Back to Journals » Journal of Asthma and Allergy » Volume 15
Expert Opinion on Biological Treatment of Chronic Rhinosinusitis with Nasal Polyps in the Gulf Region
Authors Al-Ahmad M , Alsaleh S , Al-Reefy H, Al Abduwani J, Nasr I, Al Abri R , Alamadi AMH, Fraihat AA, Alterki A , Abuzakouk M, Marglani O, Rand HA
Received 2 June 2021
Accepted for publication 1 September 2021
Published 4 January 2022 Volume 2022:15 Pages 1—12
DOI https://doi.org/10.2147/JAA.S321017
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Mona Al-Ahmad,1 Saad Alsaleh,2 Heba Al-Reefy,3 Janan Al Abduwani,4 Iman Nasr,5 Rashid Al Abri,6 Ahmed Mohd Haider Alamadi,7 Ayman Ali Fraihat,8 Abdulmohsen Alterki,9 Mohamed Abuzakouk,10 Osama Marglani,11 Hussain Al Rand12
1Al-Rashed Allergy Centre, Kuwait, State of Kuwait; 2Rhinology and Endoscopic Skull Base Surgery Division, Otolaryngology – Head and Neck Surgery Department, King Saud University, Riyadh, Saudi Arabia; 3Awali Hospital, Manama, Bahrain; 4Al Nahda Hospital, MoH, Muscat, Oman; 5Royal Hospital, MoH, Muscat, Oman; 6Sultan Qaboos, University Hospital, Muscat, Oman; 7Al Kuwait Hospital, MoH, Dubai, United Arab Emirates; 8Sulaiman Al Habib Hospital, Dubai, United Arab Emirates; 9Department Otolaryngology, Head and Neck Surgery, Zain and Al Sabah Hospitals, Medical Department, Dasman Diabetes Institute, Kuwait, State of Kuwait; 10Cleveland Clinic, Abu Dhabi, United Arab Emirates; 11Umm Al Qura University, Makkah and KFSH&RC, Jeddah, Saudi Arabia; 12Dubai HealthCare City, Dubai, United Arab Emirates
Correspondence: Hussain Al Rand Ministry of Health, P. O. Box 1853, Dubai, United Arab Emirates
Tel +971 50-6464643
Email [email protected]
Abstract: Chronic rhinosinusitis (CRS) is defined as the inflammation of nose and paranasal sinuses, affecting the patients’ quality of life and productivity. Chronic rhinosinusitis with nasal polyps (CRSwNP) is a principal clinical entity confirmed by the existence of chronic sinonasal inflammation and is characterized by anterior or posterior rhinorrhea, nasal congestion, hyposmia and/or facial pressure or facial pain. Several epidemiologic studies have revealed wide variations in the incidence of CRS among regions globally ranging from 4.6% to 12%. The Gulf countries are also witnessing an unprecedented burden of CRSwNP. According to the current clinical guidelines, glucocorticosteroids and antibiotics are the principal pharmacotherapeutic approaches. Endoscopic sinus surgery is recommended for those who have failed maximal pharmacotherapy. Recently, biologics are considered as an alternative best approach due to the complications associated with medical therapy and surgery. However, precise data on the clinical position of biologic agents in the management of CRSwNP in the Gulf region is not available. The present review article addresses the current diagnostic and management approaches for CRSwNP and also emphasizes the role of emerging biologics in the current treatment strategies for CRSwNP in the Gulf region. Further, a consensus protocol was convened to rationalize the guideline recommendations, strategize the best practices with biologics, and develop clinical practice guidelines for all primary-care specialists in the Gulf region. The consensus-based report will be a useful reference tool for primary-care physicians in primary-healthcare settings, regarding the appropriate time for the initiation of biological treatment in the Gulf region.
