Back to Journals » Patient Preference and Adherence » Volume 13
Exploring stroke survivors’ self-efficacy in understanding and taking medication and determining associated factors: a cross-sectional study in a neurology clinic in Malaysia
Authors Appalasamy JR , Joseph JP, Seeta Ramaiah S, Quek KF, Md Zain AZ , Tha KK
Received 10 May 2019
Accepted for publication 20 July 2019
Published 28 August 2019 Volume 2019:13 Pages 1463—1475
DOI https://doi.org/10.2147/PPA.S215271
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Jamuna Rani Appalasamy,1 Joyce Pauline Joseph,2 Siva Seeta Ramaiah,3 Kia Fatt Quek,1 Anuar Zaini Md Zain,1 Kyi Kyi Tha1
1Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Bandar Sunway, Selangor, Malaysia; 2Department of Neurology, Hospital Kuala Lumpur, Ministry of Health, Kuala Lumpur, Malaysia; 3Medical Department, Subang Jaya Medical Center, Sunway, Malaysia
Correspondence: Jamuna Rani Appalasamy
Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Jalan Lagoon Selatan, Bandar Sunway, Selangor 47500, Malaysia
Tel +60 12 325 3775
Email [email protected]
Background and aim: Evidence-based prescribing practices for stroke-preventive medication have benefited stroke survivors; however, medication-nonadherence rates remain high. Medication understanding and use self-efficacy (MUSE) has shown great importance in medication-taking behavior, but its relationship with medication nonadherence in stroke-preventive regimens lacks exploration. The aim of this study was to determine the prevalence of MUSE and its association with nonadherence causes and other potential factors among stroke survivors in Malaysia.
Methods: This cross-sectional study was conducted among 282 stroke patients who provided informed consent and were in follow-up at the Neurology Outpatient Department of Hospital Kuala Lumpur, Malaysia. The study employed a data-collection form that gathered information on sociodemographics, clinical treatment, outcome measures on MUSE, and medication-nonadherence reasons.
Results: The prevalence of poor medication understanding and use self-efficacy among stroke patients was 46.5%, of which 29.1% had poor “learning about medication” self-efficacy, while 36.2% lacked self-efficacy in taking medication. Beliefs about medicine (74.02%) was the commonest reason for medication nonadherence, followed by medication-management issues (44.8%). In the multivariate model, independent variables significantly associated with MUSE were health literacy (AOR 0.2, 95% CI 0.069–0.581; P=0.003), medication-management issues (AOR 0.073, 95% CI 0.020-0.266; P<0.001), multiple-medication issues (AOR 0.28, 95% CI 0.085–0.925; P=0.037), beliefs about medicine (AOR 0.131, 95% CI 0.032–0.542; P=0.005), and forgetfulness/convenience issues (AOR 0.173, 95% CI 0.050–0.600; P=0.006).
Conclusion: The relatively poor learning about medication and medication-taking self-efficacy in this study was highly associated with health literacy and modifiable behavioral issues related to nonadherence, such as medication management, beliefs about medicine, and forgetfulness/convenience. Further research ought to explore these underlying reasons using vigorous techniques to enhance medication understanding and use self-efficacy among stroke survivors to determine cause–effect relationships.
Keywords: medication understanding, medication taking, self-efficacy, poststroke, cross-sectional
Plain-language summary
Medication-taking behavior is an important criterion in optimizing the effect of preventive treatment in chronic illness, such as stroke. This study depicts patterns and factors determining the importance of self-efficacy that influence medication-taking behavior among stroke patients in Malaysia. The study was carried out on 282 informed and consented stroke patients from September 2017 to November 2017 via two valid and reliable survey measures: medication understanding and use self-efficacy (MUSE scale) and medication-nonadherence reasons (eleven-item Medication Adherence Reasons scale). The MUSE scale identified that 29.1% of the patients had poor “learning about medication” self-efficacy, while 36.2% lacked self-efficacy in taking medication. These characteristics are highly related to health-literacy and medication-adherence factors, such as medication management, beliefs about medicine, and forgetfulness/convenience. This findings helped to identify a focus area in the development of future patient-education interventions.
Background
Stroke has been identified to cause significant disability among its survivors, and its global prevalence has been projected to double by 2035.1,2 Approximately 23% of acute stroke cases recorded yearly in Malaysia are patients with recurrent stroke.3 Ischemic incidence has been reported to increase annually by 29.5% and almost 18.7% for hemorrhagic stroke.4 Stroke-prevention medication, such as antiplatelets and anticoagulants, have improved the morbidity and mortality of stroke patients.5–7 Nevertheless, it is crucial to sustaining medication adherence to achieve optimal treatment effects.8 However, medication-nonadherence prevalence is high among major chronic diseases, including stroke, despite patient-education innovations.9,10
The World Health Organization defines “adherence” as “the extent to which a person’s behavior — taking medication (TM), following a diet, and/or executing lifestyle changes — corresponds with agreed recommendations from a health-care provider”.11 Many Asian countries report 40%–80% medication-nonadherence rates in chronic illness.12,13 There are possibilities that medication nonadherence results in stroke-prevention deficits, leading to stroke recurrences.14 Also, poor adherence to medication predisposes these patients to complications, more hospital admissions, and higher health-care expenditure.15
Self-efficacy is defined as faith and confidence in oneself to be able to perform a specific action to achieve a goal.16 Social cognitive theory and the health-belief model propose that medication adherence is often influenced by “belief modifying factors” that relate to how one perceives their health problem, which determines individual self-efficacy toward likelihood to adhere to prescribed medications.17,18 Research has shown that nonadherence to stroke-preventive medications is associated with lack of medication understanding and use self-efficacy (MUSE) among stroke survivors, despite health education efforts.19 The need for a patient’s behavioral change is influenced by psychosocial factors, such as attitudes, other than demographic factors or health attributes, and it is difficult to confirm a consistent association between them due to related confounding causes, such as beliefs and perceptions, which are not easily influenced by education.20 Recurrent stroke-preventive medication is a long-term and asymptomatic therapy that requires constant medication adherence for optimal health outcomes. It is vital to recognize and identify self-efficacy cues that influence medication adherence, and hence efforts toward understanding patient self-efficacy in medication management, especially in medication-taking behavior, are warranted.
