Back to Journals » Clinical and Experimental Gastroenterology » Volume 11
Exercise in patients with inflammatory bowel diseases: current perspectives
Authors Engels M, Cross RK, Long MD
Received 9 June 2017
Accepted for publication 27 October 2017
Published 22 December 2017 Volume 2018:11 Pages 1—11
DOI https://doi.org/10.2147/CEG.S120816
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Koulaouzidis
Michael Engels,1 Raymond K Cross,1 Millie D Long2
1Department of Medicine, Division of Gastroenterology and Hepatology, University of Maryland School of Medicine, Baltimore, MD, USA, 2Department of Medicine, Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, NC, USA
Abstract: Inflammatory bowel diseases (IBDs), including both Crohn’s disease (CD) and ulcerative colitis (UC), are chronic autoimmune diseases. Both CD and UC have relapsing and remitting courses. Although effective medical treatments exist for these chronic conditions, some patients do not respond to these traditional therapies. Patients are often left frustrated with incomplete resolution of symptoms and seek alternative or complementary forms of therapy. Patients often search for modifiable factors that could improve their symptoms or help them to maintain periods of remission. In this review, we examine both the published evidence on the benefits of exercise clinically and the pathophysiological changes associated with exercise. We then describe data on exercise patterns in patients with IBDs, potential barriers to exercise in IBDs, and the role of exercise in the development and course of IBDs. While some data support physical activity as having a protective role in the development of IBDs, the findings have not been robust. Importantly, studies of exercise in patients with mild-to-moderate IBD activity show no danger of disease or symptom exacerbation. Exercise has theoretical benefits on the immune response, and the limited available data suggest that exercise may improve disease activity, quality of life, bone mineral density, and fatigue levels in patients with IBDs. Overall, exercise is safe and probably beneficial in patients with IBDs. Evidence supporting specific exercise recommendations, including aspects such as duration and heart rate targets, is needed in order to better counsel patients with IBDs.
Keywords: inflammatory bowel diseases, Crohn’s disease, ulcerative colitis, exercise, physical activity
Introduction
Inflammatory bowel diseases (IBDs), including both Crohn’s disease (CD) and ulcerative colitis (UC), are heterogeneous, chronic inflammatory conditions characterized by a relapsing and remitting disease course. Approximately 1,600,000 individuals in the US have IBDs.1 IBD-associated direct health care costs are over $6 billion annually in the US alone.2
Symptoms of IBDs are associated with substantial patient morbidity, missed days from work and school, and diminished quality of life.3–9 Symptoms include but are not limited to diarrhea, rectal bleeding, abdominal pain, fatigue, and extraintestinal manifestations of disease. Effective medical therapy exists to treat symptoms and to prevent relapses; however, most patients require continuous treatment. This can be problematic as IBD is typically diagnosed in the second to fourth generation of life. As a result, adherence to therapy can be as low as 40%.10 In addition, uncommon serious side effects of therapies exist, especially with use of immunosuppressants and biologic agents.11 Patients and caregivers can have significant fears about side effects of therapies, which likely contributes to nonadherence as well as anxiety. Therefore, it is not surprising that many patients look to alternative treatments to their disease.
Prior studies have demonstrated that 44%–56% of patients with CD seek alternative or complimentary treatments as primary or adjunctive treatment for their CD.12,13 Exercise is one candidate complementary intervention that may prevent relapses of disease. Further, lack of exercise may be an important risk factor for the development of IBDs.14 Questions by patients about the role of exercise in the development and course of IBDs are therefore common, as patients are often interested in modifiable factors to improve outcomes.
We first will summarize the data on the benefits of exercise in 1) the general population and 2) in those with chronic gastrointestinal (GI) conditions. We will then review currently available exercise guidelines in the general population and for patients with IBDs. We will describe the biological rationale for exercise as a modifiable factor that could affect the disease course of IBDs. Finally, we will summarize the currently available data on patterns of exercise and potential barriers to exercise in IBD patients, and the role of exercise in both the development and course of IBDs.
