Back to Journals » International Journal of Nanomedicine » Volume 15
Exosomes as Actively Targeted Nanocarriers for Cancer Therapy
Authors Wang Y, Zhang Y , Cai G, Li Q
Received 21 November 2019
Accepted for publication 25 May 2020
Published 17 June 2020 Volume 2020:15 Pages 4257—4273
DOI https://doi.org/10.2147/IJN.S239548
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
Yan Wang,1,* Yingru Zhang,1,* Gang Cai,1 Qi Li1,2
1Department of Medical Oncology & Cancer Institute of Integrative Medicine, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, People’s Republic of China; 2Academy of Integrative Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qi Li Email [email protected]
Abstract: In recent years, it has been found that exosomes can be used as nanocarriers, which can be used in the treatment of tumors by carrying contents. The exosomes are derived from the secretion of the organism’s own cells and are characterized by a phospholipid bilayer structure and a small particle size. These characteristics guarantee that the exosomes can carry a wide range of tumor drugs, deliver the drug to the cancer, and reduce or eliminate the tumor drug band. The toxic side effects were significantly eliminated; meanwhile, the therapeutic effects of the drug on the tumor were remarkably improved. This paper reviewed the strategies and drugs presented by different scholars for the treatment of tumors based on the drugs carried by exosomes.
Keywords: exosomes, nanocarriers, function, tumor therapy
Introduction
Exosomes are a class of natural nanoscale membrane vesicles formed by living cells through a series of regulatory processes, such as “endocytosis-fusion-efflux”.1 In brief, exosomes were first discovered around 40 years ago.2 In recent decades, people’s understanding of exosomes has remarkably grown. At the beginning, it was thought that exosomes were such a path of cell excretion,3 and further researches revealed that exosomes are also a medium participating in information exchange and material transportation between cells by carrying proteins,4 lipids,5 nucleic acids,6 and other substances of host cells. Consequently, exosomes are used as a kind of nanocarrier to transport nucleic acids (such as miRNA7) or drugs (such as paclitaxel8) for the treatment of various diseases, such as tumors. The mechanism of better-utilizing exosomes and construct low-toxic or non-toxic granules with high-efficiency exosomes-loading, which were used in cancer treatment, has quietly become a research hotspot.9,10 The present study systematically expounds exosomes, and summarizes the application of exosomes as nanocarrier-loaded drugs in tumor therapy, with the aim of providing a reference for future treatment of cancer.
Structure
Exosomes are a class of round-shaped lipid bilayer vesicles with a diameter of ranging from 30–150 nm, and in the image of exosomes EM, we can see that their shape is round-shaped and the distribution is monodisperse.11–14 As early as 1981, Trams et al2 discovered the existence of exosomes. Pan et al15 reported the formation of exosomes via electron microscopy in 1985. Until 1987, the term “exosome” was first officially introduced by Johnstone et al.16
The ingredients of exosomes (Figure 1) include multiple proteins,4,17 lipids,5 and nucleic acids, such as mRNA,18–20 tRNA,21 miRNAs,22–25 LncRNA,26–28 and DNA.6,29
 |
Figure 1 The ingredients of exosomes include multiple proteins, lipids, and nucleic acids, including RNA and DNA. And the scanning electron microscopy image of exosomes175 was made by ourselves. |
Typically, almost all mammalian cells could secrete exosomes (Figure 2), 30 including T and B lymphocytes,31,32 epithelial cells,33 endothelial cells,34 dendritic cells,35 mesenchymal stem cells,36–38 platelets,39,40 tumor cells,41,42 and act as transmitters and couriers in cellular crosstalk.43 The source of exosomes is broad, and they can be found in several body fluids, such as tears,44 nasal mucus,45 saliva,46 breast milk,47 urine,48–50 semen,51,52 lymph,53 and plasma,54 etc. Sokolova et al55 found that the size and integrity of the exosomes were strongly dependent on the storage conditions: the exosome diameter significantly decreased within 2 days at 37 ◦C and 4 days at 4 ◦C, but storage at −20 ◦C to −80◦C can be stored for months to years.
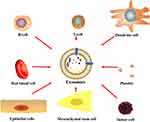 |
Figure 2 Usually, exosomes can be naturally secreted by a variety of cells, such as T and B lymphocytes,31,32 epithelial cells,33 endothelial cells,34 dendritic cells,35 mesenchymal stem cells,36–38 platelets,39,40 tumor cells.41,42 |
It was revealed by pulse tracking and electron microscopy that exosomes were generated by endocytic pathway.56 The specific generation process is as follows (Figure 3):57 early endosomal stage, the cell membrane forms early endosomes through endocytosis; late endosomal stage: on the basis of the early endosomes, ESCRT-0 first bind to the specific receptors on the surface of the early endometrial membrane through the ubiquitination binding site, as well as selectively splicing part of the cytoplasm to form intraluminal vesicles by budding, ESCRT-I binds to ESCRT-0 and induces ESCRT-II to bind to ESCRT-I. Then, ESCRT-I is synergized with ESCRT-II to promote the formation of ILVs, followed by ESCRT-III shearing the bud of the neck. The ILVs are separated from the endosomal membrane, thereby releasing the ILVs encapsulating specific proteins, nucleic acids, and other substances into the endosomal cavity, as well as completing the budding process, thereby forming mature late endosomes, and due to the late endosomes containing multiple ILVs, they are also called multi-vesicle bodies (MVBs).58 Exocytosis: afterwards, some MVBs are degraded by fusion with lysosomes, while a number of them are fused with the plasma membrane, in which the internal vesicles of the MVBs are released into the extracellular medium as exosomes. Besides, there are some MVBs combined with the Golgi body for recycling.
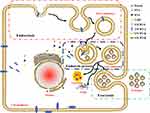 |
Figure 3 The process of exosomes is divided into three parts, namely endocytic process, endosome process, and exocytosis.56 The endosome is stroked by endocytosis, and then matured into a late endosome containing multiple intraluminal vesicles,57 which is multi-vesicle bodies (MVBs). The small vesicles secreted by MVBs to the extracellular membrane are exosomes. Some MVBs are degraded by lysosome phagocytosis, and a small part is degraded by Golgi and then recycled.58 |
The Methods for Isolation and Identification of Exosomes
At present, the methods for isolation of exosomes are mainly divided into centrifugation,59 precipitation,60 ultrafiltration,61 and immunoassay.62 Their main purpose is to remove the mixed cells and their fragments and other small molecular impurities in the exosomes. Among those methods, centrifugation is a differential and a sucrose density gradient centrifugation method.63 The principle of differential centrifugation64 is based on the difference in sedimentation rates of different protein molecules, vesicles, cells, and cell debris in a homogeneous suspension. The sucrose density gradient centrifugation method65 is based on the difference in density of each component in the sample. Under the action of centrifugal force, particles with low density are lifted upward, and particles with high density are sedimented downward. The density of enriched exosomes is at the range of 1.13~1.19 g/mL. The precipitation60,66,67 is mainly a polyethylene glycol precipitation method. Because PEG is extremely hydrophilic, it can bind to the hydrophobic lipid bilayer and change the solubility or dispersibility of the exosomes to precipitate. Ultrafiltration method61,68 uses the ultrafiltration membrane with the corresponding molecular weight cut off according to the size of the exosomes. Ultrafiltration technology is used to remove residual cells by centrifugation, in which a 0.22 μm filtration membrane is used to remove cell debris, macromolecular vesicles, etc., and pure exogenous secretion is isolated. With any method mentioned above, the exosome can be separated. The cystic bodies with double membrane secreted by the cell membrane not only involve the component of the exosome, but vesicles and microvesicles (MVs) (with the diameter of 100nm–1000nm) dropped off from the cell membrane after becoming activated, injured or dead.69,70 It is difficult to separate them from exosome with common separation methods; however, there is a promising one, the emerging microfluidic system, a microscale separation based on the physical and biochemical properties, such as immune affinity, size and density, that can also achieve the innovative separation mechanism for acoustics,71 electrophoresis72 and electromagnetism.73 With this technique, components of exosome and other microvesicles with different physical properties can be effectively separated. However, due to the lack of standardization and large-scale clinical sample testing, the microfluidic system has failed to be wildly used.
While, the identification of exomes mainly focuses on the identification of morphological structure, size, number, and surface protein of exosomes.74 Using electron microscopy,75 we can clearly observe that the morphological structure of exosomes is mostly disc-shaped, which is composed of two layers of membranes, with light internal staining and deep external staining. The Nanoparticle Tracking Analysis can be used to measure and analyze the number and size of exosomes without destroying the structure and function of exosomes.76,77 As a kind of Nanosight-related technique, the NTA technique is based on laser light scattering microscopy that can visualise and dynamically size populations of particles in the particle size range of 10 nm–1000 nm under a liquid state on an individual basis. And the Brownian motion of each and every particle is tracked separately but simultaneously using a CCD camera, from which a high-resolution plot of the particle size distribution profile is obtained. Jin et al78 determined the size and concentration of exosomes through Nanosight Tracking Analysis by utilizing Zeta View PMX 110 according to previous protocol. Western blotting,79 flow cytometry,80 and mass spectrometry81 can detect the type of exosome surface protein expression, in addition to its amount.