It is vital to recognize and identify specific medication-nonadherence cues as per local environments that influence MUSE, so as to guide in developing personalized behavioral interventions to enhance medication-taking behavior. To our knowledge, there has been limited evaluation of medication-taking self-efficacy tasks, such as understanding and using medication appropriately, among stroke patients. As such, to address this gap, the objective of this study was to explore self-efficacy prevalence and determine potential medication-nonadherence factors associated with self-efficacy in learning about medicine (LM) and TM in stroke patients. Limited resources are at hand regarding tailored intervention to enhance self-efficacy in medication management. Therefore, the study’s findings are necessary to elicit cues that influence self-efficacy in terms of medication-taking actions. These cues would help to ascertain appropriate outcome measures for personalized interventions and be of advantage in the development of patient-education tools for recurrent stroke.
Methods
Study settings and population
This cross-sectional single-center study was conducted from September 2017 to November 2017 among stroke in patients who were on follow-up at the Neurology Outpatient Department of Hospital Kuala Lumpur (HKL), Malaysia. HKL is the oldest and foremost tertiary hospital in Malaysia, and receives a high number of stroke patients and referred stroke patients from throughout Malaysia: about 1,000 in patients with neurological disorders, including acute and recurrent stroke, annually.21
Patient invitation was done via convenience sampling from a list of the neurology clinic’s medical record. Inclusion criteria were adults (age >18 years), diagnosed with first stroke within six months from the initial screening, and on stroke-preventive medication, such as antiplatelets or anticoagulants. Those who had been diagnosed with depression and impaired memory were excluded. Only those able to converse, read, and write in Malay or English were selected. Patients who agreed to participate were informed about the study objectives, and their consent were obtained. They were given a choice of responding to the questionnaires during their visit to the clinic or at home. Responses were retrieved on the same day or by post.
Sample size
There is a lack of research on stroke patients. Therefore, references on appropriate sample size for significant end results were not available. However, a meta-analysis on beliefs and medication adherence of 94 studies quoted an average sample of 266.22 Based on a sample-size calculation for a study of finite population23 and with consideration of the annual number of inpatients at HKL, a sample of 278 patients was considered adequate to elucidate significance in this study with a margin of error of ±5%. An attrition rate of 15% was considered to compensate for missing data and nonresponse, for a final sample of 320.24,25
Ethics statement
Ethical approvals were obtained from the Malaysian Medical Research and Ethics Committee, Ministry of Health Malaysia (National Medical Research Register ID 15–851-24,737) and the Monash University Human Research Ethics Committee (ID 9640). This study contributes to preliminary findings of the MyStrokeStory trial, which was registered with the Australian New Zealand Clinical Trials Registry (Australian clinical trials registration number 12618000174280) under Universal Trial Number U1111-1201–3955.
Study instruments
Patient information was derived from an interviewer-assisted data-collection form consisting of two sections: sociodemographic information (sex, age, ethnicity, education attainment, and health literacy) and clinical health information documented in medical records. Health literacy was assessed using the Newest Vital Sign.26 Medical records included such information as type of stroke, stroke severity, stroke-risk factors (eg, diabetes, hypertension, or hyperlipidemia) and baseline blood parameters that defined disease comorbidities. Hypertension was defined by blood pressure >140/90 mmHg for those with no diabetes and >130/80 mmHg for those with diabetes. Patients were diagnosed as diabetic if their A1c was >6%, venous fasting plasma >7 mmol/L, and random plasma >10 mmol/L.27 Those with hyperlipidemia were defined by low-density-lipoprotein cholesterol >3.4–4.2 mmol/L and triglycerides >8.3 mmol/L. International normalized ratio control for patients with atrial fibrillation was 2–3.28–30
The primary outcome for this study was MUSE using a validated self-rated scale developed by Cameron et al.31 MUSE is a brief eight-item questionnaire able to measure patient self-efficacy in LM and taking them appropriately. Patients were asked to give ratings of 8–32 on the four-item scale: if they agreed or disagreed, and the extent to which they agreed or disagreed slightly or strongly. Scale scores ≥3 for each item were associated with higher self-efficacy. The TM domain constituted four items: “It is easy for me to take my medicine on time”, “It is easy to remember to take all my medicines”, “It is easy for me to set a schedule to take my medicines each day”, and “It is easy for me to take my medicines every day”. The LM domain consisted of the items “It is easy for me to ask my pharmacist questions about my medicine”, “It is easy for me to understand instructions on medicine bottles”, “It is easy for me to get all the information I need about my medicine”, and “It is easy for me to understand my pharmacist’s instructions for my medicine”. This scale has been found suitable to be used for primary-care outpatients regardless of age, sex, education, or number of medications, and has been adapted for patients with diabetes.32 However, due to the small study sample, the authors translated MUSE according to standard guidelines33 and pretested it among 150 stroke patients prior to this study. Good comprehension and relevance to construct (item content–validity index [i-cvi] values >0.83 were obtained from ten bilingual patients during the face- and content-validity phase. Principal-component analysis with item-factor loading >0.5, and internal consistency Cronbach’s α approximately 0.7, for both the LM and TM domains confirmed no modifications were required for the translated version. Test–retest reliability (within 2 weeks apart) in a sample of 36 patients resulted in an intraclass correlation coefficient >0.7 being derived for both domains. Therefore, the English and Malay MUSE versions were considered valid and reliable to be used for stroke patients in Malaysia.
