJ, et al. Clinicopathologic correlation of ocular surface squamous neoplasia from a university hospital in North Taiwan 1994 to 2014. J Formos Med Assoc. 2019;118(4):776–782. Epub 2018 Sep 25. doi:10.1016/j.jfma.2018.09.001
13. Salgado R, Junius S, Benoy I, et al. Circulating interleukin‐6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103(5):642–646. doi:10.1002/ijc.10833
14. Wei L-H, Kuo M-L, Chen C-A, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22(10):1517. doi:10.1038/sj.onc.1206226
15. Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57(1):141–146.
16. Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4(4):221–233. doi:10.1158/1541-7786.MCR-05-0261
17. Cardillo MR, Sale P, Di FS. Heat shock protein-90, IL-6 and IL-10 in bladder cancer. Anticancer Res. 2000;20(6B):4579–4583.
18. El-Salahy EM. Evaluation of cytokeratin-19 & cytokeratin-20 and interleukin-6 in Egyptian bladder cancer patients. Clin Biochem. 2002;35(8):607–613.
19. Wei L-H, Kuo M-L, Chen C-A, et al. The anti-apoptotic role of interleukin-6 in human cervical cancer is mediated by up-regulation of Mcl-1 through a PI 3-K/Akt pathway. Oncogene. 2001;20(41):5799. doi:10.1038/sj.onc.1204733
20. Zhong Z, Wen Z, Darnell JE. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264(5155):95–98. doi:10.1126/science.8140422
21. Plante M, Rubin SC, Wong GY, Federici MG, Finstad CL, Gastl GA. Interleukin‐6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer. 1994;73(7):1882–1888. doi:10.1002/1097-0142(19940401)73:7<1882::aid-cncr2820730718>3.0.co;2-r
22. Cohen T, Nahari D, Cerem LW, Neufeld G, Levi B-Z. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi:10.1074/jbc.271.2.736
23. Goswami S, Gupta A, Sharma SK. Interleukin-6-mediated autocrine growth promotion in human glioblastoma multiforme cell line U87MG. J Neurochem. 1998;71:1837–1845. doi:10.1046/j.1471-4159.1998.71051837.x
24. Jee SH, Shen SC, Chiu HC, Tsai WL, Kuo ML. Overexpression of interleukin-6 in human basal cell carcinoma cell lines increases anti-apoptotic activity and tumorigenic potency. Oncogene. 2001;20:198–208. doi:10.1038/sj.onc.1204076
25. Hirst LW. Randomized controlled trial of topical mitomycin C for ocular surface squamous neoplasia: early resolution. Ophthalmology. 2007;114(5):976–982. doi:10.1016/j.ophtha.2006.09.026
26. Shields CL, Kaliki S, Kim HJ, et al. Interferon for ocular surface squamous neoplasia in 81 cases: outcomes based on the American Joint Committee on Cancer classification. Cornea. 2013;32(3):248–256. doi:10.1097/ICO.0b013e3182523f61
27. Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochemi J. 1998;334(2):297–314. doi:10.1042/bj3340297
28. Yadav A, Kumar B, Lang JC, Teknos TN, Kumar P. A muscle-specific protein ‘myoferlin’modulates IL-6/STAT3 signaling by chaperoning activated STAT3 to nucleus. Oncogene. 2017;36(46):6374. doi:10.1038/onc.2017.245
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.