Biological Functions of Exosomes
Previously, the understanding of exosomes was limited to transport some non-essential proteins and other molecules from the donor cells, thus, people originally thought exosomes are the only path of cellular excretion of waste.82 With the increase of evidence, exosomes have been confirmed to play an important role in the body’s physiological, pathological processes, such as carrying material, as well as exchanging information between local and distant cells.17,83,84 Normally, these two roles of exosomes complement each other.
With the deepening of research, it is found that exosomes can be used as a biomarker85,86 in clinical practice. Iaccino et al87 studied exosome secreted by multiple myeloma and found that these exosomes express the immunoglobulin B-cell receptor, which binds to the unique type of binding peptide. The results of this study can be used as one of the biomarkers for the early diagnosis of multiple myeloma. What’s more, exosomes play an important role in neural signal conduction,88 immune response,89,90 inflammation,91 coagulation,92 cell proliferation and differentiation,93 tumor invasion and metastasis94,95 and angiogenesis96 etc.
Due to the basic characteristics of the stability of the lipid membrane structure encapsulating the genetic information material, the widespread distribution in body fluids, and the ease of availability, and their nanoscale and great biocompatibility, exosomes could be used as a potential nanocarrier for clinical tumor therapy. In this review, we focused on the application and progress of exosomes as nanocarriers in tumor therapy.
Comparing with Conventional Nanocarriers
In general, traditional nanocarriers can be divided into two categories (Tables 1 and 2): organic nanocarriers (such as liposomes,97–99 micelles,100–102 etc.) and inorganic nanocarriers (eg, mesoporous silica nanoparticles,103–105 graphene,106,107 magnetic nanoparticles,108–110 gold nanoparticles,111–113 quantum dots,114,115 and layered double hydroxides,116–118 etc.). Compared with conventional nanocarriers, exosomes have several their own advantages and enormous potential in clinical tumor treatment.
 |
Table 1 The Summary of Inorganic Nanocarriers |
 |
Table 2 The Summary of Organic Nanocarriers |
First of all, due to the phospholipid bilayer structure, exosomes could carry drugs stably to avoid enzymes degrading drugs and extend the half-life of drugs during delivering, and their membranes could mix well with target cells. Correspondingly, the bioavailability of the loaded drug improved as well.
Then, compared with traditional drug carriers like liposomes, the immunogenicity, and toxicity of exosomes were poor. Moreover, we cannot ignore their petite body. The nanosize of exosomes allows them to be extravasated in tumor vessels and spread in tumor tissue to treat tumors. Thus, exosomes can also overcome some physiological barriers (eg, blood-brain barrier).119–122 Another advantage is that when the drug is loaded in exosomes, the efficacy of the drug is enhanced.
However, the only drawback is the difficulty of large-scale extraction. However, a number of scholars have discovered that other sources of exosomes can also be as same as human exosomes, can be utilized as anti-tumor drug carriers, and successfully applied to the treatment of cancer. Munagala et al9 found that bovine milk-derived exosomes can be used as nanocarriers for the delivery of chemotherapeutic/chemopreventive agents for the treatment of lung cancer, and can increase antitumor activity. Katakowski et al123 exploited the characteristics of exosomes capable of loading miRNAs, in which they used exosomes as nanocarriers carrying miRNAs to treat gliomas after loading miR-146b.
The Function of Exosomes in Tumor
Tumor-derived exosomes are involved in pathogenesis and microenvironmental establishment of cancer. To explore the specific function of exosomes in tumors, it is necessary to explore its complexity and functional heterogeneity. Exosomes have been widely proved to play an important role in the formation of tumor microenvironment,124 tumor invasion and metastasis,125,126 angiogenesis127 and tumor immunity.128 There is no doubt that exosomes secreted by cancers are one of the main biological mediators of tumor progression but some exosomes have been found to inhibit the occurrence and development of tumors. Which kind of exosomes to be choose is the most critical point in treatment. Logozzi et al129 suggest that nanomaterials getting to tissues are scavenged by macrophages that then release them through exosomes. Iessi et al130 devised a novel strategy involving the use of exosomes as carriers which is purified from culture supernatant of macrophages isolated from peripheral blood of healthy donors. The exosome delivery system showed to actually enhance the tumoricidal effect of Acridine Orange (AO), by increasing the exposure time of the biological targets. This evidence suggests that the exosomes release by macrophages potentially useful material. Exosomes have been detected in a variety of body fluids such as urine, saliva, bile, and blood. In tumor patients, the content of exosomes has become an important information to judge the occurrence and development of tumors.131–134 Therefore, preventing the secretion and circulation of tumor exosomes can effectively inhibit the progression of cancer. The results show that the high exosomes content in tumor tissues may be related to the acid anoxic microenvironment124,135 and Ca2+.136 The presence of an anoxic environment results in an acidic microenvironment, then results in an increased secretion of exosomes. Some studies showed that the exosomes of CD63+, CD9+ and ALIX+ increased by five times when the calcium content increased. It is therefore a useful option to suppress acidic microenvironments or to use Ca2+ channel blockers before using exosomes as carriers to treat tumors.
Application of Exosomes as Nanocarriers in Tumor Treatment
It was revealed that utilizing exosomes as nanocarriers to load antitumor drugs or siRNAs into exosomes may cause them possess the following characteristics, such as enhancing the efficacy of drugs, as well as expanding the current therapeutic range; increasing the bioavailability of drugs; targeting; being non-toxic or low-toxic, etc.137–139 We know that, Hadla et al140 made exosomal doxorubicin (ExoDOX) by electroporation, in which ExoDOX is more potent than free doxorubicin. Qi et al141 developed a dual-functional exosome-based superparamagnetic nanoparticle cluster using exosomes as a targeted drug carrier for the treatment of tumors. At present, the anti-tumor effect of exo-drugs mainly represents the occurrence and development of tumors by inhibiting cell proliferation, inducing apoptosis, inhibiting drug resistance, inhibiting tumor angiogenesis, and inhibiting cell invasion and metastasis. Simultaneously, exo-drugs can also treat tumors by regulating immunity (Figure 4).
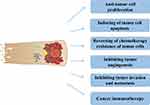 |
Figure 4 The anti-tumor effect of exo-drugs mainly represents the occurrence and development of tumors by inhibiting cell proliferation,10,123,148-150 inducing apoptosis,151–154 reversing chemotherapy resistance of tumor cells,155,158 inhibiting tumor angiogenesis,119,159 and inhibiting cell invasion and metastasis.11,160 Simultaneously, exo-drugs can also treat tumors by regulating immunity.161–166 |
Although some unanswered problems and methodological challenges remain, this rapidly advancing field have already provided important insights into the relevance of EVs in the clinical setting. The great clinical impact in nanomedicine of exosomes has been explored,142–144 The clinical research data have already demonstrated that exosomes secreted by immune cells stimulate the immune system and can be exploited as antitumor vaccines.145,146 Several clinical trials involving the use of extracellular vesicle-based delivery are ongoing, for example for the treatment of lung cancer and melanoma, that may become part of an immunotherapy approach that has great potential for patients with advanced cancers.147
Anti-Tumor Cell Proliferation
Compared with the mono drug action, the exosomes could enhance the anti-tumor cell proliferation characteristic of the drug via generating the exo-drugs with drug loading. F. Aqil et al10 mixed 10% of curcumin ethanol with acetonitrile to form exosomal curcumin (ExoCUR) with a drug loading rate of 18–24%. In vivo experiments showed that ExoCUR was absorbed compared to free curcumin, and the rate increased by 3–5 times; in vitro experiments showed that ExoCUR inhibited tumor cell proliferation, which was also significantly enhanced. More meaningful, ExoCUR has no systemic toxicity. Similarly Aqil et al148 used milk-derived exosomes to encapsulate berry anthocyanins to form ExoAnthos, which demonstrated that in vivo experiments Exo preparations may increase drug stability, uptake ratio, and half-life, allowing Anthos to be slowly released from exosomes to increase the anti-cancer effect in ovarian cancer.
Moreover, the exosomes play the role as the carrier for doxorubicin to generate the Exo-Dox, which functioned on MFC-7 cells and significantly inhibited the cell growth under Yang et al149 exploration. This effect is significantly superior to that of free doxorubicin. After combining Exo-Dox with heat-stress, Exo-Dox-HS showed better inhibition than Exo-Dox, and the content of doxorubicin in cells was higher as well. Subsequent examines using exosomes to load drugs not only revealed their own advantages, but also other technologies (eg, heat-stress) were employed to further enhance the anti-tumor efficacy of Exo-drugs.
In addition, the exosomes could also contribute to the other mediator function, such as the miR-146b and iRG peptides. Katakowski et al123 used the plasmid expressing miR-146b to transfect the marrow stromal cell to secrete M146-exosomes (M146-exo). M146-exo may inhibit the growth of gliomas by inhibiting the expression of EGFR, thereby treating tumors. Tian et al150 first modified the murine immature dendritic cells to produce exosomes carrying iRGD peptides on the surface, namely iRGD-Exos. iRGD-Exos was then loaded with DOX by electroporation to make iRGD-Exos-Dox. iRGD-Exos-Dox can target αv Integrin-positive tumor cells and can specifically bind to MDA-MB-231 cells and inhibit cells proliferation.