The authors believed that a good medication-adherence measure would be able to elucidate perceived reasons contributing to medication nonadherence. The self-administered eleven-item Medication Adherence Reasons Scale (MAR-Scale) in Malay and English was used to list reasons for medication nonadherence apart from assessing its level in this study. The 15-items MAR-Scale was originally developed in English by Unni.34 It was then translated to Malay and modified to the eleven-items MAR-Scale by Shima et al,35 which was pretested among 665 patients diagnosed with chronic diseases from government health-care settings. Initially, 15 items were retained via exploratory factor analysis, of which five extracted factors inclusive of “availability issues” achieved eigenvalues >1. However, on confirmatory factor analysis), only eleven items demonstrated good convergent validity and adequate discriminant validity. The scale also demonstrated adequate factorial invariance across sex and ethnicity. The eleven items were consolidated into four domains: managing issues, four items; multiple-medication issues, two items; beliefs about medication issues, three items; and forgetfulness and convenience issues, two items. Since patients in this study had also been diagnosed with chronic diseases as per inclusion criteria, the eleven-item Malaysian MAR-Scale was considered valid and reliable to be used among stroke patients. Patients who had missed taking their medications were requested to quantify the number of days that they had been nonadherent and to indicate the reasons for missing their medications using a five-point Likert scale, with patients who scored ≤11 for no medication-adherence issues classed as adherent and >11 as nonadherent.
Data-collection procedures
Patients were approached with information sheets and informed-consent forms at the Neurology Outpatient Clinic while they were waiting for their appointments. Those who had consented to participate in the study were asked to fill both self-administered questionnaires, which took <10 minutes to complete. Patients who opted to complete the questionnaires at home were asked to send their replies via a self-addressed envelope provided to them.
Statistical analysis
Descriptive statistics were used for all variables and outcomes in this study, with χ2 tests via cross-tabulation analysis used to explore significant associations between sociodemographic variables, eg, age, sex, and health literacy, and stroke-treatment characteristics, eg, blood-pressure control, prescribed number of medications, and exposure to previous stroke education with MUSE (poor vs good). Similar analysis was also performed to explore associations between medication-nonadherence categories with MUSE, and the strength of all these relationships was determined by Cramér’s V and φ. Mann–Whitney U tests and Kruskal–Wallis test were performed to determine significant differences (P<0.001) between dependent variables: MUSE and medication adherence with two or more groups of independent variables (potential associated medication-nonadherence factors). Multinomial logistic regression was performed to assess associations between MUSE LM and TM (three categories) and MAR-Scale constructs and related sociodemographic and treatment characteristics. Results were considered significant if P<0.05 using two-sided t-tests or Wald tests. Statistical analyses were performed using SPSS 24.0.
Results
Sample characteristics
A total of 320 patients with stroke were informed and invited to participate in the study, and 282 (88.1% response rate) consented to participate: 189 (67%) males and 93 (33%) females. The mean age of the patients was 57±12 (27–92) years. The bulk of them 155 (55%) were Malay, followed by 74 (26.2%) Indian and 46 (16.3%) Chinese. Almost half the sample had completed secondary education (55.3.1%), 19.5% had completed tertiary education, and those with good health literacy accounted for 182 (64.5%) of the study population. With regard to disease comorbidities, 66.7% had been diagnosed with ischemic stroke, followed by TIA (30.9%), with hypertension being the main stroke-risk factor (97.2%). A majority of patients perceived themselves to have been exposed to stroke education or were familiar with stroke knowledge and its preventive management (78.4%, Table 1).
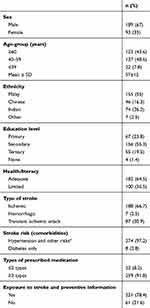 |
Table 1 Sociodemographics and health information of patients with stroke (n=282) |
MUSE and medication-nonadherence attributes
Cronbach’s α-coefficient for MUSE was >0.7, whereas that for the MAR-Scale was 0.57, which suggests that these measures had acceptable internal consistency suitable for the Malaysian stroke-patient population. The prevalence of poor MUSE among patients with stroke in this study was 46.5% with mean MUSE score of 25.32±4.18, whereas prevalence of medication nonadherence was 53.9% with mean number of issues of 1.24±1.41. Within-case analysis showed prevalence of low LM self-efficacy of 29.1% with mean score of 10.01±1.16, whereas prevalence of low self-efficacy in TM was 36.2%, with a mean score of 9.96±1.09. Beliefs about medicine (74.02%) was the commonest reason for medication nonadherence, followed by medication-managing issues (44.8%) and forgetful and convenience issues (40.25%). A total of 97 nonadherent patients (62.9%) had a minimum of one belief about medicine as a nonadherence factor. The primary factor for ischemic stroke for the majority of these patients was hypertension. As such, only blood pressure was analyzed and reported. Other blood parameter, eg, HBA1c, low-density lipoprotein, and international normalized ratio, are not reported, as some medical records were unavailable and the sample too small for significant analysis.