Health benefits of exercise
In healthy populations, exercise has been associated with favorable physical, psychological/social, and cognitive health indicators.15 Exercise is well known to decrease all-cause mortality independent of body mass index (BMI), likely through a reduction in cardiovascular disease and cancer risk.16,17 Exercise has demonstrated beneficial effects on bone mineral density (BMD), muscle mass, and functional capacity.18–22 This is also of particular interest to the field of IBDs, as IBD patients are known to suffer from issues with low BMD and low muscle mass due to side effects of medications and the disease process itself.23–26
Exercise has well-established benefits in GI diseases. Mild-to-moderate-intensity exercise has been linked to a decreased risk of constipation, diverticular disease, and cholelithiasis.16,27 Moderate exercise is associated with a reduced risk of colorectal cancer with an average risk reduction of 20%–25%.28–32 This may be an important consideration in IBD patients with a history of longstanding colonic inflammation, who are at higher risk for development of colorectal dysplasia. Interestingly, the strength of the evidence for this risk reduction in the general population is stronger for colon cancer than for any other type of cancer.32 Furthermore, exercise has improved quality of life and functional capacity in colorectal cancer patients.33–35 Finally, prospective studies have also suggested that moderate-intensity physical exercise has a beneficial effect on both overall survival and progression-free survival in colorectal cancer patients.36,37
Guidelines on exercise
The latest joint guidelines from the American Heart Association and the American Academy of Sports Medicine recommend either 1) moderate-intensity aerobic exercise for a minimum of 30 minutes 5 days each week or 2) vigorous-intensity physical activity for 20 minutes 3 days a week. These recommendations are for all persons between the ages of 18 and 65 years in order to promote and maintain health.38 However, there has only been one published set of guidelines for exercise in patients with IBDs. These guidelines were based on exercise data from healthy individuals, not from data specific to IBD patients. This lack of validation in an IBD subpopulation calls into question the applicability of these guidelines.39
Biologic rationale for benefits of exercise in IBDs
Studies in non-IBD populations have demonstrated that low- to moderate-intensity exercise improves immune function.40 It has been hypothesized that moderate-intensity exercise exerts an anti-inflammatory effect by both decreasing visceral fat and subsequent release of pro-inflammatory cytokines and releasing myokines such as interleukin 6 (IL-6) with each exercise session. IL-6 released during exercise has been shown to increase the release of glucagon-like peptides, which are trophic factors associated with repair of damaged intestinal mucosa.41 Studies in animal models of colitis have demonstrated that exercise decreases expression of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and IL-1β, increases expression of IL-6 and the anti-inflammatory cytokine IL-10, attenuates stress-induced barrier dysfunction, and ameliorates colitis.42 Through these mechanisms, exercise may have benefits in both the development and course of IBDs (Figure 1).
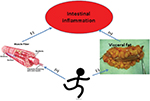  | Figure 1 Biologic rationale for benefits of exercise in inflammatory bowel diseases. |
Exercise patterns in patients with IBDs
There have been several studies that attempted to quantify exercise levels in the IBD patient population. Data are mixed, with variations by country in regard to overall reported rates and types of exercise. There are three studies that compared rates of exercise in IBD patients to the general population, and these studies showed similar or lower rates of exercise in adult and pediatric IBD populations as compared to general populations. A case–control study by Sorensen et al of 106 Danish CD patients and 75 age- and sex-matched controls showed that rates of high, moderate, and low exercise were similar in both populations, with 25%–39% participating in high levels of physical activity and 64%–69% of both groups participating in moderate levels of physical activity.43 Another study of BMD in a group of 22 CD and 33 UC pediatric patients found that mean physical activity was 8.3 hours per week for boys and 5.9 hours per week for girls, similar to the control group (9.2 and 7.1 hours per week, respectively).44 A recent study of objectively measured exercise via accelerometer activity trackers worn for 7 days in 48 CD patients (42% with active disease) and 30 healthy controls showed that CD patients were slightly more sedentary (97.7% vs 96.2%) and completed significantly fewer bouts of moderate–vigorous-intensity exercise (1.0 vs 5.0, p<0.01) than controls over 7 days. Long disease duration, low BMI, low vitamin D3, and inflammation as evidenced by an elevated serum C-reactive protein (CRP) (odds ratio [OR]: 22.6, confidence interval [CI]: 1.1–479.3, p=0.045) were associated with low physical activity in CD.45
Further studies have also demonstrated relatively low self-reported rates of exercise in patients with IBDs. A cross-sectional study of 552 IBD patients it Italy found that only 25.6% of patients regularly exercised.46 A separate Italian study of 475 IBD patients found an exercise rate of 20.6%.47 A study of Canadians with CD (n=474) and UC (n=637) found that patients with CD were more likely to classify their physical activity level as “inactive” (OR: 1.34; 95% CI: 1.12–1.61) and less likely to classify themselves as “active” (OR: 0.69; CI: 0.55–0.87) than the 113,685 respondents to the survey who did not have IBDs. Similar trends were seen in patients with UC. The most common modes of physical activity reported were walking, gardening, and home-based exercise. A total of 53%–58% of IBD patients classified themselves as inactive while 24%–25% described moderate activity levels and 18%–20% reported high activity levels.48 A recent survey of 877 patients with IBDs by Tew et al showed that 17% of patients were highly physically active, 50% were minimally active, and 33% were inactive.49
By comparison, separate studies have shown higher rates of exercise in IBD populations. A survey of 918 voluntary respondents in the Crohn’s and Colitis Foundation of the UK found that 66% of patients participated in exercise, with 32% reporting exercising daily, 57% weekly, 5% monthly, and 5% less than monthly.50 Another survey of study of 140 CD and 87 UC patients in a US academic cohort found that 16.4% never exercised, 32.8% exercised 1–2 times per week, 23.6% exercised 3–4 times per week, and 18.0% exercised >4 times per week. Of the patients who exercised at least weekly, 51% reported moderate exercise intensity, 33% reported light intensity, and 16% reported vigorous intensity.51
The rates of exercise in IBD patients thus vary widely between cohorts. Importantly, there is considerable heterogeneity in how various studies quantified exercise (via self-report or direct measurement). Additionally, in many of these survey-based studies, there is the potential for selection bias, therefore limiting external generalizability. Disease activity is another factor that may explain the wide range of reported exercise patterns of these cohorts, though only a few of the above-mentioned studies quantified this. The study by Defilippis et al found that rates of exercise were higher than had been previously reported in the general population without IBDs. However, these patients mostly had quiescent disease as evidenced by a low mean Harvey Bradshaw Index score of 4 and low mean CRP of 0.5.51 It is possible that exercise rates also fluctuate with a patient’s disease activity. The authors of the accelerometer study saw no significant association between disease activity measures and physical activity. However, the overall sample size was small and elevated CRP was associated with lower physical activity on multivariate analysis.45 In the cross-sectional study by Tew et al, a higher CD activity was independently associated with lower physical activity (p=0.038) on multivariate analysis, although this was not the case for patients with UC.49 Overall, rates of exercise in IBD patients are similar to somewhat less than those reported in the general population. Rates of exercise in IBDs do appear to be somewhat linked to disease activity.