Inducing of Tumor Cell Apoptosis
Studies have shown that exosomes also have a good effect in inducing tumor cell apoptosis. At present, exosomes can induce tumor cell apoptosis in two ways. One is to carry a content to directly induce apoptosis of tumor cells. When exosomes are combined with drugs that induce apoptosis, the ability of the drug to induce apoptosis is enhanced, and the toxicity of normal tissue cells can be remarkably reduced. Interestingly, the manufactured EXO-drugs possess some features, such as targeting and pH sensitivity. For example, Srivastava et al151 creatively combined gold nanoparticles with apoptosis-inducing doxorubicin and made Nano-Dox for the first time. Afterwards, Nano-Dox was incubated with exosomes to make Exo-GNP-Dox. Exo-GNP-Dox has the following characteristics: it can be effective up taken by tumor cells, while it is hardly up taken by normal cells; it may cause tumor cell apoptosis by activating caspase-9, as well as inducing DNA damage; it also can induce ROS in tumor cells, and interfere with mitochondrial membrane potential, eventually leading to increased DNA damage and apoptosis in cancer cells, thereby protecting normal cells. And Xu et al152 screened exosomes expressing miR-29c and acted on bladder cancer BIU-87 cells. It was revealed that miR-29c carried by this exosome can down-regulate BCL-2 and MCL-1 to induce cell apoptosis.
There are also exosomes that, after being secreted by cells, the proteins carried by the exosomes themselves can induce the apoptosis of tumor cells without needing to re-design the exosomes. For instance, Zhu et al153 purified the exosomes secreted by NK cells and obtained NK-cell-derived exosomes (NK-Exo), which contained perforin and granzymes. That’s to say, NK-Exo active apoptosis pathway of glioblastoma via increased formation of apoptosome and caspase-3 activation with the help of perforin and granzymes. At the same time, Hosseini et al154 immobilized SEB to produce EXO/SEB. Then, they used EXO/SEB in estrogen receptor-negative breast cancer cells to induce apoptosis by inhibiting the expression of anti-apoptotic gene BCL-2, as well as promoting the expression of the Bax and Bak genes.
Reversing of Chemotherapy Resistance of Tumor Cells
Several tumors are resistant to chemotherapeutic drugs after chemotherapy. For example, drug-resistant cells produce drug-resistant proteins, such as P-glycoprotein,155 which can drain the chemotherapeutic drugs out of the cells, reduce the concentration of chemotherapeutic drugs in cells, and eventually cause cell resistance. At present, EXO-drug can reverse the chemotherapy resistance of tumor cells mainly through two ways, one way is to reduce the expression of drug resistance proteins in drug-resistant cells. Munoz et al156 found that in glioblastoma multiforme cells, due to the increase of miR-9, the expression of P-glycoprotein was also increased, resulting in tumor cell resistance to temozolomide. They utilized exosomes secreted by mesenchymal stem cells to carry anti-miR-9, targeting resistant cells, as well as inhibiting the expression of drug resistance proteins, in order to enhance the sensitivity of tumor cells to chemotherapy drugs, thereby reversing the resistance of tumor cells.
And the other way is to increase the sensitivity of drug-resistant cells to chemotherapy drugs. Kim et al157 loaded Paclitaxel (PTX) into exosomes by ultrasonic technology, so that exosomes encapsulated PTX and made PTX-exosome (exoPTX). Compared with free PTX, they found that exoPTX increased the toxicity of drug-resistant cells by 50-fold, indicating that exoPTX can enhance the sensitivity of drug-resistant cells to chemotherapeutic drugs, thereby improving the therapeutic effects of the drug on drug resistant cancers. Lou et al158 used miR-122 to transfect adipose tissue-derived mesenchymal stem cells, secreted exosomes coated with miR-122, and exosomes were used for the treatment of hepatocellular carcinoma by improving the sensitivity of liver cancer cells to chemotherapeutic drugs.
Inhibiting Tumor Angiogenesis
As we know, the continuous growth of tumors depends on the supply of tumor blood vessels, and inhibiting tumor angiogenesis has a very appropriate inhibitory effect on tumor growth. In fact, in the process of tumor angiogenesis, exosomes themselves are involved in this process, and studies have found that exosomes can promote tumor angiogenesis by carrying miRNAs such as miR-25-3p. For example, Zeng et al96 found that miR-25-3p was transfected into endothelial cells by exosomes in colon cancer cells, and miR-25-3p up-regulated expression of VEGFR2, p-AKT, p-ERK, and down-regulated ZO-1, occludin and Claudin5 by silencing KLF2 and KLF4, thereby inducing angiogenesis.
Based on this research, it was found that exosomes can inhibit tumor angiogenesis by carrying siRNA such as HGF siRNA. Such as the research of Zhang et al.159 They loaded HEK293T-derived exosomes with hepatocyte growth factor siRNA (HGF siRNA), and found that the exosomes could inhibit the expression of HGF/VEGF in SGC-7901 cells, thereby inhibiting the growth of tumor blood vessels. The exosomes can also inhibit the growth and metastasis of tumor cells. In addition, Yang et al119 found that siRNAs of vascular endothelial growth factor (VEGF) were transfected into exosomes derived from brain endothelial bEND.3 cells to produce exosome-delivered siRNAs. In the brain cancer model of zebrafish, exosome-delivered siRNAs can inhibit the expression of VEGF in brain cancer cells through the blood-brain barrier, thereby inhibiting tumor angiogenesis.
Inhibiting Tumor Invasion and Metastasis
Studies have shown that exosomes themselves are involved in the process of tumor invasion and metastasis. QinLan et al94 found that in colorectal cancer, M2 macrophage-derived exosomes carrying miR-21-5p and miR-155-5p are transferred to colorectal cancer cells, which bind to the BRG1 coding sequence and inhibit BRG1 expression, thereby promoting the invasion and metastasis of colorectal cancer. Chen et al95 also demonstrated that tumor invasion and metastasis are related to exosomes.
On this basis, people think that when exosomes are loaded with therapeutic substances, they can also inhibit the metastasis of tumors. Kamerkar et al11 presented exosomes carrying siRNA or shRNA to specifically target the oncogenic KRASG12D, which can inhibit the metastasis of pancreatic cancer and prolong the survival rate by reducing the expression of KRAS gene in a variety of pancreatic cancer mouse models. In addition, Shim et al160 transfected miR-143 into mesenchymal stem cells to secrete exosomes loaded with miR-143 to make exosome-formed miR-143. Then, they applied exosome-formed miR-143 to osteosarcoma cells, which inhibited the metastasis of osteosarcoma cells.
Cancer Immunotherapy
After engineering exosomes, a specific tumor antigen was expressed on the surface. Such exosomes can activate immune cells in vivo and inhibit the growth of tumors expressing antibodies corresponding to their antigens, so as to achieve efficient treatment of tumors. Cho et al161 investigated whether the autologous or allogeneic exosomes express their specific tumor antigen human MUC1 on the surface, and they can activate immune cells and inhibit the growth of MUC1-expressing tumors, in order to achieve the tumor treatment. These scholars first constructed the recombinant lentivirus pLXIN-muc1, and then infected mouse-derived CT26 and TA3HA cells with pLXIN-muc1 to obtain two types of cells, including CT26-MUC1 and TA3HA-hMUC1. These cells can be secreted, CT26-hMUC1 exosomes and TA3HA-hMUC1 exosomes. These two exosomes can promote the proliferation and activation of immune cells, and effectively inhibit the growth of tumors expressing MUC1. Morishita et al162 transfected a plasmid vector fused to streptavidin-lactocin with murine melanoma B16BL6 cells, engineered an exosome expressing streptavidin-lactocin (SAV-LA), and then used SAV-LA-expressing exosomes (SAV-exo). After integration with biotinylated CPG-DNA, CPG-DNA-modified SAV-exo was made, which was CpG-SAV-exo. They found that CPG-sav-exo can effectively activate mouse dendritic dc2.4 cells compared with CPG and SAV-exo, thereby enhancing the tumor antigen presentation ability of dc2.4 cells, indicating that CPG-sav-exo has a proper anti-tumor effect.