An initial analysis (unadjusted) using Pearson's χ2 was performed to identify associations between patient sociodemographic characteristics, stroke treatment, and medication adherence with MUSE (Table 2). In this analysis, MUSE was dichotomized to “poor” and “good”, whereby total scores >22 were considered good, provided that LM and TM total scores were each >11 (with the assumption that each question was scored 3 or 4 according to the authors’ preliminary clinical practice observation at HKL). Similarly for the MAR-Scale, those patients who have scored 11 with the assumption that each question was scored 1 were considered to have good medication adherence compared to those whose score was >11, who were classified as having poor medication adherence.
 |
Table 2 Association of sociodemographics, stroke treatment, and medication-nonadherence reasons with Medication Understanding and Use Self-Efficacy Scale (MUSE) scores |
MUSE scores were significantly associated with positive and strong relationships with medication-adherence scores (Cramér’s V/φ=0.548, P<0.001). Patients with poor MUSE scores were more likely to develop poor medication adherence (OR 12.44, P<0.001) than those with good MUSE scores. MUSE scores were significantly associated (negative correlation) with all adherence-category scores; however, the strength of their relationship was stronger for management issues and beliefs about medication than multiple-medication issues and forgetfulness/convenience issues. Interestingly, only health literacy was significantly associated with MUSE (Cramér’s V/φ=–0.276, P<0.001) compared to other sociodemographic and treatment criteria, such as age, sex, medication quantity, exposure to stroke education, and blood-pressure control. Further bivariate analysis showed a significant association with positive correlation of moderate strength (Cramér’s V/φ=0.472, P<0.001) between health literacy and education background.
Similarly to MUSE analysis, health literacy and education background were factors significantly associated with medication adherence; however, age (Cramér’s V=0.182, P=0.003) and exposure to stroke education (Cramér’s V=0.123, P=0.043) were also significantly associated with medication adherence, but with weak negative correlation. There were significant differences in distribution (P<0.001) between MUSE categories with medication adherence, health literacy, and education attainment, whereas for medication-adherence categories, there were additional significant differences in distribution (P<0.001) with age and exposure to stroke education apart from MUSE scores, health literacy, and education background.
Independent variables of sociodemographic criteria, stroke treatment, and medication adherence were examined concurrently in the multinomial logistic regression, as presented in Table 3. The final model with adequate R2 and the model fitting information (Nagelkerke's R2=0.515, P<0.001) predicting percentage correct (71.3%) estimated gross effects of selected independent variables on MUSE. Likelihood-ratio tests identified four variables that were significantly associated with MUSE categories. They were health literacy (P=0.010) and three adherence categories: managing issues (P<0.001), beliefs about medicine (P=0.007) and forgetfulness/convenience issues (P=0.009). There were marginal discrepancies between the “Both LM and TM poor” and “Either LM or TM poor” categories with reference to the “Both LM and TM good” category. In the “Both LM and TM poor” category, patients with stroke were more likely to have health-literacy problems and all medication-adherence issues than patients who were in the “Either LM or TM poor” group, who were only more likely to have two medication-adherence issues: managing issues and forgetfulness/convenience issues. Further analysis discovered that with other independent variables held constant, the odds of patients not having medication-managment issues and forgetfulness/convenience issues and being in the “Either LM or TM poor” group rather than the “Both LM and TM poor” group were approximately twice those of patients with these issues.
 |
Table 3 Factors associated with Medication Understanding and Use Self-efficacy Scale using multinomial logistic regression analyses |
Discussion
The prevalence of medication nonadherence in this study (53.9%) was comparable with another Malaysian study36 and relatively lower if compared to a recent study by Ganasegaran et al.13 However, there have been no substantial reports on poor MUSE prevalence (46.5%) in other studies of similar population. Differences in medication-nonadherence prevalence could be attributed to discrepancies in population samples, methodologies, and outcome measures across all related studies locally and internationally.12 For example, it is a norm for Asian and developing nations with different health systems to adopt self-report measures to assess medication adherence compared to their counterparts from developed nations, who have resources and access to established pharmacy refill data. Nevertheless, since medication nonadherence is a dynamic process that involves multiple behavioral attributes,37 the use of reliable outcome measures is warranted.
This cross-sectional study supports the hypothesis and findings of Cameron et al,31 higher scores on MUSE correspond with higher medication adherence, despite variances in population sample and methodology. It is more likely that a patient with high self-efficacy in understanding and using prescribed medication appropriately has a higher tendency to adhere to medications and vice versa.38 According to Bandura,17 self-efficacy influences a person to reflect and make efforts that then emotionally and motivationally react toward a particular action.39 It is a crucial construct as a cognitive process of the social cognitive theory,40 which can predict positive health behavior in patients with chronic illnesses, despite various challenges in being adherent.41–43 However, self-efficacy is influenced by such factors as experiences and gained skills, role models and motivation, verbal persuasion, or physiological symptoms.44 As such, it was essential to acknowledge the effect of each construct that contributed to the final MUSE scores related to medication adherence. This effect is apparent in Table 3, whereby potential factors influencing low MUSE scores — poor LM and poor TM self-efficacy — were health literacy, beliefs about medicine, multiple medication issues, medication-management issues, and forgetfulness/convenience issues.