Keywords: paranasal sinuses, glucocorticosteroids, endoscopic sinus surgery, inflammation, rhinorrhea
Introduction
Chronic rhinosinusitis (CRS) is defined as an inflammation of the nose and paranasal sinuses, which is characterized by the presence of two or more symptoms affecting both adults and children1,2 (Table 1). Chronic rhinosinusitis (CRS) is a very rampant inflammatory disease affecting the patient’s quality of life and productivity. The classification of CRS is based on the presence or absence of nasal polyps. Chronic rhinosinusitis is categorized into two major phenotypes: chronic rhinosinusitis with nasal polyps (CRSwNP) or chronic rhinosinusitis without nasal polyps (CRSsNP).3,4 Chronic rhinosinusitis with nasal polyposis (CRSwNP) is a chronic inflammatory syndrome of the nasal passage linings or sinuses leading to soft tissue growth known as nasal polyps in the upper nasal cavity. The resultant swellings can grow in both nostrils (bilateral), resulting from chronic rhinosinusitis, greatly impacting a patient’s morbidity due to nasal obstruction, loss of smell, facial pain, facial pressure, and nasal discharge.5 Severe CRSwNP is defined as:
bilateral CRSwNP with a nasal polyp score (NPS) of at least 4 of 8 points and persistent symptoms despite long-term intranasal corticosteroids (INCSs) with the need for add-on treatment.6
 |
Table 1 Definition of Chronic Rhinosinusitis in Adults |
Uncontrolled CRSwNP is:
the persistent or recurring CRSwNP despite long-term treatment with INCSs and having received at least 1 course of systemic corticosteroids in the preceding 2 years or having a medical contraindication or intolerance to systemic corticosteroids and/or previous sinonasal surgery.6
Overall, CRSwNP is a leading cause of significant morbidity globally and is considered as a more severe form as compared to CRSsNP.4
The global incidence of CRS is rapidly increasing, and the recent CRS prevalence surveys based on the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) definition have depicted prevalence of 12% in the United States of America,10,11 4.6% in Canada,10 5.5% in Brazil,10,11 7.8% in Denmark,10 10.9% in Europe,10 8% in China,10,11 and 11% in Korea.11 Various studies in the Gulf region also indicated high prevalence of CRS. The findings of the Global Allergy and Asthma European Network (GA2LEN) study that was conducted in 5201 participants who had been living for at least one year in the urban areas of Bushehr, Iran, concluded that the overall CRS prevalence was 28.4% based on the EPOS criteria, while the self-reported physician-diagnosed CRS prevalence was 20.0%. This study also demonstrated a strong association between asthma and CRS.11 An estimated prevalence of CRSwNP in common people is 1–4% and in patients with CRS is 25–30%.12 As CRSwNP is a growing concern in CRS patients, it has to be appropriately treated to overcome CRSwNP-associated complications.
Chronic rhinosinusitis with nasal polyposis, a type 2, Th2-dominant inflammatory disease, is also associated with other inflammatory diseases, including airway diseases such as asthma,13,14 allergic rhinitis, and non-steroidal anti-inflammatory drug-exacerbated respiratory disease/aspirin-exacerbated respiratory disease (NSAID-ERD/AERD), and non-airway diseases such as atopic dermatitis and food allergy.14,15 Coexisting type 2 inflammatory airway diseases are common in patients with CRSwNP with reported prevalence of approximately 50%, 15%, and 67% for asthma, NSAID-ERD/AERD, and allergic rhinitis, respectively.15 Allergic fungal rhinosinusitis (AFRS) and NSAID-ERD are comorbidities that are frequent in patients with CRSwNP. A multicenter retrospective case–control study conducted from March 2010 to October 2018 on 262 CRSwNP patients concluded that the prevalence of AFRS is 45% in patients with NPs in the western region of Saudi Arabia.5 A cross-sectional study conducted from February 2007 to September 2009 in 162 patients with documented chronic sinusitis demonstrated that the prevalence of fungal infection is about 7.4%. A study conducted among 80 Iranian patients with CRSwNP, to determine the frequency of aspirin (ASA) hypersensitivity by oral aspirin challenge (OAC) test, depicted that 48.8% of CRSwNP patients presented with ASA hypersensitivity.16