Some exosomes are secreted and purified from the cells in the body, and the tumor antigen is directly expressed on the surface without modification. Besides, the exosomes suppress the growth of the tumor by mediating the immune reaction. For example, in the study of liver cancer conducted by Rao et al163 human hepatocellular carcinoma HepG2 cell–derived exosomes were isolated and purified, which could induce dendritic cells to produce a strong immune response. Thus, they could inhibit the growth of the tumors by increasing the number of T lymphocytes, elevating the levels of interferon-c, as well as decreasing the levels of interleukin-10 (IL-10) and tumor growth factor-β (TGF-β1) in tumor sites. Bu164 et al loaded the exosomes from dendritic cells into chaperone rich cell lysates (CRCLs) and made DEX (CRCL-GL261)-DCs, in which in vivo experiments showed that DEX (CRCL-GL261)-DCs could promote the proliferation of cytotoxic T cells, leading to increase their activity, stimulate the production of anti-tumor factors IL-2 and IFNγ, and eventually inhibit tumor growth. Wang et al165 found that exosomes derived from CD40 ligand gene-modified 3LL tumor cells have a stronger immune effect and can induce more IFN-γ and IL-2 secretion after dendritic cells become more mature. Furthermore, Gehrmann et al166 produced an exosome that not only expressed the antigen ovalbumin (OVA), but also loaded the invariant NKT (iNKT) immune cell ligand α-galactosylceramide (αGC), by a series of immune responses, such as activation and proliferation of iNKT, NK, gamma delta (γδ) T cells (γδ T cells), as well as proliferating OVA-specific CD8+ T cells, in order to inhibit tumor growth.
In addition to these common chemical treatments for tumors, it has also been found that tumors can be treated by exosome combined with physical therapy. Altanerova et al167 found that exosomes carrying magnetic nanoparticles were effectively engulfed by tumor cells by endocytosis. Under the induction of external alternating magnetic fields, magnetic nanoparticles produced high temperatures, causing tumor cell ablation.
Conclusion
Chemotherapy and targeted therapy can hardly achieve the desired curative effect and result in the process of oncotherapy due to various defects, and patients may also have various uncomfortable physiological responses due to large amounts of drug intake. Exosome has been widely used as a biologically carrier of therapeutic materials, as its great drug loading capacity, high specificity and low immunogenicity are natural advantages that other nanometer materials do not have. The application of further customized exosome drugs in oncotherapy provides an important platform for the research and development of next-generation antineoplastic drugs. However, such drugs should be produced in a larger scale at more strictly controlled quality for application. In addition to rigorous toxicity observation, the drugs also need to be supported by the ultimate clinical tests. As far as the current research progress is concerned, the application of exosomes, in addition to low yield, needs to be studied in the following aspects: 1, The structure and mechanism of action of exosomes have not been thoroughly studied, and further research is needed; 2, Exo-drugs are targeted, but the stability of their targeting has not been studied in depth, and there may be off-target phenomenon.3, Exosomes from different sources carry a variety of substances from donor cells, leading to exogenous secretion. There are differences between the bodies, and it is necessary to standardize the use of exosomes. 4, In some studies, the researchers use tumor cell-derived exosomes. While, exosomes in from tumor patients contain tumor support components which could cause tumor changes in recipient cells or organisms.168–171 Cossetti et al171 found that exosomes are capable of transferring substances of tumor cells to recipient cells. Plasma-derived exosomes need to identify and remove tumor support components during the purification process. In view of this, animal-derived exosomes are more or less problematic, and Pedro Perez-Berm’udez et al have already found exosomes in food,172 such as milk, fruits, vegetables, etc. They not only have been isolated exosomes from these foods by centrifugation, but also found that exosomes in milk may be able to act on the human body. For example, milk-derived exosomes can affect the body’s immune function173 by carrying proteins, and can also regulate the intestinal barrier function.174 Perhaps we can use milk as a source of exosomes.
Even if these problems exist, with the breakthrough of research, it is just around the corner to use exosomes as nanocarriers for the treatment of tumors.
Abbreviations
EM, electron microscope; miRNA, micro ribonucleic acid; mRNA, messenger ribonucleic acid; tRNA, transfer ribonucleic acid; miRNA, microRNA; LncRNA, long noncoding RNA; DNA, deoxyribonucleic acid; ESCRT, endosomal sorting complexes required for the transport; ILVs, intraluminal vesicles; MVBs, multi-vesicle bodies; PEG, polyethylene glycol; TSG101, tumor susceptibility gene 101; ELISA, enzyme-linked immunosorbent assay; NTA, nanoparticle tracking analysis; EGFR, epidermal growth factor receptor; Dox, doxorubicin; RGD, Arg-Gly-Asp; ROS, reactive oxygen species; NK cells, natural killer cells; SEB, staphylococcal enterotoxin B; PTX, paclitaxel; VEGFR, vascular endothelial growth factor receptor; IL-2, interleukin-2; IFNγ, interferon-γ.
Data Sharing Statement
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Authors declare no conflicts of interest for this article.
References
1. Lee BR, Kim JH, Choi ES, Cho JH, Kim E. Effect of young exosomes injected in aged mice. Int J Nanomedicine. 2018;13:5335–5345. doi:10.2147/ijn.s170680
2. Trams EG, Lauter CJ, Salem N
3. Johnstone RM, Ahn J. A common mechanism may be involved in the selective loss of plasma membrane functions during reticulocyte maturation. Biomed Biochim Acta. 1990;49:S70–S75.
4. Yu G, Jung H, Kang YY, Mok H. Comparative evaluation of cell- and serum-derived exosomes to deliver immune stimulators to lymph nodes. Biomaterials. 2018;162:71–81. doi:10.1016/j.biomaterials.2018.02.003
5. Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841:108–120. doi:10.1016/j.bbalip.2013.10.004
6. Kahlert C, Melo SA, Protopopov A, et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289:3869–3875. doi:10.1074/jbc.C113.532267
7. Ding Y, Cao F, Sun H, et al. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett. 2018. doi:10.1016/j.canlet.2018.10.039
8. Kim MS, Haney MJ, Zhao Y, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14:195–204. doi:10.1016/j.nano.2017.09.011
9. Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61. doi:10.1016/j.canlet.2015.10.020
10. Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Gupta R. Exosomes for the enhanced tissue bioavailability and efficacy of curcumin. AAPS J. 2017;19(6):1691–1702. doi:10.1208/s12248-017-0154-9
11. Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi:10.1038/nature22341
12. Nash LA, McFall ER, Perozzo AM, et al. Survival motor neuron protein is released from cells in exosomes: a potential biomarker for spinal muscular atrophy. Sci Rep. 2017;7(1):13859. doi:10.1038/s41598-017-14313-z
13. Chen C, Zong S, Wang Z, et al. Visualization and intracellular dynamic tracking of exosomes and exosomal miRNAs using single molecule localization microscopy. Nanoscale. 2018;10(11):5154–5162. doi:10.1039/c7nr08800k
14. Mun D, Kim H, Kang J-Y, et al. Expression of miRNAs in circulating exosomes derived from patients with persistent atrial fibrillation. FASEB J. 2019;33(5):5979–5989. doi:10.1096/fj.201801758R
15. Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–948. doi:10.1083/jcb.101.3.942
16. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420.