Sociodemographic factors, such as age and exposure to stroke education, were only significantly associated with medication adherence compared to MUSE, so we considered these attributes as potential confounding factors. However, surprisingly blood-pressure control was not at all associated with both medication adherence and MUSE. There have been mixed results from previous studies refuting the view that older age correlates with medication nonadherence.45 Nonetheless, knowing that stroke risk increases with age and comorbidity incidence is higher among the elderly, the possibility of medication nonadherence was significant in our study outcomes and thus debatable.46 Exposure to stroke awareness and educational materials play an important role in self-efficacy and medication adherence, but on the contrary our findings were insignificant for MUSE if compared to other studies.47–50 This paradoxical result could be attributed to a likelihood that the success of patient education depended on the type of information and mode of delivery. A recent video-based educational intervention with patients with stroke resulted in a positive association with improved self-efficacy,51 which helped to explain this insignificant association. Our sample of patients received various types of information on stroke, hypertension, diabetes, and, hyperlipidemia. The most common mode of delivery was printed materials and oral presentations. Therefore, we had a pool of patients with unpersonalized exposure toward learned skills, whereby merely reading and receiving information was insufficient to boost self-efficacy among stroke patients who were susceptible emotionally. In terms of blood-pressure control, our analysis contradicted recent studies that found poor medication-adherence rates negatively to affect blood-pressure control.36,52–54 Discrepancies would have possibly occurred due to variance in study objectives, patient samples, and type of intervention. For example, Tan et al compared medication-adherence intervention against blood-pressure improvement among hypertensive patients. The majority of our patient sample were hypertensive and had varying degrees of comorbidity that were not stratified or controlled for comparison. Our study assessed general medication adherence, which also involved various type of medication inclusive of stroke-preventive medication. Therefore, it is necessary that each type of medication is explored for its association with MUSE and medication adherence.
In this study, health literacy showed a positive association with MUSE and medication adherence, which corresponded with other research.55–57 In contrast, some studies contradict these findings, as they found no association, even though a similar health-literacy scale (Newest Vital Sign) was used in one of the studies. Plausible explanations of discrepancies have been sample size, different population and disease characteristics, and dissimilar self-efficacy measures.58,59 Interestingly, study analysis also found a significant relationship between health literacy and educational attainment, which were positively associated with MUSE and medication adherence.60,61 However, making education attainment a prerequisite focus for medication self-efficacy interventions would require further research, as its outcome may be subject to confounding factors, such as socioeconomic and motivation status and skill–practice sustainability.
The self-efficacy concept is also an addition to the health belief model, which proposes a readiness to act of individuals based on perception: perceived susceptibility, perceived severity, perceived benefits, and perceived barriers.18 Perceived susceptibility and severity are driven by knowledge, health practices, and beliefs about the illness and the symptoms experienced by the individuals or family.17 In terms of treatment, these perceptions influence one's actions in so far as thinking about the advantages of adhering to medication outweighing its disadvantages. As such, beliefs about medicines are strongly associated with medication adherence. Our data on the association of self-efficacy in understanding and taking medication appropriately with beliefs about medicine were comparable with studies that proved a similar effect on medication nonadherence.62–64 However, according to McCulley et al,65 patients with higher self-efficacy had poor adherence. Their findings tallied with patients from the “Either LM or TM poor” group compared to patients from other groups. It is possible these results cannot be generalized to the whole population, due to a diverse sample and research methodology. Nonetheless, we concluded that if this relationship remains constant, a specific group of stroke patients were perhaps confident in their health practices or alternative treatments, and thus opted not to adhere to prescribed medications.66 These discoveries show that further research is necessary to explore underlying factors leading to these phenomena.
Medication-management issues were associated with all MUSE categories, which depended very much on the ability of individuals to be responsible in applying skills to learn, plan, and ensure the right prescribed medications were taken appropriately for optimum health outcomes. These findings are consistent with studies that proved the ability to reflect medication-taking actions and to seek resources to improve one’s condition is of utmost importance in differentiating individuals with high self-efficacy from those without.67–69 Despite these impressive results, we concluded medication-management issues were not a stand-alone influencer, as they could also be related to other underlying confounding attributes, such as forgetfulness and convenience issues, which requires further exploration. There were possibilities that forgetfulness issues were caused by the aging factor.70 However, another study contradicts this, proving that it was perceived low memory causing perceived forgetfulness wherein literacy and mental health were predisposing factors.71 Nonetheless, this situation was unavoidable, as the study would have had a patient sample with deteriorating cognitive effects, as they were stroke patients who had higher risks of low memory capacity, causing skewed data.72,73 Our study showed that multiple-medication issues had a significant positive association with poor MUSE and medication nonadherence. A recent study by Addo et al74 supports our findings, but another study by Grant et al does not support this association.75 This study found no association with the number of medications, even though the majority of patients took more than three types of medication. Due to the diversity in types of disease, illness severity, and dosing regimens, this observation is interpreted with caution. As such, it is very subjective to conclude that medication quantity prescribed to stroke patients influences MUSE. Cost-effectiness data for our study were excluded, because all our patient sample received prescribed regimens provided by the hospital from the Ministry of Health’s subsidized health scheme.
Study limitations
This cross-sectional study could not establish temporal relationships. Susceptibility of biases of outcome measures could have existed, causing overestimation or underestimation of MUSE, medication-nonadherence prevalence, and determination of factor associations. Generalizability of the results was not established (as depicted by inconsistent confidence intervals), most probably due to samples being from a single site, although significant odds ratios for factors were determined. Other than that, the majority of patients were >50 years old, due to the nature of the disease, which explains the distribution of the final data. Also, our outcome measures were self-administered, which adds more bias. It should be noted that these significant factor interrelations, which were retained in the multinomial analysis of MUSE and medication nonadherence, are still debatable, whereas those excluded factors that existed as confounding elements would require further exploration as potential predictors. The possibility of overlapping questions of different constructs in both outcome measures would have caused close correlation. Nevertheless, self-efficacy in understanding and taking medication, especially when related to medication nonadherence, is a dynamic behavior concern, and thus it was impossible to explore all factors in one study, as this involves cognitive skills, abilities, and beliefs.76
Conclusion
This study enabled the identification and determination of potentially modifiable characteristics of medication nonadherence in terms of MUSE among stroke patients. These findings are suggestive that MUSE and medication nonadherence are interrelated, and thus new emerging personalized behavioral interventions are warranted to address the need for improved medication-taking behavior for a specific niche among stroke survivors. It would be interesting to explore interventions based on patient stratification related to MUSE-outcome measures. With this, hopefully, MUSE could be enhanced to promote stroke risk–factor control and long-term reduction of stroke recurrence.