Barriers to exercise in patients with IBDs
Although there are theoretical benefits of exercise in IBDs, there are also barriers to exercise that are specific to IBDs. Given ongoing GI symptoms, patients may be hesitant to seek exercise options outside the home or in areas without ready bathroom access. Symptom exacerbation is another main concern of patients and providers. In healthy patients, strenuous, high-intensity exercise reduces blood flow to the gastrointestinal system, which can be exacerbated by hypovolemia due to dehydration during prolonged high-intensity exercise.16,52 These mechanisms may be responsible for the rare cases of ischemic colitis and the relatively high rates of occult blood positivity in high-intensity endurance athletes such as marathoners and triathletes.16,52
In a survey of 900 British CD and UC patients, 23% of respondents reported that exercise exacerbated their IBD symptoms with 41% of those patients reporting increased fatigue, 12% reporting increased need for the toilet, and 17% reporting increased abdominal pain. Additional complaints included increased joint pain, other IBD symptoms, and increased recovery time.50 However, it is important to note that the majority of patients (72%) felt that exercise improved their symptoms.
In another single-center, cross-sectional study of the prevalence of exercise and limitations in patients with IBDs, 44% of patients reported that their IBDs limited their exercise for reasons including fatigue, joint pain, embarrassment, and weakness.51 Tew et al showed even higher rates of reported symptoms, with 79% of respondents reporting that their IBDs limited their participation in physical activity/exercise. The most common reasons were abdominal or joint pain, fatigue, disease flare-up, and increased fecal urgency.49
There are objective data supporting the perception that IBDs may limit an individual’s ability to exercise. Objective markers of exercise capacity are reduced in the IBD patient population when compared to healthy controls. Anaerobic threshold, muscle strength, and heart rate recovery are lower in IBD populations, although the mechanism is not clear.53–58 One study of 29 CD patients who had small bowel surgery and proctocolectomy found that all patients had reduced exercise capacity, but the degree of reduction was greater in those with greater lengths of small bowel resected.55 Interestingly, 72 UC patients who underwent ‘curative’ proctocolectomy had normal exercise capacity 12 months after ileal pouch-anal anastomosis.59 Other factors, such as disease-related anemia, may also play a role in exercise capacity reductions.
Experimental animal models of colitis show inconsistent results for the safety of exercise. One study by Saxena et al showed that exercise exacerbated inflammation in a dextran sulfate sodium (DSS) model of colitis. However, exercise improved inflammatory symptoms in an adiponectin knockout mice colitis model.42 Another study utilizing the mouse DSS colitis model showed increased diarrhea and inflammatory markers with moderate-intensity forced treadmill running. However, voluntary lower-intensity exercise was found to be protective against inflammation in the same study.60 A separate study using a DSS model of colitis in rats specifically found swimming to be beneficial. Swimming improved DSS-induced decrease in crypt depth, increases in myeloperoxidase activity, infiltration of Ly6G+ neutrophils and TNF-α- and IFN-γ-expressing CD3+ T cells, as well as fecal calprotectin and lactoferrin levels. Proposed mechanisms included modulating inflammation, oxidative stress, and apoptosis.61
Data in patients with IBDs are more reassuring, without detrimental effects of exercise. Prospective studies have demonstrated little danger of symptom exacerbation. Ploeger et al studied the effects of 30 minutes of cycling at 50% of peak capacity and high-intensity intermittent exercise in 15 pediatric CD patients and 15 matched healthy controls. They found similar increases in immune cells, growth factors, and inflammatory cytokines in both healthy controls and CD patients. The only difference in the healthy controls as compared to the IBD patients was a transiently larger rise in monocytes in the CD patients (p<0.001). However, the most significant finding from this study is that all studied inflammatory markers returned to baseline within a maximum of 60 minutes after cessation of exercise. The transient nature of these elevations strongly suggests that there is little danger of symptom exacerbation with exercise in patients with IBDs.62
No long-term complications have been associated with exercise in IBDs. A single exercise session at a maximum of 60% oxygen consumption did not increase gastrointestinal symptoms, change intestinal permeability, or negatively affect transit time in 6 CD patients in clinical remission.63 A low-intensity walking program caused no exacerbation of symptoms in 10 patients with inactive or mildly active CD, results that have been duplicated in a separate study of 32 quiescent or mildly active CD patients.64,65 A study of low-impact exercise in 117 CD patients demonstrated no serious adverse effects from the exercise regimen and no worsening of disease activity scores in the exercise group.66 Klare et al had 30 CD patients with mild-to-moderately active disease participate in a moderate-intensity 10-week exercise program, which did not cause any exacerbations of disease or symptoms, while this program substantially increased quality-of-life scores.67
Limited recent data suggest that even high-intensity endurance sports may be safe in some IBD patients. A recent small, prospective study 10 IBD patients participating in high intensity aerobic exercise such as triathlons, marathons, and long distance bike races had no change in their fecal calprotectin levels.68 Eight of the ten total patients had no change in their symptoms or disease activity scores. The two patients with CD who had elevations in their disease activity scores initially with exercise had these scores return to baseline within 1 week.