Further, the presence of comorbid type 2 inflammatory diseases is associated with an increased risk of recurrence of nasal polyps post-surgery in patients with CRSwNP,17 as well as an increased number of sinus surgeries.18 In a retrospective cohort study in patients with CRSwNP (n = 299 with 338 consecutive surgeries), comorbid asthma was associated with a significantly increased risk of polyp recurrence over time following surgery (hazard ratio [HR] 1.71; p < 0.05). Furthermore, the presence of comorbid NSAID-ERD/AERD was associated with a more aggressive rate of polyp recurrence (HR 1.79; p < 0.05).17 A comparison of the number of sinus surgeries in patients with CRSwNP versus those with CRSwNP and asthma showed that the presence of comorbid asthma is associated with a significantly increased number of sinus surgeries versus CRSwNP without comorbidities (p < 0.001).15 The presence of NSAID-ERD/AERD impacted on the severity of CRSwNP disease further, being associated with significantly more sinus surgeries than both CRSwNP and comorbid asthma (p < 0.001) and CRSwNP without comorbidities (p < 0.001).18 On the whole, patients with CRSwNP and type 2 comorbidities have a greater prevalence and recurrence of nasal polyps post-surgery, and a greater treatment burden versus patients with CRSwNP alone.17
The underlying hypothetical mechanisms that may contribute to the disease pathology include alterations in mucociliary clearance, epithelial barrier dysfunction, abnormalities in the host immune response, and tissue remodeling.19 Although a clear etiology for the development of CRSwNP is unknown, allergy and viral, bacterial or fungal infections have all been indicated as feasible initial etiological factors that may stimulate the development of nasal polyps.5 Chronic rhinosinusitis with nasal polyposis is characterized by extensive tissue eosinophilia. This is generally accompanied by an increase in tissue mast cells, local IgE, basophils, T-helper-2 cells (Th2 cells), as well as type 2 cytokines (IL-4, IL-5, IL-9, IL-13, IL-25, and IL-33).20,21
The most common symptoms associated with CRSwNP include prominent nasal obstruction, post-nasal drip, loss of smell (anosmia) or hyposmia, facial pain, headache, facial pressure, and nasal discharge.8 The differential diagnosis includes allergic and non-allergic rhinitis, structural abnormalities including hypertrophied turbinates and septal deviations, inflammatory conditions of the nose including vasculitis disease and tumors.4 The warning signs and symptoms that need immediate referral to doctor include: unilateral symptoms, blood-stained rhinorrhea, bleeding, crusting, cacosmia, orbital symptoms, periorbital edema, displaced globe, reduced or double vision, ophthalmoplegia, severe unilateral or bilateral frontal headache, frontal swelling, and signs of meningitis or focal neurologic signs.4 The treatment strategies for the management of CRSwNP include local and systemic corticosteroids followed by standardized surgical interventions.6 There is a lack of highly efficacious treatment approaches beyond surgery and is the significant unmet need for the management of CRSwNP. The advent of novel biologic agents in the therapeutic armamentarium of CRSwNP offered new approaches for clinicians and a ray of hope for patients to manage this complicated disease condition.6
In the Gulf settings, regional guidelines and consensus statements are lacking on the decision-making criteria for the diagnosis and management of CRSwNP. The aim of the present expert opinion article was to establish a regional/local algorithm for CRSwNP and coexisting comorbidities management in the Gulf region by evaluating EPOS 2020 guidelines and the current practices in the diagnosis and management of CRSwNP.
Methods
An Expert Group Advisory Committee Meeting (EGACM), involving 13 experts from United Arab Emirates (UAE), Kuwait, Bahrain, the Kingdom of Saudi Arabia (KSA), and Oman, was convened in September 2020 and experts discussed about comprehensive and pragmatic approaches of positioning of biologics in the treatment algorithm of CRSwNP in the primary-care setting in the Gulf region. Based on the biologics algorithm and management guidelines from EPOS 2020, the experts arrived at a consensus on the management of CRSwNP with biologics. The flow chart representation of consensus development is summarized in Figure 1.
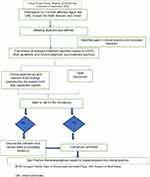 |
Figure 1 Flow chart representation of consensus development. |
Results
Based on the results of the literature review, specifically guidelines and the expert discussions, the expert consensus was derived.