Data-sharing statement
All data available are within the judiciary of the Director General of Health Malaysia and are the intellectual property of Monash University.
Acknowledgments
The authors would like to thank the Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia for their financial, material, and other support. The authors wish to acknowledge the contributions of doctors, clinic nurses and patients from the Neurology Clinic, Hospital Kuala Lumpur. We are grateful to the head of the Clinical Research Centre of Hospital Kuala Lumpur and the head of the Neurology Clinic of Hospital Kuala Lumpur for their support and approval for conducting this study. We would like to thank the Director General of Health Malaysia for his permission to publish this article. This study was part of research performed by JRA in fulfillment of her PhD from Monash University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. The top 10 causes of death. 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
2. Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global burden of diseases, injuries, risk factors study 2010 (GBD 2010); GBD stroke experts group. Lancet Global Health. 2013;1(5):e259–e281. PMID:25104492. doi:10.1016/S2214-109X(13)70089-5
3. Loo KW, Gan SH. Burden of stroke in Malaysia. Int J Stroke. 2012;7(2):165–167. doi:10.1111/j.1747-4949.2011.00767.x
4. Aziz ZA, Lee YY, Ngah BA, et al. Acute stroke registry Malaysia, 2010–2014: results from the national neurology registry. J Stroke Cerebrovasc Dis. 2015;24(12):2701–2709. doi:10.1016/j.jstrokecerebrovasdis.2015.07.025
5. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–1935. doi:10.1016/s0140-6736(14)60584-5
6. Antithrombotic Trialists' Collaboratio.n Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7330):141. doi:10.1136/bmj.324.7330.141
7. Orourke F. Current and future concepts in stroke prevention. Can Med Assoc J. 2004;170(7):1123–1133. doi:10.1503/cmaj.1031185
8. Brown MT, Bussells JK. Medication adherence: WHO cares? SciVee. 2011. doi:10.4016/27949.01
9. Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open. 2018;8(1):e016982. doi:10.1136/bmjopen-2017-016982
10. Stenberg U, Vågan A, Flink M, et al. Health economic evaluations of patient education interventions a scoping review of the literature. Patient Educ Couns. 2018;101(6):1006–1035. doi:10.1016/j.pec.2018.01.006
11. Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003.
12. Akeroyd JM. Adherence to cardiovascular medications in the South Asian population: a systematic review of current evidence and future directions. World J Cardiol. 2015;7(12):938. doi:10.4330/wjc.v7.i12.938
13. Ganasegeran K, Rashid A. The prevalence of medication nonadherence in post-myocardial infarction survivors and its perceived barriers and psychological correlates: a cross-sectional study in a cardiac health facility in Malaysia. Patient Prefer Adherence. 2017;11:1975–1985. doi:10.2147/ppa.s151053
14. Schryver ELLM, Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Non–adherence to aspirin or oral anticoagulants in secondary prevention after ischaemic stroke. J Neurol. 2005;252(11):1316–1321. doi:10.1007/s00415-005-0858-0
15. Ganasegeran K, Perianayagam W, Manaf RA, Jadoo SAA, Al-Dubai SAR. Patient satisfaction in malaysia’s busiest outpatient medical care. Sci World J. 2015;2015:1–6. doi:10.1155/2015/714754
16. Bandura A, Al E, Williams SL, Mefford IN, Barchas JD. Catecholamine secretion as a function of perceived coping self-efficacy. J Consult Clin Psychol. 1985;53(3):406–414. doi:10.1037//0022-006x.53.3.406
17. Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–164. doi:10.1177/1090198104263660
18. Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the health belief model. Health Educ Q. 1988;15(2):175–183. doi:10.1177/109019818801500203
19. Jamison J, Sutton S, Mant J, Simoni AD. Barriers and facilitators to adherence to secondary stroke prevention medications after stroke: analysis of survivors and caregivers views from an online stroke forum. BMJ Open. 2017;7:7. doi:10.1136/bmjopen-2017-016814
20. Li S-C. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag. 2008;4:269–286. doi:10.2147/tcrm.s1458
21. Neurology [HKLWeb].Available from: http://www.hkl.gov.my/index.php?option=com_content&view=article&id=%20151.
22. Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS One. 2013;8:12. doi:10.1371/journal.pone.0080633
23. Creative Research Systems [Sample size calculator]; 2012. Available from: http://www.surveysystem.com/sscalc.htm.
24. Rashid A, Conroy R, Ahmad Z. I Hate Statistics! George Town. Malaysia: Penang Medical College; 2012.
25. Naing L, Winn T, Rusli BN. Practical issues in calculating the sample size for prevalence studies. Arch Orofac Sci. 2006;1:9–14.