Overall, these data demonstrate that exercise capacity may be reduced in IBD patients.53–59 Despite theoretical concerns and conflicting data from animal models, moderate- to low-intensity exercise appears to be safe with minimal risk of symptom exacerbation when prospectively studied in patients with quiescent to moderately active disease. Very limited data suggest that even high-intensity exercise may be safe in selected IBD patients.
Associations between exercise and IBD onset
A host of environmental factors may contribute to the onset of IBDs. There have been several studies examining the role of physical activity and an active lifestyle as potential protective factors in the development of IBDs. Persson et al compared weekly and daily exercise levels in 152 CD patients and 145 UC patients compared to 305 randomly selected controls selected matched for age and sex. A statistically significant inverse relationship between weekly and daily exercise and the risk of CD was noted (OR: 0.6; 95% Cl: 0.4–0.9 and OR: 0.5; 95% Cl: 0.3–0.9, respectively). However, there was no association between exercise and onset of UC.69 Similarly, Ng et al found a protective effect of exercise and onset of CD (OR: 0.58; 95% CI: 0.4–1.0), but none for UC, when comparing 186 CD patients, 256 UC patients, and 940 controls matched for age, sex, and geographical location.70 A case–control study in a Slovakian population found a statistically significant association between lower weekly rates of sport participation as an adolescent and the development of IBDs (OR: 2.7, 95% CI: 1.5–5.0).71 An Israeli study of 55 UC and 33 CD patients found that there was an association (p<0.001) between lower levels of physical activity in the pre-illness period for IBD patients when matched with clinic-based controls.72
The first cohort study to examine the role of exercise in IBD development was performed by Sonnenberg in 1990. He performed a retrospective review of 12,014 patients in a German Social Security database and found that pre-illness occupations that were outdoors and involved physical activity were protective against IBD development when compared to more sedentary and indoor occupations.73 Bøgglid et al then studied over 2.3 million Danish citizens.74 The authors found that only one of the eight specific sedentary occupations identified by Sonnenberg (female office workers) had a statistically increased risk of being hospitalized for IBDs with a standardized hospitalization ratio (SHR) of 111 (CI: 102–121). When they aggregated sedentary occupations and compared them to aggregated active occupations they found that there was a statistically significant increased risk of being hospitalized with new-onset IBDs for those with sedentary occupations (SHR 125, Cl: 116.9–133.1).
Cucino and Sonnenberg examined the association between occupation and mortality in 2,419 UC and 2,399 CD patients in a social security database in the US. They found higher mortality rates in patients with sedentary occupations than in those with physically active occupations. However, data were only available for occupations at or around the time of death; thus, there was no way to control for the fact that these occupations may have been chosen in response to pre-mortality increased IBD disease activity.75
Recently, additional studies have investigated the strength of the association between fitness and IBD onset. Chan et al found that there was no association between onset of IBDs and physical activity levels in the large, prospective European Prospective Investigation into Cancer and Nutrition cohort (n=300,724), where patients who developed IBDs were matched with healthy controls.76 The Nurses’ Health Study I and II likewise showed no statistically significant relationship between exercise levels and development of UC in a female population, although there was a statistically significant (p=0.02) inverse association between the risk of developing CD and physical activity. Specifically, they showed a hazard ratio (HR) of 0.56 (95% CI: 0.37–0.84) for patients with at least 27 mets of physical activity weekly when compared to controls.77 Another recent cohort study examined 240,984 Swedish male military conscripts, showing that low physical fitness in adolescence was associated with an increased risk of developing both CD and UC (HR: 1.62; CI: 1.31–2.00 and HR: 1.36; CI: 1.17–1.59, respectively).78 However, there was significant attenuation of this association when the analysis was adjusted for markers of prodromal disease activity such as BMI, height, and sedimentation rate (adjusted HR: 1.32; 95% CI: 1.05–1.66 for CD and adjusted HR: 1.25; CI: 1.06–1.48 for UC). The authors speculated that this attenuation indicated that early subclinical disease activity could have affected exercise capacity and physical fitness. Thus, some or all of the association between IBDs and disease onset could be explained by a voluntary reduction in physical activity secondary to symptoms associated with IBDs before the diagnosis is recognized.