Overview of Literature on Current Diagnostic Approaches of CRSwNP
The diagnosis of CRSwNP is made according to the EPOS 2020: Rhinosinusitis (including nasal polyps) is defined as inflammation of the nose and the paranasal sinuses characterized by two or more symptoms that include blockage/congestion, anterior/post-nasal drip, facial pain/pressure, reduction or loss of smell; and either endoscopic signs that include polyps, mucopurulent discharge from middle meatus, oedema/mucosal obstruction primarily in middle meatus; and/or computed tomography (CT) charges that include mucosal changes within the ostiomeatal complex and/or sinuses.7
The most common investigative modalities in the assessment of CRS are anterior rhinoscopy, fiberoptic endoscopy, and paranasal sinus CT.4,7 Anterior rhinoscopy is useful in the detection of nasal discharge or large nasal polyps. Using a simple otoscope, middle meatus and middle turbinate can be viewed for the inspection of edema, purulence, and nasal polyps. Nasal endoscopy can facilitate the diagnosis of CRSwNP by endoscopic visualization of nasal polyps in the nasal cavity.4 Preoperative endoscopic scoring was performed by the bilateral endoscopic nasal polyp score (NPS). For this, the polyp score is allotted as: 0, no polyps; 1, polyps confined to the middle meatus; 2, multiple polyps occupying the middle meatus; 3, polyps extending beyond middle meatus; 4, polyps completely obstructing the nasal cavity.22
Tests Assessing Symptom Severity
Various tests were proposed to quantify the severity of patient-reported symptoms, as these allow more effective monitoring of treatment responses. Major nasal symptoms, including nasal obstruction, nasal discharge, olfactory dysfunction, facial pain, and headache, were scored on a visual analog scale (VAS) of 0 to 10. This VAS is ideal in the classification of symptoms of rhinosinusitis into mild (VAS 0–3), moderate (>3–7), and severe (>7–10).4,23 The validated Arabic version of sinonasal outcome test-22 (SNOT-22) questionnaire is a disease-specific, health-related questionnaire and is also the most effective tool currently available for grading the severity and impact of clinical symptoms of CRS. It contains 22 questions, the first 12 items covering physical symptoms (1–12) and the last ten (13–22) covering aspects of health-related quality of life. Symptom severity was graded from 0 to 5 as follows: no problem (0), very mild problem (1), mild or slight problem (2), moderate problem (3), severe problem (4), and problem as bad as it can be (5). The SNOT-22 total scores range from 0 to 110, with higher SNOT-22 total scores indicating worse symptoms.24–26
Smell Assessment Tests
The most widely used olfactory tests for the diagnosis of anosmia in CRSwNP patients, primarily for monitoring the outcomes of anti-inflammatory or surgical treatment, include Sniffin’ Sticks test, and University of Pennsylvania Smell Identification Test (UPSIT). These tests may also be useful during the follow-up of CRS patients.7
Sniffin’ Sticks test encompasses three sub-tests: odor threshold, odor discrimination, and odor identification tests. The threshold test was performed using dilutions of n-butanol in a single-staircase, triple-forced choice procedure. The discrimination test used triplets of pens presented in a random order with 2 containing the same odorant and the third a different odorant. The identification test utilized 16 odorants presented at suprathreshold intensity using a multiple-choice procedure. The overall results were also combined for an overall score called the “composite threshold-discrimination-identification score” (TDI), which ranged from 1 to 48.27 Patients were considered as following based on the olfactory status using total score: ≤15 anosmic, ≤30 hyposmic, and >30 normosmic.28
The UPSIT is a scratch and sniff smell identification test consisting of four booklets. Each booklet contains 10 different microencapsulated odors with each having four multiple-forced choices; each participant was informed and forced to select one of the choices, and only tests with 40 answers were evaluated. The UPSIT scores and outcomes are clearly enlisted in Table 2.29 Although the UPSIT is one of the best-validated tests in some countries, UPSIT has not been validated for use in Gulf region.
 |
Table 2 University of Pennsylvania Smell Identification Test (UPSIT) Scores and Outcomes |
Bilateral endoscopic nasal polyp score (NPS) is a physician-reported scoring system to estimate the extent or severity of nasal polyps based on assessments by nasal endoscopy. Each nostril is scored on a scale of 0 to 4, with the total score being the sum of left and right nostril scores (range: 0–8). Higher scores depict severe congestion or obstruction.22
Computed tomography (CT) is the preferred imaging modality in the diagnosis of CRS for patients failing medical therapy or those with atypical or severe disease, ie, unilateral symptoms, blood-stained discharge, displacement of the eye, and severe pain. The Lund–Mackay CT score is used for the quantification of radiologic disease severity as indicated by sinus CT scans. The extent of radiologic disease is graded from 0 to 2 for each paranasal sinus and for the ostiomeatal complex (OC). The CT scan scored by the Lund–Mackay scoring system is as follows: 0, no opacification; 1, partial opacification; 2, complete opacification. These points are then applied to the maxillary, anterior ethmoid, posterior ethmoid, sphenoid, and frontal sinus on each side. The OC is graded separately as 0, not occluded or 2, occluded, deriving a maximum score of 12 per side. The Lund–Mackay CT score is a composite score, ranging from 0 to 12 per side, with a maximum of 24 for both sides. Higher scores indicate more severe disease.23 Other specific tests to assess nasal function include measurement of olfactory function, nasal airflow using a peak nasal inspiratory flow rate, and nasal cavity volume using rhinomanometry or acoustic rhinometry.4 The current clinical guidelines for the diagnosis of CRSwNP is summarized in Table 3.