26. Powers BJ, Trinh JV, Bosworth HB. Can this patient read and understand written health information? Jama. 2010;304(1):76. doi:10.1001/jama.2010.896
27. Ganasegeran K, Renganathan P, Manaf RA, Al-Dubai SAR. Factors associated with anxiety and depression among type 2 diabetes outpatients in Malaysia: a descriptive cross-sectional single-centre study. BMJ Open. 2014;4:4. doi:10.1136/bmjopen-2014-004794
28. Moon MA. Recurrent stroke prevention guidelines get an update. Internal Med News. 2010;43(18):38. doi:10.1016/s1097-8690(10)70936-7
29. Venketasubramanian N, Pwee KH, Chen CPL. Singapore ministry of health clinical practice guidelines on stroke and transient ischemic attacks. Int J Stroke. 2011;6(3):251–258. doi:10.1111/j.1747-4949.2011.00602.x
30. Karalis I. Antithrombotic therapy for atrialfibrillation. Thrombosis Clin Pract. 2005;97–118. doi:10.3109/9780203640357-7
31. Cameron KA, Ross EL, Clayman ML, et al. Measuring patients’ self-efficacy in understanding and using prescription medication. Patient Educ Couns. 2010;80(3):372–376. doi:10.1016/j.pec.2010.06.029
32. Abboud SAA. Validation of Malaysian versions of Perceived Diabetes Self-Management Scale (PDSMS), Medication Understanding and use Self- Efficacy Scale (MUSE) and 8-Morisky Medication Adherence Scale (MMAS-8) using partial credit rasch model. J Clin Diagn Res. 2016. doi:10.7860/jcdr/2016/15079.8845
33. Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46(12):1417–1432. doi:10.1016/0895-4356(93)90142-n
34. Unni EJ. Development of models to predict medication non-adherence based on a new typology [thesis]. Available at: http://ir.uiowa.edu/cgi/viewcontent.cgi?article=1195&context=etd. Accessed October 8, 2012. doi:10.17077/etd.ep3ttkid
35. Shima R, Farizah H, Majid H. The 11-item medication adherence reasons scale: reliability and factorial validity among patients with hypertension in Malaysian primary healthcare settings. Singapore Med J. 2015;56(08):460–467. doi:10.11622/smedj.2015069
36. Paraidathathu T, Ramli A, Sufiza AN. Medication adherence among hypertensive patients of primary health clinics in Malaysia. Patient Prefer Adherence. 2012;6:613. doi:10.2147/ppa.s34704
37. Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance. Med Care. 1998;36(8):1138–1161. doi:10.1097/00005650-199808000-00004
38. Redding CA, Rossi JS, Rossi SR, et al. Health behavior models. Int Electron J Health Educ. 2000;3:180–193.
39. Ironson G, Weiss S, Lydston D, et al. The impact of improved self-efficacy on HIV viral load and distress in culturally diverse women living with AIDS: the SMART/EST womens project. AIDS Care. 2005;17(2):222–236. doi:10.1080/09540120512331326365
40. Wood R, Bandura A. Social cognitive theory of organizational management. Acad Manage Rev. 1989;14(3):361. doi:10.2307/258173
41. Kott KB. Self-efficacy, outcome expectation, self-care behavior, and glycosylated hemoglobin level in persons with type 2 diabetes. West J Nurs Res. 2008;30(8):1028–1029. doi:10.1177/0193945908323637
42. Sleath B, Blalock SJ, Robin A, et al. Development of an instrument to measure glaucoma medication self-efficacy and outcome expectations. Eye. 2009;24(4):624–631. doi:10.1038/eye.2009.174
43. Wallston KA, Rothman RL, Cherrington A. Psychometric properties of the Perceived Diabetes Self-Management Scale (PDSMS). J Behav Med. 2007;30(5):395–401. doi:10.1007/s10865-007-9110-y
44. Bandura A. Organizational applications of social cognitive theory. Aust J Manage. 1988;13(2):275–302. doi:10.1177/031289628801300210
45. Krueger K, Botermann L, Schorr SG, Griese-Mammen N, Laufs U, Schulz M. Age-related medication adherence in patients with chronic heart failure: a systematic literature review. Int J Cardiol. 2015;184:728–735. doi:10.1016/j.ijcard.2015.03.042
46. Kissela BM, Khoury JC, Alwell K, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79(17):1781–1787. doi:10.1212/wnl.0b013e318270401d
47. Allison K. Adherence through education: a call to clinicians to educate all patients on medication use. Mental Health Clinician. 2012;2(4):83–85. doi:10.9740/mhc.n117761
48. Taibanguay N, Chaiamnuay S, Asavatanabodee P, Narongroeknawin P. Effect of patient education on medication adherence of patients with rheumatoid arthritis: a randomized controlled trial. Patient Prefer Adherence. 2019;13:119–129. doi:10.2147/ppa.s192008
49. Ndosi M, Johnson D, Young T, et al. Effects of needs-based patient education on self-efficacy and health outcomes in people with rheumatoid arthritis: a multicentre, single blind, randomised controlled trial. Ann Rheum Dis. 2015;75(6):1126–1132. doi:10.1136/annrheumdis-2014-207171
50. Lawrance L, Mcleroy KR. Self-efficacy and health education. J Sch Health. 1986;56(8):317–321. doi:10.1111/j.1746-1561.1986.tb05761.x
51. Denny MC, Vahidy F, Vu KYT, Sharrief AZ, Savitz SI. Video-based educational intervention associated with improved stroke literacy, self-efficacy, and patient satisfaction. PLoS One. 2017;12:3. doi:10.1371/journal.pone.0171952
52. Tan BY, Shafie AA, Hassali MAA, Saleem F. Assessment of medication adherence and the costs associated with a calendar blister pack intervention among hypertensive patients in Malaysia: a randomized controlled trial. SAGE Open Med. 2017;5:205031211770918. doi:10.1177/2050312117709189