A meta-analysis of the above literature evaluated six studies.79 The pooled results demonstrated that higher levels of physical activity were associated with a reduced risk (RR of 0.63; 95% CI: 0.50–0.79) of CD compared to those with a low physical activity. There was no association between exercise and the development of UC.69,70,76,77
Inherent biases exist in studies that attempt to demonstrate an association between an exposure (exercise) and onset of disease (IBDs). However, there appears to be a significant association between low physical activity and onset of CD but not UC. A summary of the studies to date investigating the association between exercise and IBD development is provided in Table 1. One explanation for these findings could be that exercise results in an anti-inflammatory environment decreasing the onset of CD. Alternately, this association may be related to residual confounding. Patients that exercise regularly are more likely to have other healthy habits that may decrease the onset of CD. Such factors could include a healthier diet (less-processed carbohydrates, more fruits and vegetables, and less red meat), decreased rates of smoking, improved sleep, and decreased stress. Given that there can be a disconnect between symptoms and intestinal inflammation in CD and that the diagnosis can often be delayed, it is plausible that symptoms from yet-to-be-diagnosed CD could result in a decrease in physical activity before the diagnosis of CD is made. It is also plausible that these symptoms result in patients choosing occupations that are less physically demanding to better accommodate their symptoms. One study attempted to control for some of these factors by using discordant twin pairs, and overall found no association with exercise and IBD onset.80 Given the significant health benefits of regular exercise in general, it is possible that physical activity may have some benefit in prevention of development of CD.
Associations between exercise and IBD course
To date there are only a few prospective or retrospective studies examining the question of whether IBD course is affected by exercise (Table 2). The available studies have also focused on varying outcome measures.
Patients with IBDs have higher rates of osteoporosis than the general population, likely due to a combination of inflammation, malabsorption, and medications such as corticosteroids.81,82 One of the first prospective interventional studies on exercise in IBDs was performed by Robinson et al. This was a randomized control trial of 117 patients with CD in clinical remission. A 12-month low-intensity exercise program had beneficial effects on BMD in this population. The intervention increased BMD at both the greater trochanter (4.67; CI: 0.86–8.48) and femoral neck (0.28; CI: 0.10–0.45), with a nonsignificant trend of increased BMD in all areas measured.66
A separate study looked at the effects of moderate-intensity exercise on various physiologic parameters in IBD patients undergoing a 1-hour exercise session at a max of 60% oxygen consumption. There was no significant difference in lipoperoxidation or orocecal transit time in the six patients with quiescent CD when compared to healthy controls, and there was no exacerbation of symptoms immediately after the session or at 6 months of follow-up.63
Exercise has also shown to be an effective intervention when part of a multifaceted wellness program. Ellensbruch and coauthors performed a case–control study of a 60-hour mind body therapy program that included stress management and self-care training, moderate exercise, and a Mediterranean diet on 30 UC patients in remission or with minimally active disease. There was a significant increase in health-related quality-of-life scores (p<0.05) as well as in the IBD questionnaire (IBDQ) disease-related quality-of-life scores (p<0.01) in the intervention as compared to the control group.83
Exercise seems to improve quality of life. Loudon et al performed a study of a 12-week moderate-intensity walking program in 12 patients with inactive or only mildly active CD. They found that disease activity scores, quality-of-life scores, aerobic fitness levels, and VO2 max all had statistically significant improvements over the study period with an average of 2.9 sessions of 32 minutes a week.65
Ng et al also assessed the effect of an exercise intervention on IBD patients’ quality of life. Thirty-two patients with quiescent or minimally active CD activity and low physical activity levels were randomized to standard care or a 3 times weekly walking program at 40% of aerobic capacity (VO2 max) for 3 months. There was a statistically significant improvement in quality of life as measured by the IBDQ. Other improvements included physical stress and symptom activity (p<0.05).64 A separate study randomized 30 patients with mild-to-moderate CD to usual care or moderate-intensity running 3 times weekly for 10 weeks. The intervention group saw a nonsignificant trend toward improvement in the IBDQ score 13.8 (CI: 1.8–29.4) when compared to controls. They also saw a significant increase in the social subscores of the IBDQ (p=0.026).
A survey of 918 IBD patients found that patients attributed significant symptom improvement to exercise. A total of 72% of respondents reported that exercise made them feel better. Additionally, participants credited exercise for contributing to improved well-being, confidence, boosted energy levels (12%), feeling fitter or healthier (9%), and weight control and improved sleep (12%). A total of 23% of respondents said that exercise made them feel worse.50 Another study of cognitive fatigue scores in 181 CD patients, 113 UC patients, and 85 controls found that there was a nonsignificant trend toward improvement in physical and cognitive fatigue scores in patients who instituted a regular exercise program (OR: 15.5, 95% CI: 1.20–200.70).84
In a recent large prospective study of 1,308 CD and 518 UC patients in clinical remission utilizing the Crohn’s and Colitis Foundation of America Partners internet-based cohort, authors investigated independent effects of exercise on the risk of relapse 6 months later. Risk of active CD at follow-up was reduced in those with higher levels of exercise in multivariate analysis for both CD and UC (adjusted RR 0.72, 95% CI: 0.55–0.94 and adjusted RR: 0.78, 95% CI: 0.54–1.13, respectively).85
Conclusion
In summary, exercise may play an important role as a modifiable factor in the development and course of IBDs. Current self-reported and measured exercise rates vary widely in different IBD patient cohorts. Data suggest that rates of exercise are inversely related to current disease activity.86 Exercise may play a preventive role, along with other factors, in the development of IBDs. While there are some concerns as to barriers to exercise in IBD patients, moderate-intensity exercise has demonstrated safety, and potential benefits, for patients with nonsevere disease. The safety of higher-intensity exercise in patients with severely active disease is currently not clear. Retrospective and prospective studies indicate that exercise may have beneficial effects on both the disease course and the quality of life of patients with IBDs. Exercise may provide additional benefits such as improvements sleep, mood, and BMD in IBD patients. Further studies are needed to guide specific recommendations on the intensity, frequency, and type of exercise that may be most beneficial to patients with IBDs.