 |
Table 3 Clinical Guidelines for the Diagnosis of CRSwNP |
Key Expert Consensus on Diagnostic Approaches of CRSwNP in the Gulf Region
The EPOS criteria are indeed preferred for diagnosing type 2 inflammation in CRSwNP patients. The assessment of blood eosinophils, IgE, and particularly tissue eosinophils should be considered in routine practice for the diagnosis of type II inflammation. Histopathology also plays a vital role in endotyping of inflammatory diseases, thereby directing potential therapies for the management of CRSwNP.
Smell test should be done to assess anosmia in CRSwNP patients.
Subjective methods, such as sinonasal outcome test (SNOT-22) and visual analog scales (VAS), must be used to assess the quality of life; questionnaires of olfactory disorders (QOD) must be used to assess the sense of smell in patients with CRSwNP.
The UPSIT score is the most widely used test to objectively evaluate anosmia. Although the subjective and objective scoring evaluation methods of olfactory function are crucial for accurate diagnosis of patients with CRSwNPs, history of anosmia should also be explored. The clinician must know how and when the patient developed anosmia (pre-surgery, during the surgery, or post-surgery).
The diagnosis of asthma should be assessed by a pulmonologist and an immunologist or an allergist if available. Collaborative efforts of a multidisciplinary team, including a pulmonologist, an immunologist, and an ear, nose and throat (ENT) specialist, are essential for the treatment of patients with CRSwNPs and severe asthma.
Overview of Literature on Current Treatment Approaches for CRSwNP
The current standard treatment for CRSwNPs is intranasal corticosteroids and, for severe cases, intermittent courses of oral systemic corticosteroids. Surgical removal of polyp tissues may also be indicated for severe cases. Sinus surgery should also be considered in patients who are symptomatic after maximal medical treatment. However, polyps have a strong tendency to reoccur, often leading to repeat surgery. Nasal washes, saline irrigations, and systemic antibiotics are also a part of pharmacotherapy for CRSwNP.8,20 However, nasal corticosteroids are the first-line treatment options for CRSwNP. Nasal drops are likely to be more effective than sprays because of their distribution within the sinus cavities, specifically postoperatively. Long-term high-dose treatment is discouraged owing to the significant increase in associated side effects with the dose and duration of treatment.10 The clinical guidelines for the management of CRSwNP are summarized in Table 4.
 |
Table 4 Clinical Guidelines for the Management of CRSwNP |
Biologics: A Novel Approach for Treatment of CRSwNP
Long-term application of oral corticosteroids deteriorates the health status of patients. Repeated sinus surgeries associated with increased risks of complications could not be the only possible option in these patients. As patients with CRSwNP are associated with severe Th2 inflammation with a high probability of recurrence after surgery and comorbid asthma, a valuable innovative alternative approach targeting the Th2 bias with biologicals could be a new dimension of effectiveness in comparison with glucocorticosteroids and sinus surgeries. Dupilumab, mepolizumab, benralizumab and omalizumab are the biologic agents investigated in CRSwNP patients.31
Evidence Supporting the Impact of Biologics on CRSwNP Treatment
Multiple factors have to be considered for the selection of biologics in the treatment of CRSwNP, including disease severity, risk of polyp recurrence with medical or surgical treatment, patient preferences and goals, safety, and cost-effectiveness.32 Dupilumab is the first drug approved by the United States Food and Drug Administration (US FDA) followed by omalizumab to treat adults with CRSwNP.31 Various studies that assessed the efficacy of dupilumab and omalizumab are detailed in Table 5.32
 |
Table 5 Studies on Dupilumab and Omalizumab in CRSwNP |
Clinical Rationale of Use of Biologics in the Current Management of CRSwNP
If surgery in combination with appropriate medical treatment fails, additional therapy can be considered that includes the use of aspirin treatment after aspirin desensitization (ATAD), longer (tapering) treatment with OCS, long-term antibiotics, and/or biologics when indicated.