53. Romanelli RJ, Schiro TA, Jukes T, Wong KS, Ishisaka DY. Disparities in blood pressure control within a community-based provider network: an exploratory analysis. Ann Pharmacother. 2011;45(12):1473–1482. doi:10.1345/aph.1q523
54. Pan J, Lei T, Hu B, Li Q. Post-discharge evaluation of medication adherence and knowledge of hypertension among hypertensive stroke patients in northwestern China. Patient Prefer Adherence. 2017;11:1915–1922. doi:10.2147/ppa.s147605
55. Lee Y-M, Yu HY, You M-A, Son Y-J. Impact of health literacy on medication adherence in older people with chronic diseases. Collegian. 2017;24(1):11–18. doi:10.1016/j.colegn.2015.08.003
56. Park NH, Song MS, Shin SY, Jeong J-H, Lee HY. The effects of medication adherence and health literacy on health-related quality of life in older people with hypertension. Int J Older People Nurs. 2018;13:3. doi:10.1111/opn.12196
57. Osborn CY, Cavanaugh K, Wallston KA, et al. Health literacy explains racial disparities in diabetes medication adherence. J Health Commun. 2011;16(sup3):268–278. doi:10.1080/10810730.2011.604388
58. Huang Y-M, Shiyanbola O, Smith P. Association of health literacy and medication self-efficacy with medication adherence and diabetes control. Patient Prefer Adherence. 2018;12:793–802. doi:10.2147/ppa.s153312
59. Kim S, Love F, Quistberg DA, Shea JA. Association of health literacy with self-management behavior in patients with diabetes. Diabetes Care. 2004;27(12):2980–2982. doi:10.2337/diacare.27.12.2980
60. Jansen T, Rademakers J, Waverijn G, Verheij R, Osborne R, Heijmans M. The role of health literacy in explaining the association between educational attainment and the use of out-of-hours primary care services in chronically ill people: a survey study. BMC Health Serv Res. 2018;18:1. doi:10.1186/s12913-018-3197-4
61. Heide IVD, Wang J, Droomers M, Spreeuwenberg P, Rademakers J, Uiters E. The relationship between health, education, and health literacy: results from the Dutch adult literacy and life skills survey. J Health Commun. 2013;18(sup1):172–184. doi:10.1080/10810730.2013.825668
62. Porteous T, Francis J, Bond C, Hannaford P. Temporal stability of beliefs about medicines: implications for optimising adherence. Patient Educ Couns. 2010;79(2):225–230. doi:10.1016/j.pec.2009.07.037
63. Wei L, Champman S, Li X, et al. Beliefs about medicines and non-adherence in patients with stroke, diabetes mellitus and rheumatoid arthritis: a cross-sectional study in China. BMJ Open. 2017;7:10. doi:10.1136/bmjopen-2017-017293
64. Achaval SD, Suarez-Almazor ME. Treatment adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosus. Int J Clin Rheumtol. 2010;5(3):313–326. doi:10.2217/ijr.10.15
65. Mcculley C, Katz P, Trupin L, Yelin EH, Barton JL. Association of medication beliefs, self-efficacy, and adherence in a diverse cohort of adults with rheumatoid arthritis. J Rheumatol. 2018;45(12):1636–1642. doi:10.3899/jrheum.171339
66. Shiyanbola OO, Unni E, Huang Y-M, Lanier C. Using the extended self-regulatory model to characterise diabetes medication adherence: a cross-sectional study. BMJ Open. 2018;8(11):e022803. doi:10.1136/bmjopen-2018-022803
67. Worley MM, Hermansen-Kobulnicky CJ. Outcome and self-efficacy expectations for medication management of patients with diabetes: influence of the pharmacist-patient relationship. J Am Pharm Assoc. 2008;48(5):621–631. doi:10.1331/japha.2008.07090
68. Náfrádi L, Nakamoto K, Schulz PJ. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS One. 2017;12:10. doi:10.1371/journal.pone.0186458
69. Oshotse C, Zullig LL, Bosworth HB, Tu P, Lin C. Self-efficacy and adherence behaviors in rheumatoid arthritis patients. Prev Chronic Dis. 2018;15. doi:10.5888/pcd15.180218
70. Mcdougall G, Kang J. Memory self-efficacy and memory performance in older males. Int J Mens Health. 2003;2(2):131–147. doi:10.3149/jmh.0202.131
71. Mol ME, Ruiter RA, Verhey FR, Dijkstra J, Jolles J. A study into the psychosocial determinants of perceived forgetfulness: implications for future interventions. Aging Ment Health. 2008;12(2):167–176. doi:10.1080/13607860801972503
72. Al-Qazzaz N, Ali S, Ahmad SA, Islam S, Mohamad K. Cognitive impairment and memory dysfunction after a stroke diagnosis: a post-stroke memory assessment. Neuropsychiatr Dis Treat. 2014;10:1677. doi:10.2147/ndt.s67184
73. Aggarwal B, Pender A, Mosca L, Mochari-Greenberger H. Factors associated with medication adherence among heart failure patients and their caregivers. J Nurs Educ Pract. 2014;5:3. doi:10.5430/jnep.v5n3p22
74. Addo B, Sencherey S, Babayara MNK. Medication noncompliance among patients with chronic diseases attending a primary health facility in a Periurban district in Ghana. Int J Chronic Dis. 2018;2018:1–10. doi:10.1155/2018/7187284
75. Grant RW, Devita NG, Singer DE, Meigs JB. Polypharmacy and medication adherence in patients with type 2 diabetes. Diabetes Care. 2003;26(5):1408–1412. doi:10.2337/diacare.26.5.1408
76. Adefolalu AO. Cognitive-behavioural theories and adherence: application and relevance in antiretroviral therapy. South Afr J HIV Med. 2018;19:1. doi:10.4102/sajhivmed.v19i1.762
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