17. Fan Q, Yang L, Zhang X, et al. The emerging role of exosome-derived non-coding RNAs in cancer biology. Cancer Lett. 2018;414:107–115. doi:10.1016/j.canlet.2017.10.040
18. Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi:10.1038/ncb1800
19. Mao L, Li X, Gong S, et al. Serum exosomes contain ECRG4 mRNA that suppresses tumor growth via inhibition of genes involved in inflammation, cell proliferation, and angiogenesis. Cancer Gene Ther. 2018;25:248–259. doi:10.1038/s41417-018-0032-3
20. Del Re M, Marconcini R, Pasquini G, et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br J Cancer. 2018;118(6):820–824. doi:10.1038/bjc.2018.9
21. Li M, Zeringer E, Barta T, et al. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130502. doi:10.1098/rstb.2013.0502
22. Srivastava A, Moxley K, Ruskin R, et al. A non-invasive liquid biopsy screening of urine-derived exosomes for miRNAs as Biomarkers in endometrial cancer patients. AAPS J. 2018;20:82. doi:10.1208/s12248-018-0220-y
23. Kim R, Lee S, Lee J, et al. Exosomes derived from microRNA-584 transfected mesenchymal stem cells: novel alternative therapeutic vehicles for cancer therapy. BMB Rep. 2018;51:406–411. doi:10.5483/BMBRep.2018.51.8.105
24. Momen-Heravi F, Bala S, Kodys K, Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci Rep. 2015;5(1):9991. doi:10.1038/srep09991
25. Lin XJ, Fang J-H, Yang X-J, et al. Hepatocellular carcinoma cell-secreted exosomal microRNA-210 promotes angiogenesis in vitro and in vivo. Mol Ther Nucleic Acids. 2018;11:243–252. doi:10.1016/j.omtn.2018.02.014
26. Patel NA, Moss LD, Lee JY, et al. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J Neuroinflammation. 2018;15:204. doi:10.1186/s12974-018-1240-3
27. Li B, Xu H, Han H, et al. Exosome-mediated transfer of lncRUNX2-AS1 from multiple myeloma cells to MSCs contributes to osteogenesis. Oncogene. 2018;37:5508–5519. doi:10.1038/s41388-018-0359-0
28. Lin LY, Yang L, Zeng Q, et al. Tumor-originated exosomal lncUEGC1 as a circulating biomarker for early-stage gastric cancer. Mol Cancer. 2018;17:84. doi:10.1186/s12943-018-0834-9
29. Fricke F, Lee J, Michalak M, et al. TGFBR2-dependent alterations of exosomal cargo and functions in DNA mismatch repair-deficient HCT116 colorectal cancer cells. Cell Commun Signal. 2017;15:14. doi:10.1186/s12964-017-0169-y
30. Liu C, Guo J, Tian F, et al. Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano. 2017;11:6968–6976. doi:10.1021/acsnano.7b02277
31. Mazzeo C, Calvo V, Alonso R, Merida I, Izquierdo M. Protein kinase D1/2 is involved in the maturation of multivesicular bodies and secretion of exosomes in T and B lymphocytes. Cell Death Differ. 2016;23:99–109. doi:10.1038/cdd.2015.72
32. Hazan-Halevy I, Rosenblum D, Weinstein S, et al. Cell-specific uptake of mantle cell lymphoma-derived exosomes by malignant and non-malignant B-lymphocytes. Cancer Lett. 2015;364(1):59–69. doi:10.1016/j.canlet.2015.04.026
33. Riches A, Campbell E, Borger E, Powis S. Regulation of exosome release from mammary epithelial and breast cancer cells - a new regulatory pathway. Eur J Cancer. 2014;50:1025–1034. doi:10.1016/j.ejca.2013.12.019
34. van Balkom BW, de Jong OG, Smits M, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:
35. Zheng L, Li Z, Ling W, et al. Exosomes derived from dendritic cells attenuate liver injury by modulating the balance of treg and Th17 cells after ischemia reperfusion. Cell Physiol Biochem. 2018;46(2):740–756. doi:10.1159/000488733
36. Cho BS, Kim JO, Ha DH, Yi YW. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res Ther. 2018;9:187. doi:10.1186/s13287-018-0939-5
37. Kim HY, Kumar H, Jo M-J, et al. Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment. Nano Lett. 2018;18(8):4965–4975. doi:10.1021/acs.nanolett.8b01816
38. Safwat A, Sabry D, Ragiae A, et al. Adipose mesenchymal stem cells-derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J Circ Biomark. 2018;7:1849454418807827. doi:10.1177/1849454418807827
39. Klaihmon P, Lertthammakiat S, Anurathapan U, et al. Activated platelets and leukocyte activations in young patients with beta-thalassemia/HbE following bone marrow transplantation. Thromb Res. 2018;169:8–14. doi:10.1016/j.thromres.2018.07.007
40. Li J, Tan M, Xiang Q, Zhou Z, Yan H. Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thromb Res. 2017;154:96–105. doi:10.1016/j.thromres.2017.04.016
41. Fu Q, Zhang Q, Lou Y, et al. Primary tumor-derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via SMAD3 in liver cancer. Oncogene. 2018;37(47):6105–6118. doi:10.1038/s41388-018-0391-0
42. Xu H, Jiao X, Wu Y, et al. Exosomes derived from PM2.5treated lung cancer cells promote the growth of lung cancer via the Wnt3a/betacatenin pathway. Oncol Rep. 2018. doi:10.3892/or.2018.6862
43. Zhang W, Jiang X, Bao J, et al. Exosomes in pathogen infections: a bridge to deliver molecules and link functions. Front Immunol. 2018;9:90. doi:10.3389/fimmu.2018.00090
44. Grigor’eva AE, Tamkovich SN, Eremina AV, et al. [Characteristics of exosomes andmicroparticles discovered in human tears]. Biomed Khim. 2016;62:99–106. doi:10.18097/pbmc20166201099. Russian.
45. Wu G, Yang G, Zhang R, et al. Altered microRNA expression profiles of extracellular vesicles in nasal mucus from patients with allergic rhinitis. Allergy Asthma Immunol Res. 2015;7(5):44s9–457. doi:10.4168/aair.2015.7.5.449
46. Sun Y, Huo C, Qiao Z, et al. Comparative proteomic analysis of exosomes and microvesicles in human saliva for lung cancer. J Proteome Res. 2018;17(3):1101–1107. doi:10.1021/acs.jproteome.7b00770
47. Liao Y, Du X, Li J, Lonnerdal B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol Nutr Food Res. 2017;61(11):1700082. doi:10.1002/mnfr.201700082
48. Wang L, Skotland T, Berge V, Sandvig K, Llorente A. Exosomal proteins as prostate cancer biomarkers in urine: from mass spectrometry discovery to immunoassay-based validation. Eur J Pharm Sci. 2017;98:80–85. doi:10.1016/j.ejps.2016.09.023
49. Franzen CA, Blackwell RH, Foreman KE, et al. Urinary exosomes: the potential for biomarker utility, intercellular signaling and therapeutics in urological malignancy. J Urol. 2016;195:1331–1339. doi:10.1016/j.juro.2015.08.115
50. He L, Zhu D, Wang J, Wu X. A highly efficient method for isolating urinary exosomes. Int J Mol Med. 2018. doi:10.3892/ijmm.2018.3944
51. Madison MN, Welch JL, Okeoma CM. Isolation of exosomes from semen for in vitro uptake and HIV-1 infection assays. Bio-Protocol. 2017;7. doi:10.21769/BioProtoc.2216.
52. Welch JL, Kaddour H, Schlievert PM, Stapleton JT, Okeoma CM. Semen exosomes promote transcriptional silencing of HIV-1 by disrupting NF-kB/Sp1/Tat circuitry. J Virol. 2018;92. doi:10.1128/jvi.00731-18.
53. Kojima M, Gimenes-Junior JA, Chan TW, et al. Exosomes in postshock mesenteric lymph are key mediators of acute lung injury triggering the macrophage activation via Toll-like receptor 4. FASEB J. 2018;32:97–110. doi:10.1096/fj.201700488R
54. Xu N, Wang L, Guan J, et al. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol. 2018;117:102–107. doi:10.1016/j.ijbiomac.2018.05.066
55. Sokolova V, Ludwig A-K, Hornung S, et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surfaces B Biointerfaces. 2011;87(1):146–150. doi:10.1016/j.colsurfb.2011.05.013
56. Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16(4):415–421. doi:10.1016/j.ceb.2004.06.003
57. Adell MAY, Vogel GF, Pakdel M, et al. Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation. J Cell Biol. 2014;205(1):33–49. doi:10.1083/jcb.201310114
58. Tamai K, Tanaka N, Nakano T, et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun. 2010;399:384–390. doi:10.1016/j.bbrc.2010.07.083
59. Shojapour M, Mosayebi G, Hajihossein R, et al. A simplified protocol for the purification of schwann cells and exosome isolation from C57BL/6 mice. Rep Biochem Mol Biol. 2018;7:9–15.
60. Mastoridis S, Bertolino GM, Whitehouse G, et al. Multiparametric analysis of circulating exosomes and other small extracellular vesicles by advanced imaging flow cytometry. Front Immunol. 2018;9:1583. doi:10.3389/fimmu.2018.01583
61. Oeyen E, Van Mol K, Baggerman G, et al. Ultrafiltration and size exclusion chromatography combined with asymmetrical-flow field-flow fractionation for the isolation and characterisation of extracellular vesicles from urine. J Extracell Vesicles. 2018;7:1490143. doi:10.1080/20013078.2018.1490143
62. Yoshida T, Ishidome T, Hanayama R. High purity isolation and sensitive quantification of extracellular vesicles using affinity to TIM4. Curr Protoc Cell Biol. 2017;77:
63. Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem. 2008;105(5):1211–1218. doi:10.1002/jcb.21923
64. Liu Z, Cauvi DM, Bernardino EM, et al. Isolation and characterization of human urine extracellular vesicles. Cell Stress Chaperones. 2018;23:943–953. doi:10.1007/s12192-018-0902-5
65. Gupta S, Rawat S, Arora V, et al. An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells. Stem Cell Res Ther. 2018;9(1):180. doi:10.1186/s13287-018-0923-0
66. Deregibus MC, Figliolini F, D’antico S, et al. Charge-based precipitation of extracellular vesicles. Int J Mol Med. 2016;38(5):1359–1366. doi:10.3892/ijmm.2016.2759
67. Martins TS, Catita J, Rosa IM, e Silva OADC, Henriques AG. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS One. 2018;13:e0198820. doi:10.1371/journal.pone.0198820
68. Guerreiro EM, Vestad B, Steffensen LA, et al. Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size-exclusion chromatography. PLoS One. 2018;13(9):e0204276. doi:10.1371/journal.pone.0204276
69. Borges FT, Reis L, Schor N. Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res. 2013;46(10):824–830. doi:10.1590/1414-431X20132964
70. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi:10.1083/jcb.201211138
71. Lee K, Shao H, Weissleder R, Lee H. Acoustic purification of extracellular microvesicles. ACS Nano. 2015;9:2321–2327. doi:10.1021/nn506538f
72. Davies RT, Kim J, Jang SC, et al. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip. 2012;12(24):5202–5210. doi:10.1039/c2lc41006k
73. Wang Z, Wu H-J, Fine D, et al. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip. 2013;13(15):2879–2882. doi:10.1039/c3lc41343h
74. Wang J, Yao Y, Wu J, Li G. Identification and analysis of exosomes secreted from macrophages extracted by different methods. Int J Clin Exp Pathol. 2015;8:6135–6142.