The emergence of biologics or monoclonal antibodies (mAbs) has catalyzed significant changes in the therapeutic landscape of CRSwNP treatment. The recent EPOS 2020 guidelines have provided recommendations on the positioning of biologics in the integrated care pathway of CRS. The biologics are indicated in a patient with bilateral polyps, who had sinus surgery or was not fit for surgery and who had three of the following characteristics:
- Evidence of type 2 disease (tissue eosinophils ≥10/high power field (HPF) or blood eosinophils ≥250 OR total IgE ≥100).
- Need for at least two courses of systemic corticosteroids or continuous use of systemic corticosteroids (≥2 courses per year OR long-term [>3 months] low-dose steroids OR contraindication to systemic steroids).
- Significantly impaired quality of life (SNOT-22 ≥40)
- Anosmic on smell test and/or a diagnosis of comorbid asthma needing regular inhaled corticosteroids.
Further, patients who have recurrence despite surgery and patients who want to avoid surgery or are not eligible for surgery may benefit from a biologic agent.32 The EPOS guidelines have also developed a paradigm for the response to biologic treatment. The following parameters or criteria are assessed after 16 weeks and after one year for evaluation of treatment response: reduced nasal polyp size, reduced need for systemic corticosteroids, improved quality of life, improved sense of smell, and reduced impact of co-morbidities. The patient responses to biologic treatment are classified, based on fulfilment of parameters assessed, into excellent response (5 criteria), moderate response (3–4 criteria), poor response (1–2 criteria), and no response (0 criteria). Patients are recommended to discontinue the treatment if no response in any of the criteria is seen.7
The approval of dupilumab, a biologic agent for the treatment of CRSwNP by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) in 2019, has substantially changed the treatment opportunities in type 2 CRS.
Key Expert Consensus on the Use of Biologic vs Surgery in CRSwNP Patients in the Gulf Region
Type 2 biologics should be indicated in severe uncontrolled CRSwNP patients despite at least one previous endoscopic sinus surgery.
Biologics are promising treatments for CRSwNP and there is a need to provide to all patients in need. If the EPOS criteria are fulfilled, there are greater chances of insurance companies accepting the applications of CRSwNP patients. The criteria include SNOT-22 for assessing quality of life of CRSwNP patients, but the Lund–Mackay score, a widely used method for radiologic staging of chronic rhinosinusitis, is not included in the criteria.
Despite surgery, there are still 40% to 50% chances of recurrence; this might explain the high unmet medical need for patients who might prefer biological treatment.
Algorithm for the Biological Treatment in CRSwNP Patients in the Gulf Region
Based on the insights of the panel experts and the recent EPOS 2020 guidelines, an algorithm for biologic treatment in CRSwNP patients has been developed and presented in Figure 2.
 |
Figure 2 Algorithm for the biological treatment in CRSwNP patients. |
Key Expert Consensus for Consideration of Biologics for CRSwNP Patients in the Gulf Region
The biologic, dupilumab, was first approved for atopic dermatitis and later for various other indications including asthma, adolescent atopic dermatitis, nasal polyps, and pediatric atopic dermatitis in the Gulf region. The US FDA approved dupilumab33 has been licensed for use in CRSwNP patients in all Gulf countries and KSA.
Dupilumab has shown promising results in the following three groups of patients:
Patients with nasal polyposis and asthma
Patients with CRSwNP associated with poor quality of life, and multiple surgeries but has no asthma
Surgery-naïve patients with no comorbidities but only anosmia: treatment with dupilumab has shown up to 98% success in restoring sense of smell as well as taste in these patients in six to eight weeks.
Biologics may become the first-line treatment for CRSwNP in near future. However, cost is one of the limiting factors as some of the patients might not have insurance. Currently, there are no studies in the Gulf region comparing the cost-effectiveness of medications with that of surgery in CRSwNP patients. There is a lack of published data comparing the cost-effectiveness of medication versus surgery in CRSwNP patients even in the United States and Europe. A publication on indirect cost considerations while treating CRSwNP patients may provide a rough idea. However, comparative cost-effectiveness data are still needed for private payers or insurance companies to gain a clear understanding.
Conclusion
Chronic rhinosinusitis is a challenging disease to manage as treatment with corticosteroids or endoscopic sinus surgery contribute to various complications. Based on available data, biologic agents may be beneficial in patients with persistent polyps despite maximal medical therapy, have recurrence despite surgery, or have allergic diseases including moderate-to–severe asthma or AERD. Qualitative evidence regarding the position of biologic agents in the management of CRSwNP in the Gulf region is lacking. Hence, an expert advisory board meeting was conducted and helped in the establishment of the biologics treatment algorithm in the treatment strategy of CRSwNPs in the Gulf region.