Disclosure
Dr Long has consulted for AbbVie, UCB, Takeda, Pfizer, Target Pharmasolutions. She has no relevant conflicts of interest for this work. The other authors report no conflicts of interest in this work.
References
Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV Jr. Incidence and prevalence of crohn’s disease and ulcerative colitis in olmsted county, Minnesota From 1970 Through 2010. Clin Gastroenterol Hepatol. 2017;15(6):857–863. | ||
Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727. | ||
Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135(6):1907–1913. | ||
Van Limbergen J, Russell R, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135(4):1114–1122. | ||
Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology. 2008;135(4):1106–1113. | ||
Longobardi T, Jacobs P, Bernstein CN. Work losses related to inflammatory bowel disease in the United States: results from the National Health Interview Survey. Am J Gastroenterol. 2003;98(5):1064–1072. | ||
Ferguson A, Sedgwick DM, Drummond J. Morbidity of juvenile onset inflammatory bowel disease: effects on education and employment in early adult life. Gut. 1994;35(5):665–668. | ||
Cohen R. The quality of life in patients with Crohn’s disease. Aliment Pharmacol Ther. 2002;16(9):1603–1609. | ||
Casellas F, Arenas JI, Baudet JS, et al. Impairment of health-related quality of life in patients with inflammatory bowel disease: a Spanish multicenter study. Inflamm Bowel Dis. 2005;11(5):488–496. | ||
Kane SV, Cohen RD, Aikens JE, Hanauer SB. Prevalence of nonadherence with maintenance mesalamine in quiescent ulcerative colitis. Am J Gastroenterol. 2001;96(10):2929–2933. | ||
Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol. 2012;107:1409–1422. | ||
Weizman AV, Ahn E, Thanabalan R, et al. Characterisation of complementary and alternative medicine use and its impact on medication adherence in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35(3):342–349. | ||
Cheifetz AS, Gianotti R, Luber R, Gibson PR. Complementary and alternative medicines used by patients with inflammatory bowel diseases. Gastroenterology. 2017;152:415–429. | ||
Basil M, Guo L, Groshek J, Reich J, Farraye FA. Inflammatory bowel disease: poor knowledge and high stigma of IBD in the general population: results of a National Survey. Am J Gastroenterol. 2016;111(S1):S260–S336. | ||
Poitras VJ, Gray C, Borghese MM, et al. Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Appl Physiol Nutr Metab. 2016;41(6 Suppl 3):197–239. | ||
DeOliveira EP, Burini RC. The impact of physical exercise on the gastrointestinal tract. Curr Opin Clin Nutr Metab Care. 2009;12(5):533–538. | ||
Loprinzi PD. Dose–response association of moderate-to-vigorous physical activity with cardiovascular biomarkers and all-cause mortality: considerations by individual sports, exercise and recreational physical activities. Prev Med. 2015;81:73–77. | ||
Kelley GA, Kelley KS, Tran ZV. Exercise and bone mineral density in men: a meta-analysis. J Appl Physiol. 2000;88(5):1730–1736. | ||
Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre-and postmenopausal women. Calcif Tissue Int. 2000;67(1):10–18. | ||
Nair KS. Aging Muscle. Am J Clin Nutr 2005. 2005;81(5):953–963. | ||
Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18(2):189–193. | ||
Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007;40(1):14–27. | ||
Bernstein CN, Seeger LL, Sayre JW, Anton PA, Artinian L, Shanahan F. Decreased bone density in inflammatory bowel disease is related to corticosteroid use and not disease diagnosis. J Bone Miner Res. 1995;10(2):250–256. | ||
Schoon EJ, Blok BM, Geerling BJ, Russel MG, Stockbrügger RW, Brummer RJM. Bone mineral density in patients with recently diagnosed inflammatory bowel disease. Gastroenterology. 2000;119(5):1203–1208. | ||
Rocha R, Santana GO, Almeida N, Lyra AC. Analysis of fat and muscle mass in patients with inflammatory bowel disease during remission and active phase. Br J Nutr. 2009;101(05):676–679. | ||
Bechtold S, Alberer M, Arenz T, et al. Reduced muscle mass and bone size in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16(2):216–225. | ||
Peters H, De Vries W, Vanberge-Henegouwen G, Akkermans L. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48(3):435–439. | ||
Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States). Cancer Causes Control. 1996;7(2):253–263. | ||
Boutron-Ruault M, Senesse P, Méance S, Belghiti C, Faivre J. Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence. Nutr Cancer. 2001;39(1):50–57. | ||
Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev. 2002;11(Suppl 2):S94–S100. | ||
Martínez ME. Primary prevention of colorectal cancer: lifestyle, nutrition, exercise. In: Senn H-J, Morant R, editors. Tumor Prevention and Genetics III. Berlin, Heidelberg: Springer Berlin Heidelberg; 2005:177–211. | ||