75. Lovett JAC, Durcan PJ, Myburgh KH. Investigation of circulating extracellular vesicle microRNA following two consecutive bouts of muscle-damaging exercise. Front Physiol. 2018;9:1149. doi:10.3389/fphys.2018.01149
76. McNicholas K, Michael MZ. Immuno-characterization of exosomes using nanoparticle tracking analysis. Methods Mol Biol. 2017;1545:35–42. doi:10.1007/978-1-4939-6728-5_3
77. Hikita T, Miyata M, Watanabe R, Oneyama C. Sensitive and rapid quantification of exosomes by fusing luciferase to exosome marker proteins. Sci Rep. 2018;8:14035. doi:10.1038/s41598-018-32535-7
78. Jin J, Shi Y, Gong J, et al. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. 2019;10(1):95. doi:10.1186/s13287-019-1177-1
79. Li J, Chen X, Yi J, et al. Identification and characterization of 293T cell-derived exosomes by profiling the protein, mrna and MicroRNA components. PLoS One. 2016;11:e0163043. doi:10.1371/journal.pone.0163043
80. van der Pol E, de Rond L, Coumans FAW, et al. Absolute sizing and label-free identification of extracellular vesicles by flow cytometry. Nanomedicine. 2018;14(3):801–810. doi:10.1016/j.nano.2017.12.012
81. Yuan D, Chen H, Wang S, et al. Identification of LEA, a podocalyxin-like glycoprotein, as a predictor for the progression of colorectal cancer. Cancer Med. 2018;7:5155–5166. doi:10.1002/cam4.1765
82. Johnstone RM. The Jeanne Manery-Fisher Memorial Lecture 1991. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem Cell Biol. 1992;70(3–4):179–190. doi:10.1139/o92-028
83. Agnati LF, Fuxe K. Extracellular-vesicle type of volume transmission and tunnelling-nanotube type of wiring transmission add a new dimension to brain neuro-glial networks. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130505. doi:10.1098/rstb.2013.0505
84. Wang Z, Chen JQ, Liu JL, Tian L. Exosomes in tumor microenvironment: novel transporters and biomarkers. J Transl Med. 2016;14:297. doi:10.1186/s12967-016-1056-9
85. Ludwig N, Razzo BM, Yerneni SS, Whiteside TL. Optimization of cell culture conditions for exosome isolation using mini-size exclusion chromatography (mini-SEC). Exp Cell Res. 2019;378(2):149–157. doi:10.1016/j.yexcr.2019.03.014
86. Campanella C, Rappa F, Sciumè C, et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer. 2015;121(18):3230–3239. doi:10.1002/cncr.29499
87. Iaccino E, Mimmi S, Dattilo V, et al. Monitoring multiple myeloma by idiotype-specific peptide binders of tumor-derived exosomes. Mol Cancer. 2017;16:159. doi:10.1186/s12943-017-0730-8
88. Jiang Y, Xie H, Tu W, et al. Exosomes secreted by HUVECs attenuate hypoxia/reoxygenation-induced apoptosis in neural cells by suppressing miR-21-3p. Am J Transl Res. 2018;10(11):3529–3541.
89. Cocozza F, Menay F, Tsacalian R, et al. Cyclophosphamide enhances the release of tumor exosomes that elicit a specific immune response in vivo in a murine T-cell lymphoma. Vaccine. 2019;37(12):1565–1576. doi:10.1016/j.vaccine.2019.02.004
90. Campanella C, D’Anneo A, Gammazza AM, et al. The histone deacetylase inhibitor SAHA induces HSP60 nitration and its extracellular release by exosomal vesicles in human lung-derived carcinoma cells. Oncotarget. 2016;7(20):28849–28867. doi:10.18632/oncotarget.6680
91. Zheng Y, He R, Wang P, et al. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater Sci. 2019;7:2037–2049. doi:10.1039/c8bm01449c
92. Kapustin AN, Schoppet M, Schurgers LJ, et al. Prothrombin loading of vascular smooth muscle cell-derived exosomes regulates coagulation and calcification. Arterioscler Thromb Vasc Biol. 2017;37:e22–e32. doi:10.1161/atvbaha.116.308886
93. Wu R, Huang C, Wu Q, et al. Exosomes secreted by urine-derived stem cells improve stress urinary incontinence by promoting repair of pubococcygeus muscle injury in rats. Stem Cell Res Ther. 2019;10(1):80. doi:10.1186/s13287-019-1182-4
94. Lan J, Sun L, Xu F, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79:146–158. doi:10.1158/0008-5472.can-18-0014
95. Chen X, Chen R-X, Wei W-S, et al. PRMT5 circular RNA promotes metastasis of urothelial carcinoma of the bladder through sponging mir-30c to induce epithelial-mesenchymal transition. Clin Cancer Res. 2018;24:6319–6330. doi:10.1158/1078-0432.ccr-18-1270
96. Zeng Z, Li Y, Pan Y, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395. doi:10.1038/s41467-018-07810-w
97. Du B, Han S, Li H, et al. Multi-functional liposomes showing radiofrequency-triggered release and magnetic resonance imaging for tumor multi-mechanism therapy. Nanoscale. 2015;7(12):5411–5426. doi:10.1039/c4nr04257c
98. Budai M, Szogyi M. [Liposomes as drug carrier systems. Preparation, classification and therapeutic advantages of liposomes]. Acta Pharm Hung. 2001;71:114–118. Hungarian.
99. Li G, Liu D, Kimchi ET, et al. Nanoliposome C6-ceramide increases the anti-tumor immune response and slows growth of liver tumors in mice. Gastroenterology. 2018;154(4):1024–1036.e1029. doi:10.1053/j.gastro.2017.10.050
100. Ceccon A, Schmidt T, Tugarinov V, et al. Interaction of huntingtin exon-1 peptides with lipid-based micellar nanoparticles probed by solution NMR and Q-band pulsed EPR. J Am Chem Soc. 2018;140:6199–6202. doi:10.1021/jacs.8b02619
101. Parayath NN, Nehoff H, Muller P, Taurin S, Greish K. Styrene maleic acid micelles as a nanocarrier system for oral anticancer drug delivery - dual uptake through enterocytes and M-cells. Int J Nanomedicine. 2015;10:4653–4667. doi:10.2147/ijn.s87681
102. Mahmoudzadeh M, Fassihi A, Emami J, Davies NM, Dorkoosh F. Physicochemical, pharmaceutical and biological approaches toward designing optimized and efficient hydrophobically modified chitosan-based polymeric micelles as a nanocarrier system for targeted delivery of anticancer drugs. J Drug Target. 2013;21:693–709. doi:10.3109/1061186x.2013.824455
103. Dreau D, Moore LJ, Alvarez-Berrios MP, et al. Mucin-1-antibody-conjugated mesoporous silica nanoparticles for selective breast cancer detection in a mucin-1 transgenic murine mouse model. J Biomed Nanotechnol. 2016;12(12):2172–2184. doi:10.1166/jbn.2016.2318
104. Liu X, Situ A, Kang Y, et al. Irinotecan delivery by lipid-coated mesoporous silica nanoparticles shows improved efficacy and safety over liposomes for pancreatic cancer. ACS Nano. 2016;10(2):2702–2715. doi:10.1021/acsnano.5b07781
105. Butler KS, Durfee PN, Theron C, et al. Protocells: modular mesoporous silica nanoparticle-supported lipid bilayers for drug delivery. Small. 2016;12(16):2173–2185. doi:10.1002/smll.201502119
106. Liu T, Dan W, Dan N, et al. A novel grapheme oxide-modified collagen-chitosan bio-film for controlled growth factor release in wound healing applications. Mater Sci Eng C. 2017;77:202–211. doi:10.1016/j.msec.2017.03.256
107. Zhu X, Zhang Y, Huang H, et al. Functionalized graphene oxide-based thermosensitive hydrogel for near-infrared chemo-photothermal therapy on tumor. J Biomater Appl. 2016;30(8):1230–1241. doi:10.1177/0885328215619583
108. Yin PT, Pongkulapa T, Cho H-Y, et al. Overcoming chemoresistance in cancer via combined microRNA therapeutics with anticancer drugs using multifunctional magnetic core-shell nanoparticles. ACS Appl Mater Interfaces. 2018;10:26954–26963. doi:10.1021/acsami.8b09086