The Key Consensus That Was Additionally Added Apart from EPOS 2020 Guidelines Recommendations for the Diagnosis and Management of CRSwNP in the Gulf Region Include
Biological treatments indicated in severe uncontrolled CRSwNP patients despite at least one previous endoscopic sinus surgery or in surgery-naïve patients who are contraindicated for surgery.
Biological treatments may be beneficial for severe CRSwNP with comorbidities such as bronchial asthma or Samter’s triad.
Smell test, ie, VAS score, would be useful to assess anosmia in CRSwNP patients till UPSIT test validation to be used for Gulf region.
Validated Arabic versions of established metrics (eg, SNOT-22) should be procured for nurse- or patient self-assessment to evaluate the quality of life.26
The diagnosis of asthma should be assessed by a pulmonologist and an immunologist or an allergist if available.
The concept of biologics is still novel, and there are enormous opportunities for the emergence of biologics as the cornerstone for the management of CRSwNP, soon with evolving data from future studies and real-world evidence. The established Gulf consensus report, including treatment algorithm, for CRSwNP offers an up-to–date guidance to assist the primary-care physicians with the diagnostic process and expert opinion for biologic treatment in the Gulf region.
Acknowledgments
Sanofi Genzyme offered logistical support through offering medical writing for the manuscript. This manuscript was written by BioQuest Solutions medical team. Editorial support for the preparation of this manuscript was provided by BioQuest solutions.
Disclosure
Sanofi Genzyme has provided advisory board honoraria for authors who participated in Sanofi Genzyme advisory board, but they were not paid for writing the publication. The authors report no other potential conflicts of interest for this work.
References
1. Chan Y, Kuhn FA. An update on the classifications, diagnosis, and treatment of rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17(3):204–208. doi:10.1097/MOO.0b013e32832ac393
2. Poddighe D, Brambilla I, Amelia L, et al. Pediatric rhinosinusitis and asthma. Respir Med. 2018;141:94–99. doi:10.1016/j.rmed.2018.06.016
3. Nakayama T, Yoshikawa M, Asaka D, et al. Mucosal eosinophilia and recurrence of nasal polyps -new classification of chronic rhinosinusitis. Rhinology. 2011;49:392–396. doi:10.4193/Rhino10.261
4. Slovick LJ, Hopkins C. Updates in the management of chronic rhinosinusitis. Clin Pract. 2014;11:649. doi:10.2217/cpr.14.71
5. Hussein A, Obaid A, Lotfi WT, et al. Prevalence, demographic and environmental factors of allergic fungal sinusitis among chronic sinusitis patients with nasal polyps in western Saudi Arabia. Pan Arab J Rhinol. 2019;9:26–30.
6. Bachert C, Han JK, Wagenmann M, et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: definitions and management. J Allergy Clin Immunol. 2021;147:29–36. doi:10.1016/j.jaci.2020.11.013
7. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58:1–464. doi:10.4193/Rhin20.401
8. Passali D, Cingi C, Cambi J, et al. A survey on chronic rhinosinusitis: opinions from experts of 50 countries. Eur Arch Otorhinolaryngol. 2016;273:2097–2109. doi:10.1007/s00405-015-3880-6
9. Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–S32. doi:10.1053/hn.2003.v128.amhn0312811
10. Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015;136:1431–1440. doi:10.1016/j.jaci.2015.10.010
11. Ostovar A, Fokkens WJ, Vahdat K, et al. Epidemiology of chronic rhinosinusitis in Bushehr, southwestern region of Iran: a GA2LEN study. Rhinology. 2019;57:43–48. doi:10.4193/Rhin18.061
12. Chen S, Zhou A, Emmanuel B, et al. Systematic literature review of the epidemiology and clinical burden of chronic rhinosinusitis with nasal polyposis. Curr Med Res Opin. 2020;36:1897–1911. doi:10.1080/03007995.2020.1815682
