Back to Journals » Clinical Ophthalmology » Volume 17
Evidence-Based Consensus Guidelines Series for MicroPulse Transscleral Laser Therapy - Surgical Technique, Post-Operative Care, Expected Outcomes and Retreatment/Enhancements
Authors Grippo TM , Töteberg-Harms M, Giovingo M, Francis BA, de Crom RMPC, Jerkins B, Brubaker JW, An J , Radcliffe N , Noecker R
Received 8 September 2022
Accepted for publication 21 November 2022
Published 6 January 2023 Volume 2023:17 Pages 71—83
DOI https://doi.org/10.2147/OPTH.S389198
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tomas M Grippo,1 Marc Töteberg-Harms,2 Michael Giovingo,3 Brian A Francis,4 Ronald RMPC de Crom,5 Brian Jerkins,6 Jacob W Brubaker,7 Jella An,8 Nathan Radcliffe,9 Robert Noecker10
1Grippo Glaucoma and Cataract Center, Buenos Aires, Argentina; 2Department of Ophthalmology, Medical College of Georgia, Augusta University, Augusta, GA, USA; 3Cook County Health, Chicago, IL, USA; 4Department of Ophthalmology, Doheny and Stein Eye Institutes, David Geffen School of Medicine, University of California Los Angeles (UCLA), Los Angeles, CA, USA; 5University Eye Clinic, Maastricht University Medical Centre, Maastricht, the Netherlands; 6Department of Ophthalmology, Hamilton Eye Institute, University of Tennessee Health Science Center, Memphis, TN, USA; 7Sacramento Eye Consultants, Sacramento, CA, USA; 8Department of Ophthalmology, Wilmer Eye Institute, John Hopkins School of Medicine, Bethesda, MD, USA; 9Glaucoma Department, New York Eye and Ear Infirmary, New York, NY, USA; 10Ophthalmic Consultants of Connecticut, Fairfield, CT, USA
Correspondence: Tomas M Grippo, Grippo Glaucoma and Cataract Center, 250 Luis Maria Campos, 1st Floor, Suite O, Capital Federal, 1426, Argentina, Tel +54-11-4-774-2930, Email [email protected]
Purpose: To provide expert consensus and evidence-based current guidelines on treatment technique, postoperative care, expected outcomes and retreatment for MicroPulse Transscleral Laser Treatment (TLT).
Methods: A comprehensive search of PubMed led to the identification and analysis of 61 studies on MicroPulse TLT. To provide guidance in areas where there was not enough available literature, a three-round Delphi method was conducted involving 10 international experts in MicroPulse TLT.
Results: The response rate was 70% in the first round, 70% in the second round, and 80% in the third round of the Delphi method. Once all responses were aggregated, a live meeting was held with 90% attendance, and consensus was achieved on each of the findings detailed in this manuscript.
Conclusion: Used within appropriate treatment parameters, with proper technique and patient selection, MicroPulse TLT is a safe and effective treatment for many types and severities of glaucoma. MicroPulse TLT represents a useful addition to the glaucoma armamentarium.
Keywords: glaucoma, MicroPulse, transscleral laser treatment
Introduction
MicroPulse Transscleral Laser Therapy (TLT) is a non-incisional laser treatment for glaucoma. The Cyclo G6 Laser (Iridex, Mountain View, CA, USA) and the MicroPulse P3 Delivery Device (Iridex, Mountain View, CA, USA) use an infrared diode laser at a wavelength of 810nm in which the continuous energy wave is divided into a series of pulses, minimizing tissue temperature elevation and coagulative damage. The laser is “ON” 31.3% of the time (the duty cycle) and “OFF” 68.7% of the time. The MicroPulse technology results in a lower amount of energy being applied over time and, combined with a sweeping technique, delivers a more homogeneous distribution of energy compared to traditional transscleral cyclophotocoagulation (CPC) technique. Lower temperature targets and greater thermal control with MicroPulse TLT contribute to lower the risk of complications compared to continuous wave transscleral cyclophotocoagulation.1 Furthermore, the recently modified delivery probe has an improved ergonomic design that allows for more stable positioning during the treatment. It also facilitates a more posterior application of laser energy, farther away from the anterior segment structures such as the lens and the iris root.2,3
While published studies on MicroPulse TLT have demonstrated that this procedure is a safe and effective treatment for glaucoma, recommendations on optimal treatment protocols have not existed previously.3 In an effort to refine treatment technique, patient selection, and outcomes of MicroPulse TLT, an international panel of ten glaucoma specialists was formed. The panel published its first paper, “Evidence-based consensus guidelines series for MicroPulse Transscleral Laser Therapy - Dosimetry & Patient Selection”, providing the rationale and recommendations for standard starting dose MicroPulse TLT settings using the revised MicroPulse P3 probe. This publication summarized the current conceptual understanding of MicroPulse TLT including mechanism of action, indications, cautions in patient selection, and dosimetry. The recommended starting settings for MicroPulse TLT are 2500 mW, 31.3% duty cycle, and 4 sweeps at a sweep velocity of 20 seconds per hemisphere for a total of 160 seconds per eye.3 This publication also provided guidance on dose escalation of treatment. This same international panel met again to establish consensus on treatment technique, post-procedural management, expected outcomes, and retreatments or enhancements.