109. Sargazi A, Shiri F, Keikha S, Majd MH. Hyaluronan magnetic nanoparticle for mitoxantrone delivery toward CD44-positive cancer cells. Colloids Surf B Biointerfaces. 2018;171:150–158. doi:10.1016/j.colsurfb.2018.07.025
110. Li Z, Zhang J, Li X, Guo X, Zhang Z. Preparation and evaluation of multifunctional autofluorescent magnetic nanoparticle-based drug delivery systems against mammary cancer. J Pharm Sci. 2018;107:2694–2701. doi:10.1016/j.xphs.2018.06.009
111. Poletaeva J, Dovydenko I, Epanchintseva A, et al. Non-covalent associates of siRNAs and AuNPs enveloped with lipid layer and doped with amphiphilic peptide for efficient siRNA delivery. Int J Mol Sci. 2018;19(7):2096. doi:10.3390/ijms19072096
112. Bera K, Maiti S, Maity M, Mandal C, Maiti NC. Porphyrin-gold nanomaterial for efficient drug delivery to cancerous cells. ACS Omega. 2018;3:4602–4619. doi:10.1021/acsomega.8b00419
113. Yuan X, He Y, Zhou G, et al. Target challenging-cancer drug delivery to gastric cancer tissues with a fucose graft epigallocatechin-3-gallate-gold particles nanocomposite approach. J Photochem Photobiol B. 2018;183:147–153. doi:10.1016/j.jphotobiol.2018.04.026
114. Getz T, Qin J, Medintz IL, et al. Quantum dot-mediated delivery of siRNA to inhibit sphingomyelinase activities in brain-derived cells. J Neurochem. 2016;139(5):872–885. doi:10.1111/jnc.13841
115. Degim IT, Kadioglu D. Cheap, suitable, predictable and manageable nanoparticles for drug delivery: quantum dots. Curr Drug Deliv. 2013;10:32–38. doi:10.2174/1567201811310010006
116. Zhang LX, Xie XX, Liu DQ, Xu ZP, Liu RT. Efficient co-delivery of neo-epitopes using dispersion-stable layered double hydroxide nanoparticles for enhanced melanoma immunotherapy. Biomaterials. 2018;174:54–66. doi:10.1016/j.biomaterials.2018.05.015
117. Pontes-Neto JG, Fontes DAF, de Lyra MAM, et al. Evaluation of antioxidant potencial of novel CaAl and NiAl layered double hydroxides loaded with olanzapine. Life Sci. 2018;207:246–252. doi:10.1016/j.lfs.2018.05.031
118. Xu T, Xu X, Gu Y, Fang L, Cao F. Functional intercalated nanocomposites with chitosan-glutathione-glycylsarcosine and layered double hydroxides for topical ocular drug delivery. Int J Nanomedicine. 2018;13:917–937. doi:10.2147/ijn.s148104
119. Yang T, Fogarty B, LaForge B, et al. Delivery of small interfering RNA to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain cancer. AAPS J. 2017;19(2):475–486. doi:10.1208/s12248-016-0015-y
120. Li X, Tsibouklis J, Weng T, et al. Nano carriers for drug transport across the blood-brain barrier. J Drug Target. 2017;25:17–28. doi:10.1080/1061186x.2016.1184272
121. Yang T, Martin P, Fogarty B, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32(6):2003–2014. doi:10.1007/s11095-014-1593-y
122. Katakowski M, Chopp M. Exosomes as tools to suppress primary brain tumor. Cell Mol Neurobiol. 2016;36(3):343–352. doi:10.1007/s10571-015-0280-9
123. Katakowski M, Buller B, Zheng X, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201–204. doi:10.1016/j.canlet.2013.02.019
124. Logozzi M, Mizzoni D, Angelini D, et al. Microenvironmental pH and exosome levels interplay in human cancer cell lines of different histotypes. Cancers. 2018;10(10):370. doi:10.3390/cancers10100370
125. Lugini L, Valtieri M, Federici C, et al. Exosomes from human colorectal cancer induce a tumor-like behavior in colonic mesenchymal stromal cells. Oncotarget. 2016;7(31):50086. doi:10.18632/oncotarget.10574
126. Zhao H, Achreja A, Iessi E, et al. The key role of extracellular vesicles in the metastatic process. Biochim Biophys Acta Rev Cancer. 2018;1869:64–77.
127. Park JE, Tan HS, Datta A, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–1099.
128. Anand PK. Exosomal membrane molecules are potent immune response modulators. Commun Integr Biol. 2010;3(5):405–408. doi:10.4161/cib.3.5.12474
129. Logozzi M, Mizzoni D, Bocca B, et al. Human primary macrophages scavenge AuNPs and eliminate it through exosomes. A natural shuttling for nanomaterials. Eur J Pharm Biopharm. 2019;137:23–36. doi:10.1016/j.ejpb.2019.02.014
130. Iessi E, Logozzi M, Lugini L, et al. Acridine Orange/exosomes increase the delivery and the effectiveness of Acridine Orange in human melanoma cells: a new prototype for theranostics of tumors. J Enzyme Inhib Med Chem. 2017;32(1):648–657. doi:10.1080/14756366.2017.1292263
131. Logozzi M, Angelini DF, Iessi E, et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017;403:318–329. doi:10.1016/j.canlet.2017.06.036
132. Logozzi M, De Milito A, Lugini L, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4:e5219.
133. Osti D, Del Bene M, Rappa G, et al. Clinical significance of extracellular vesicles in plasma from glioblastoma patients. Clin Cancer Res. 2019;25(1):266–276. doi:10.1158/1078-0432.CCR-18-1941
134. Rodríguez Zorrilla S, Pérez-Sayans M, Fais S, et al. A pilot clinical study on the prognostic relevance of plasmatic exosomes levels in oral squamous cell carcinoma patients. Cancers. 2019;11(3):429. doi:10.3390/cancers11030429
135. Logozzi M, Spugnini E, Mizzoni D, Di Raimo R, Fais S. Extracellular acidity and increased exosome release as key phenotypes of malignant tumors. Cancer Metastasis Rev. 2019;38(1–2):93–101. doi:10.1007/s10555-019-09783-8
136. Savina A, Furlán M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278(22):20083–20090. doi:10.1074/jbc.M301642200
137. Manfredi F, Di Bonito P, Arenaccio C, Anticoli S, Federico M. Lentiviral Vectors and Exosomes as Gene and Protein Delivery Tools. Springer; 2016:249–260.
138. Kojima R, Bojar D, Rizzi G, et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun. 2018;9(1):1–10. doi:10.1038/s41467-018-03733-8
139. Wang Q, Yu J, Kadungure T, et al. ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat Commun. 2018;9:1–7. doi:10.1038/s41467-017-02088-w
140. Hadla M, Palazzolo S, Corona G, et al. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine (London, England). 2016;11(18):2431–2441. doi:10.2217/nnm-2016-0154
141. Qi H, Liu C, Long L, et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10(3):3323–3333. doi:10.1021/acsnano.5b06939
142. Fais S, O’Driscoll L, Borras FE, et al. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano. 2016;10(4):3886–3899. doi:10.1021/acsnano.5b08015
143. Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials–an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi:10.3402/jev.v4.30087
144. Van Niel G, d’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213. doi:10.1038/nrm.2017.125
145. Besse B, Charrier M, Lapierre V, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5(4):e1071008. doi:10.1080/2162402X.2015.1071008
146. Pitt JM, Charrier M, Viaud S, et al. Dendritic cell–derived exosomes as immunotherapies in the fight against cancer. J Immunol. 2014;193:1006–1011. doi:10.4049/jimmunol.1400703
147. Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials–an ISEV position paper[J]. J Extracell Vesicles, 2015;4(1): 30087.