13. Staniorski JC, Price CPE, Weibman AR, et al. Asthma onset pattern and patient outcomes in a chronic rhinosinusitis population. Int Forum Allergy Rhinol. 2018;8:495–503. doi:10.1002/alr.22064
14. Gandhi NA, Bennett BL, Graham NM, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15:35–50. doi:10.1038/nrd4624
15. Khan A, Vandeplas G, Huynh TMT, et al. The Global Allergy and Asthma European Network (GALEN rhinosinusitis cohort: a large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57:32–42. doi:10.4193/Rhin17.255
16. Esmaeilzadeh H, Nabavi M, Arshi S, et al. Aspirin hypersensitivity in Iranian patients with chronic rhinosinusitis and nasal polyposis: prevalence and comorbid factors. Clin Transl Allergy. 2014;4:P22. doi:10.1186/2045-7022-4-S3-P22
17. Bassiouni A, Wormald PJ. Role of frontal sinus surgery in nasal polyp recurrence. Laryngoscope. 2013;123:36–41. doi:10.1002/lary.23610
18. Stevens WW, Peters AT, Hirsch AG, et al. Clinical characteristics of patients with chronic rhinosinusitis with nasal polyps, asthma, and aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2017;5:1061–1070. doi:10.1016/j.jaip.2016.12.027
19. Stevens WW, Lee RJ, Schleimer RP, et al. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol. 2015;136(6):1442–1453. doi:10.1016/j.jaci.2015.10.009
20. Avdeeva K, Fokkens W. Precision medicine in chronic rhinosinusitis with nasal polyps. Curr Allergy Asthma Rep. 2018;18:25. doi:10.1007/s11882-018-0776-8
21. Ryu G, Kim DW. Th2 inflammatory responses in the development of nasal polyps and chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2020;20(1):1–8. doi:10.1097/ACI.0000000000000588
22. Measures of disease severity in CRSwNP. Available from: https://www.type2inflammation.com/-/media/EMS/Conditions/Respiratory/Brands/DupixentAsthmaHCP-US/pdf/Measures-of-Disease-Severity-in-CRSwNP.pdf?la=en.
23. Zhu M, Gao X, Zhu Z, et al. The roles of nasal nitric oxide in diagnosis and endotypes of chronic rhinosinusitis with nasal polyps. J of Otolaryngol - Head & Neck Surg. 2020;49:68. doi:10.1186/s40463-020-00465-y
24. Abdalla S, Alreefy H, Hopkins C. Prevalence of sinonasal outcome test (SNOT-22) symptoms in patients undergoing surgery for chronic rhinosinusitis in the England and Wales National prospective audit. Clin Otolaryngol. 2012;37:276–282. doi:10.1111/j.1749-4486.2012.02527.x
25. Dejaco D, Riedl D, Huber A, et al. The SNOT-22 factorial structure in European patients with chronic rhinosinusitis: new clinical insights. Eur Arch Otorhinolaryngol. 2019;276:1355–1365. doi:10.1007/s00405-019-05320-z
26. Alanazy F, Dousary SA, Albosaily A, et al. Psychometric Arabic sino-nasal outcome test-22: validation and translation in chronic rhinosinusitis patients. Ann Saudi Med. 2018;38:22–27. doi:10.5144/0256-4947.2018.22
27. Soler ZM, Kohli P, Storck KA, et al. Olfactory impairment in chronic rhinosinusitis using threshold, discrimination, and identification scores. Chem Senses. 2016;41:713–719. doi:10.1093/chemse/bjw080
28. Kohli P, Naik AN, Harruff EE, et al. The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope. 2017;127:309–320. doi:10.1002/lary.26316
29. Kamrava SK, Farhadi M, Jalessi M, et al. University of Pennsylvania smell identification on Iranian population. Iran Red Crescent Med J. 2014;16:e7926. doi:10.5812/ircmj.7926
30. Kaplan A. Canadian guidelines for chronic rhinosinusitis: clinical summary. Can Fam Physician. 2013;59:1275–e534.
31. Alsaleh S, Alqahtani A, Alotaibi NH, et al. The use of biologics in chronic rhinosinusitis with polyps: Saudi otorhinolaryngology society position statement. Saudi J Otorhinolaryngol Head Neck Surg. 2020;22:93–94.
32. Patel GB, Peters AT. The role of biologics in chronic rhinosinusitis with nasal polyps. Ear Nose Throat J. 2021;100:44–47. doi:10.1177/0145561320964653
33. FDA approves first treatment for chronic rhinosinusitis with nasal polyps. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-chronic-rhinosinusitis-nasal-polyps.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