Kruk J, Czerniak U. Physical activity and its relation to cancer risk: updating the evidence. Asian Pac J Cancer Prev. 2013;14(7):3993–4003. | ||
Stevinson C, Lawlor DA, Fox KR. Exercise interventions for cancer patients: systematic review of controlled trials. Cancer causes Control. 2004;15(10):1035–1056. | ||
Galvão DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899–909. | ||
Peddle CJ, Au HJ, Courneya KS. Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Dis Colon Rectum. 2008;51:1242–1248. | ||
Meyerhardt JA, Giovannucci E, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. | ||
Jeon J, Sato K, Niedzwiecki D, et al. Impact of physical activity after cancer diagnosis on survival in patients with recurrent colon cancer: findings from CALGB 89803/Alliance. Clin Colorectal Cancer. 2013;12(4):233–238. | ||
Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. | ||
Ball E. Exercise guidelines for patients with inflammatory bowel disease. Gastroenterol Nurs. 1998;21(3):108–111. | ||
Brolinson PG, Elliott D. Exercise and the immune system. Clin Sports Med. 2007;26(3):311–319. | ||
Ellingsgaard H, Hauselmann I, Schuler B, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481–1489. | ||
Saxena A, Fletcher E, Larsen B, Baliga MS, Durstine JL, Fayad R. Effect of exercise on chemically-induced colitis in adiponectin deficient mice. J Inflamm (Lond). 2012;9(1):30. | ||
Sørensen VZ, Olsen BG, Binder V. Life prospects and quality of life in patients with Crohn’s disease. Gut. 1987;28(4):382–385. | ||
Boot A, Bouquet J, Krenning E, de Muinck Keizer-Schrama S. Bone mineral density and nutritional status in children with chronic inflammatory bowel disease. Gut. 1998;42(2):188–194. | ||
van Langenberg DR, Papandony MC, Gibson PR. Sleep and physical activity measured by accelerometry in Crohn’s disease. Aliment Pharmacol Ther. 2015;41(10):991–1004. | ||
D’Inca R, Garribba AT, Vettorato MG, et al. Use of alternative and complementary therapies by inflammatory bowel disease patients in an Italian tertiary referral centre. Dig Liver Dis. 2007;39(6):524–529. | ||
Bertomoro P, Renna S, Cottone M, et al. Regional variations in the use of complementary and alternative medicines (CAM) for inflammatory bowel disease patients in Italy: An IG-IBD study. J Crohn’s Colitis. 2010;4(3):291–300. | ||
Mack DE, Wilson PM, Gilmore JC, Gunnell KE. Leisure-time physical activity in Canadians living with Crohn disease and ulcerative colitis: population-based estimates. Gastroenterol Nurs. 2011;34(4):288–294. | ||
Tew GA, Jones K, Mikocka-Walus A. Physical activity habits, limitations, and predictors in people with inflammatory bowel disease: a large cross-sectional online survey. Inflamm Bowel Dis. 2016;22(12):2933–2942. | ||
Chan D, Robbins H, Rogers S, Clark S, Poullis A. Inflammatory bowel disease and exercise: results of a Crohn’s and Colitis UK survey. Frontline Gastroenterol. 2014;5(1):44–48. | ||
DeFilippis EM, Tabani S, Warren RU, Christos PJ, Bosworth BP, Scherl EJ. Exercise and self-reported limitations in patients with inflammatory bowel disease. Dig Dis Sci. 2016;61(1):215–220. | ||
Moses FM. Exercise-associated intestinal ischemia. Curr Sports Med Rep. 2005;4(2):91–95. | ||
Spooren C, Pierik MJ, Van den Heuvel T, et al. Su1320 prevalence of impaired muscle strength in an IBD outpatient cohort. Gastroenterology. 2015;148(4 Supplement 1):S473–S474. | ||
Sarli B, Dogan Y, Poyrazoglu O, et al. Heart rate recovery is impaired in patients with inflammatory bowel diseases. Med Princ Pract. 2016;25(4):363–367. | ||
Brevinge H, Berglund B, Bosaeus I, Tolli J, Nordgren S, Lundholm K. Exercise capacity in patients undergoing proctocolectomy and small bowel resection for Crohn’s disease. Br J Surg. 1995;82(8):1040–1045. | ||
Otto JM, O’Doherty AF, Hennis PJ, et al. Preoperative exercise capacity in adult inflammatory bowel disease sufferers, determined by cardiopulmonary exercise testing. Int J Colorectal Dis. 2012;27(11):1485–1491. | ||
Ploeger HE, Takken T, Wilk B, et al. Exercise capacity in pediatric patients with inflammatory bowel disease. J Pediatr. 2011;158(5):814–819. | ||
Wiroth JB, Filippi J, Schneider SM, et al. Muscle performance in patients with Crohn’s disease in clinical remission. Inflamm Bowel Dis. 2005;11(3):296–303. | ||
Ohrstrom M, Jansson O, Wohlfart B, Ekelund M. Working capacity and resting energy expenditure after ileal pouch-anal anastomosis. Br J Surg. 2004;91(5):618–624. | ||
Cook MD, Martin SA, Williams C, et al. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav Immun. 2013;33:46–56. | ||
Qin L, Yao ZQ, Chang Q, et al. Swimming attenuates inflammation, oxidative stress, and apoptosis in a rat model of dextran sulfate sodium-induced chronic colitis. Oncotarget. 2017;8(5):7391–7404. | ||