148. Aqil F, Jeyabalan J, Agrawal AK, et al. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food Funct. 2017;8(11):4100–4107. doi:10.1039/c7fo00882a
149. Yang Y, Chen Y, Zhang F, Zhao Q, Zhong H. Increased anti-tumour activity by exosomes derived from doxorubicin-treated tumour cells via heat stress. Int J Hyperthermia. 2015;31(5):498–506. doi:10.3109/02656736.2015.1036384
150. Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi:10.1016/j.biomaterials.2013.11.083
151. Srivastava A, Amreddy N, Babu A, et al. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci Rep. 2016;6:38541. doi:10.1038/srep38541
152. Xu X-D, Wu X-H, Fan Y-R, et al. Exosome-derived microRNA-29c induces apoptosis of BIU-87 cells by down regulating BCL-2 and MCL-1. Asian Pac J Cancer Prev. 2014;15(8):3471–3476. doi:10.7314/APJCP.2014.15.8.3471
153. Zhu L, Oh JM, Gangadaran P, et al. Targeting and therapy of glioblastoma in a mouse model using exosomes derived from natural killer cells. Front Immunol. 2018;9:824. doi:10.3389/fimmu.2018.00824
154. Mahmoodzadeh Hosseini H, Imani Fooladi AA, Soleimanirad J, et al. Staphylococcal enterotoxin B anchored exosome induces apoptosis in negative esterogen receptor breast cancer cells. Tumour Biol. 2014;35:3699–3707. doi:10.1007/s13277-013-1489-1
155. Wang LM, Zhang MY, Zhu QS, Lu CF, Bai X. Hyperin enhances the sensitivity of HCT8/VCR colon cancer cell line to vincristine by down-regulating P-glycoprotein. Clin Lab. 2018;64:269–275. doi:10.7754/Clin.Lab.2017.170923
156. Munoz JL, Bliss SA, Greco SJ, et al. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids. 2013;2:e126. doi:10.1038/mtna.2013.60
157. Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–664. doi:10.1016/j.nano.2015.10.012
158. Lou G, Song X, Yang F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8(1):122. doi:10.1186/s13045-015-0220-7
159. Zhang H, Wang Y, Bai M, et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018;109(3):629–641. doi:10.1111/cas.13488
160. Shimbo K, Miyaki S, Ishitobi H, et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun. 2014;445(2):381–387. doi:10.1016/j.bbrc.2014.02.007
161. Cho J-A, Yeo D-J, Son H-Y, et al. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114(4):613–622. doi:10.1002/ijc.20757
162. Morishita M, Takahashi Y, Matsumoto A, Nishikawa M, Takakura Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111:55–65. doi:10.1016/j.biomaterials.2016.09.031
163. Rao Q, Zuo B, Lu Z, et al. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology (Baltimore, Md). 2016;64(2):456–472. doi:10.1002/hep.28549
164. Bu N, Wu H, Zhang G, et al. Exosomes from dendritic cells loaded with chaperone-rich cell lysates elicit a potent T cell immune response against intracranial glioma in mice. J Mol Neurosci. 2015;56(3):631–643. doi:10.1007/s12031-015-0506-9
165. Wang J, Wang L, Lin Z, Tao L, Chen M. More efficient induction of antitumor T cell immunity by exosomes from CD40L gene-modified lung tumor cells. Mol Med Rep. 2014;9:125–131. doi:10.3892/mmr.2013.1759
166. Gehrmann U, Hiltbrunner S, Georgoudaki A-M, et al. Synergistic induction of adaptive antitumor immunity by codelivery of antigen with alpha-galactosylceramide on exosomes. Cancer Res. 2013;73:3865–3876. doi:10.1158/0008-5472.can-12-3918
167. Altanerova U, Babincova M, Babinec P, et al. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int J Nanomedicine. 2017;12:7923–7936. doi:10.2147/ijn.s145096
168. Federici C, Petrucci F, Caimi S, et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One. 2014;9(2):e88193. doi:10.1371/journal.pone.0088193
169. Campanella C, Caruso Bavisotto C, Logozzi M, et al. On the choice of the extracellular vesicles for therapeutic purposes. Int J Mol Sci. 2019;20(2):236. doi:10.3390/ijms20020236
170. Logozzi M, De Milito A, Lugini L, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4(4):e5219. doi:10.1371/journal.pone.0005219
171. Cossetti C, Lugini L, Astrologo L, et al. Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: possible transport by exosomes. PLoS One. 2014;9(7):e101629. doi:10.1371/journal.pone.0101629
172. Perez-Bermudez P, Blesa J, Soriano JM, Marcilla A. Extracellular vesicles in food: experimental evidence of their secretion in grape fruits. Eur J Pharm Sci. 2017;98:40–50. doi:10.1016/j.ejps.2016.09.022
173. Samuel M, Chisanga D, Liem M, et al. Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci Rep. 2017;7(1):5933. doi:10.1038/s41598-017-06288-8
174. Gao HN, Guo HY, Zhang H, et al. Yak-milk-derived exosomes promote proliferation of intestinal epithelial cells in an hypoxic environment. J Dairy Sci. 2019;102(2):985–996. doi:10.3168/jds.2018-14946
175. Lim J, Choi M, Lee H, et al. Direct isolation and characterization of circulating exosomes from biological samples using magnetic nanowires. J Nanobiotechnol. 2019;17(1):1. doi:10.1186/s12951-018-0433-3
176. Pulskamp K, Diabate S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168(1):58–74. doi:10.1016/j.toxlet.2006.11.001
177. Zhang W, Zhang Z, Zhang Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res Lett. 2011;6(1):555. doi:10.1186/1556-276x-6-555
178. Cui D, Tian F, Ozkan CS, Wang M, Gao H. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett. 2005;155(1):73–85. doi:10.1016/j.toxlet.2004.08.015
179. Huang H, Yuan Q, Shah JS, Misra RD. A new family of folate-decorated and carbon nanotube-mediated drug delivery system: synthesis and drug delivery response. Adv Drug Deliv Rev. 2011;63:1332–1339. doi:10.1016/j.addr.2011.04.001
180. Jayakumar S, Li H, Chen J, Yang Q. Cationic Zn-porphyrin polymer coated onto CNTs as a cooperative catalyst for the synthesis of cyclic carbonates. ACS Appl Mater Interfaces. 2018;10:2546–2555. doi:10.1021/acsami.7b16045
181. Zhao Q, Wang S, Yang Y, et al. Hyaluronic acid and carbon dots-gated hollow mesoporous silica for redox and enzyme-triggered targeted drug delivery and bioimaging. Mater Sci Eng C Mater Biol Appl. 2017;78:475–484. doi:10.1016/j.msec.2017.04.059
182. Wang Y, Wang J, Yang Y, et al. In situ biodegradable crosslinking of cationic oligomer coating on mesoporous silica nanoparticles for drug delivery. Colloids Surf B Biointerfaces. 2017;153:272–279. doi:10.1016/j.colsurfb.2017.02.033
183. Zhao J, He Z, Li B, Cheng T, Liu G. AND logic-like pH- and light-dual controlled drug delivery by surface modified mesoporous silica nanoparticles. Mater Sci Eng C Mater Biol Appl. 2017;73:1–7. doi:10.1016/j.msec.2016.12.056
184. Zhou S, Wu D, Yin X, et al. Intracellular pH-responsive and rituximab-conjugated mesoporous silica nanoparticles for targeted drug delivery to lymphoma B cells. J Exp Clin Cancer Res. 2017;36(1):24. doi:10.1186/s13046-017-0492-6
185. Beola LL, Asín L, Fratila RM, et al. Dual role of magnetic nanoparticles as intracellular hotspots and extracellular matrix disruptors triggered by magnetic hyperthermia in 3D cell culture models. ACS Appl Mater Interfaces. 2018;10(51):44301–44313. doi:10.1021/acsami.8b18270
186. Chen H, Yang Q, Ding Y, et al. Competitive and noncompetitive immunoassays for the detection of benzothiostrobin using magnetic nanoparticles and fluorescein isothiocyanate-labeled peptides. Anal Bioanal Chem. 2018. doi:10.1007/s00216-018-1478-8
187. Xue X, Lu R, Liu M, et al. A facile and general approach for the preparation of boronic acid-functionalized magnetic nanoparticles for the selective enrichment of glycoproteins. Analyst. 2018. doi:10.1039/c8an01704b
188. Wang J, Han J, Zhu C, et al. Gold Nanorods/Polypyrrole/m-SiO2 Core/Shell Hybrids as Drug Nanocarriers for Efficient Chemo-Photothermal Therapy. Langmuir. 2018;34(48):14661–14669. doi:10.1021/acs.langmuir.8b02667
189. Liu Y, Zhang X, Luo L, et al. Gold-nanobranched-shell based drug vehicles with ultrahigh photothermal efficiency for chemo-photothermal therapy. Nanomedicine. 2018. doi:10.1016/j.nano.2018.09.015
190. Fadel M, Kassab K, Abd El Fadeel DA, Nasr M, El Ghoubary NM. Comparative enhancement of curcumin cytotoxic photodynamic activity by nanoliposomes and gold nanoparticles with pharmacological appraisal in HepG2 cancer cells and Erlich solid tumor model. Drug Dev Ind Pharm. 2018;44(11):1809–1816. doi:10.1080/03639045.2018.1496451
191. Zhou Q, You C, Ling Y, Wu H, Sun B. pH and thermo dual stimulus-responsive liposome nanoparticles for targeted delivery of platinum-acridine hybrid agent. Life Sci. 2018. doi:10.1016/j.lfs.2018.11.052
192. Hu Y, Gaillard PJ, Rip J, de Lange ECM, Hammarlund-Udenaes M. In vivo quantitative understanding of PEGylated liposome’s influence on brain delivery of diphenhydramine. Mol Pharm. 2018;15(12):5493–5500. doi:10.1021/acs.molpharmaceut.8b00611
193. Zhang L, Liu Z, Kong C, et al. Improving drug delivery of micellar paclitaxel against non-small cell lung cancer by coloading itraconazole as a micelle stabilizer and a tumor vascular manipulator. Small. 2018;14(51):e1802112. doi:10.1002/smll.201802112
194. Razuvaeva E, Kulebyakina AI, Streltsov DR, et al. Effect of composition and molecular structure of Poly(L-lactic acid)/Poly(ethylene oxide) block copolymers on micellar morphology in aqueous solution. Langmuir. 2018. doi:10.1021/acs.langmuir.8b03379
195. Omolo CA, Kalhapure RS, Agrawal N, et al. A hybrid of mPEG-b-PCL and G1-PEA dendrimer for enhancing delivery of antibiotics. J Control Release. 2018;290:112–128. doi:10.1016/j.jconrel.2018.10.005
196. Long Z, Wu Y-P, Gao H-Y, et al. Functionalization of halloysite nanotubes via grafting of dendrimer for efficient intracellular delivery of siRNA. Bioconjug Chem. 2018;29(8):2606–2618. doi:10.1021/acs.bioconjchem.8b00321
197. Burkova EE, Dmitrenok PS, Bulgakov DV, et al. Exosomes from human placenta purified by affinity chromatography on sepharose bearing immobilized antibodies against CD81 tetraspanin contain many peptides and small proteins. IUBMB Life. 2018;70:1144–1155. doi:10.1002/iub.1928
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