Ploeger H, Obeid J, Nguyen T, et al. Exercise and inflammation in pediatric Crohn’s disease. Int J Sports Med. 2012;33(8):671–679. | ||
D’Inca R, Varnier M, Mestriner C, Martines D, D’Odorico A, Sturniolo GC. Effect of moderate exercise on Crohn’s disease patients in remission. Ital J Gastroenterol Hepatol. 1999;31(3):205–210. | ||
Ng V, Millard W, Lebrun C, Howard J. Low-intensity exercise improves quality of life in patients with Crohn’s disease. Clin J Sport Med. 2007;17(5):384–388. | ||
Loudon CP, Corroll V, Butcher J, Rawsthorne P, Bernstein CN. The effects of physical exercise on patients with Crohn’s disease. Am J Gastroenterol. 1999;94(3):697–703. | ||
Robinson RJ, Krzywicki T, Almond L, et al. Effect of a low-impact exercise program on bone mineral density in Crohn’s disease: a randomized controlled trial. Gastroenterology. 1998;115(1):36–41. | ||
Klare P, Nigg J, Nold J, et al. The impact of a ten-week physical exercise program on health-related quality of life in patients with inflammatory bowel disease: a prospective randomized controlled trial. Digestion. 2015;91(3):239–247. | ||
Hassid B, Lamere B, Kattah M, Mahadevan U. Effect of intense exercise on inflammatory bowel disease activity. Am J Gastroenterol. 2016;111(S1):S260–S336. | ||
Persson PG, Leijonmarck CE, Bernell O, Hellers G, Ahlbom A. Risk indicators for inflammatory bowel disease. Int J Epidemiol. 1993;22(2):268–272. | ||
Ng SC, Tang W, Leong RW, et al. Environmental risk factors in inflammatory bowel disease: A population-based case-control study in Asia-Pacific. Gut. 2015;64(7):1063–1071. | ||
Hlavaty T, Toth J, Koller T, et al. Smoking, breastfeeding, physical inactivity, contact with animals, and size of the family influence the risk of inflammatory bowel disease: a Slovak case–control study. United European Gastroenterol J. 2013;1(2):109–119. | ||
Klein I, Reif S, Farbstein H, Halak A, Gilat T. Preillness non dietary factors and habits in inflammatory bowel disease. Ital J Gastroenterol Hepatol. 1998;30(3):247–251. | ||
Sonnenberg A. Occupational distribution of inflammatory bowel disease among German employees. Gut. 1990;31(9):1037–1040. | ||
Bøggild H, Tüchsen F, Orhede E. Occupation, employment status and chronic inflammatory bowel disease in Denmark. Int J Epidemiol. 1996;25(3):630–637. | ||
Cucino C, Sonnenberg A. Occupational mortality from inflammatory bowel disease in the United States 1991–1996. Am J Gastroenterol. 2001;96(4):1101–1105. | ||
Chan SS, Luben R, Olsen A, et al. Body mass index and the risk for Crohn’s disease and ulcerative colitis: data from a European Prospective Cohort Study (The IBD in EPIC Study). Am J Gastroenterol. 2013;108(4):575–582. | ||
Khalili H, Ananthakrishnan AN, Konijeti GG, et al. Physical activity and risk of inflammatory bowel disease: prospective study from the Nurses’ Health Study cohorts. BMJ. 2013;347:f6633. | ||
Melinder C, Hiyoshi A, Hussein O, Halfvarson J, Ekbom A, Montgomery S. Physical fitness in adolescence and subsequent inflammatory bowel disease risk. Clin Transl Gastroenterol. 2015;6(11):e121. | ||
Wang Q, Xu KQ, Qin XR, Wang XY. Association between physical activity and inflammatory bowel disease risk: a meta-analysis. Dig Liver Dis. 2016;48(12):1425–1431. | ||
Halfvarson J, Jess T, Magnuson A, et al. Environmental factors in inflammatory bowel disease: a co-twin control study of a Swedish-Danish twin population. Inflamm Bowel Dis. 2006;12(10):925–933. | ||
Lee N, Radford-Smith G, Taaffe DR. Bone loss in Crohn’s disease: exercise as a potential countermeasure. Inflamm Bowel Dis. 2005;11(12):1108–1118. | ||
Scott EM, Gaywood I, Scott BB. Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. British Society of Gastroenterology. Gut. 2000;46(Suppl 1):i1–i8. | ||
Elsenbruch S, Langhorst J, Popkirowa K, et al. Effects of mind-body therapy on quality of life and neuroendocrine and cellular immune functions in patients with ulcerative colitis. Psychother Psychosom. 2005;74(5):277–287. | ||
Van Langenberg D, Della Gatta P, Warmington S, Kidgell D, Gibson P, Russell A. Objectively measured muscle fatigue in Crohn’s disease: correlation with self-reported fatigue and associated factors for clinical application. J Crohn’s Colitis. 2014;8(2):137–146. | ||
Jones PD Kappelman MD, Martin CF, Chen W, Sandler RS, Long MD. Exercise decreases risk of future active disease in inflammatory bowel disease patients in remission. Inflamm Bowel Dis. 2015;21(5):1063–1071. | ||
Bernklev T, Jahnsen J, Lygren I, Henriksen M, Vatn M, Moum B. Health-related quality of life in patients with inflammatory bowel disease measured with the short form-36: psychometric assessments and a comparison with general population norms. Inflamm Bowel Dis. 2005;11(10):909–918. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