Methods
Ten glaucoma specialists with three or more years of experience performing MicroPulse TLT were recruited from Argentina, the Netherlands, Switzerland, and the United States to form an expert panel. Additional considerations when forming the panel included having published on the topic, surgical volume, working in both private and academic settings, and representation of patients with a diversity of disease states and heterogeneous demographics. The panel co-chairs conducted a PubMed search and identified 61 studies, which were then analyzed and summarized by the entire panel for the selected topics. To form guidelines where no clear published evidence existed and to better define guidelines based on the available evidence, a three-round Delphi panel with the authors was held between December 2021 and February 2022. The first round involved polling the panel on all aspects of their individual treatment techniques, post-procedure management, expected outcomes, and retreatments/enhancements. The second round asked the panelists to consider specific treatment scenarios and refine their responses, as well as define treatment success and failure. The third round sought to clarify treatment techniques and rationales. Finally, a virtual meeting was held to reach consensus and elaborate on treatment guidelines. All responses remained anonymous until the final meeting, and the study sponsor was not privy to the questionnaires, responses, data collection, or preparation of the manuscript until it was ready for submission where a courtesy review was provided to the sponsor. Given the nature of this study, it did not require approval from an ethics committee.
Results
The first and second rounds of the Delphi Panel had response rates of 70%. The third round had a response rate of 80%, and the virtual meeting had 90% attendance with the two remaining panel members providing feedback separately. Agreement with the findings stated below was 100%.
When applicable, an evidence section was added below the finding to summarize the literature supporting the finding. When the finding was based solely on the Delphi process, no evidence section was added.
Clinical Setting for Treatment and Procedure Technique
Finding 1: Anesthesia
While it is possible to perform MicroPulse TLT in the office, it is most comfortable for the patient to perform the procedure in the OR. The preferred anesthesia techniques are topical with sedation, with or without a block. There are situations in which only topical anesthesia has been used, which is a decision which is left up to the clinician. In general, post-operative pain is minimal.
Evidence: Because MicroPulse TLT targets the ciliary body region, there is some induced ciliary muscle spasm during the procedure which can be uncomfortable for the patient. Sufficient pain control during MicroPulse TLT is necessary to maintain patient comfort and enable precise and correct delivery of treatment. There are no randomized controlled trials comparing different forms of anesthesia nor are there systematic uses of analog pain scales or quality of life questionnaires in published studies. There is one single study of MicroPulse TLT that reports on patient pain levels in the first hours after the procedure.4 A majority of the published literature reports the use of subtenons, peribulbar, and retrobulbar blocks as the method of anesthesia, but there are studies that report the use of topical anesthesia.5,6 General anesthesia was used in all published MicroPulse TLT treatments in children,7,8 and in some studies in adults.9,10 No complications due to anesthesia were reported in any of the studies.
Theoretically, all forms of anesthesia from topical to general can be used. A typical regimen involves the eye being treated with topical anesthesia pre-operatively, with lidocaine gel and/or topical tetracaine commonly used. In the operating room, the patient may receive IV anesthesia such as versed and/or propofol. A retrobulbar or peribulbar block may also be administered. A decision about whether to use topical anesthesia with sedation or block anesthesia should be made based on the anxiety level and general pain tolerance of the patient, the specific clinical scenario and the OR setting. Due to limited cooperation, general anesthesia is recommended in all cases of MicroPulse TLT in children.
Finding 2: Coupling Agent
A coupling agent should always be used. The most commonly used agent is lidocaine gel. The optically neutral coupling agent is generally applied to the eye before the start of the procedure and is usually reapplied at least once during the procedure, but reapplication is at the discretion of the surgeon.
Evidence: Adequate coupling between the probe and the globe optimizes and localizes energy delivery between the laser probe and the target tissues. Patel et al compared various solutions including balanced salt solution, tetracaine drops, lubricating ointment, and lidocaine gel. They found that while more energy was delivered when a contact medium was used on the eye, there was no significant difference in energy delivery between the various types of coupling agents.11
Finding 3: Probe Placement
Using the revised MicroPulse P3 probe, optimal energy transmission occurs when moderate pressure is evenly applied across the footplate (with a posterior bias) and the conjunctiva is gently compressed. As optimal angulation is incorporated into the design of the revised MicroPulse P3 probe, there is no need for additional probe manipulation.
Evidence: For both probes (original and revised) it is important to apply the footplate flush to the ocular surface to prevent energy loss. If the probe tip is not adequately flush to the ocular surface during treatment, laser energy will be lost or misdirected at the interface. Tactile feedback can be used for determining inadequate flushness of the footplate during treatment. The original MicroPulse P3 probe requires full contact with the surface of the eye held at an angulation of 45° tangential to the target tissue during treatment. As optimal angulation is incorporated into the design of the revised MicroPulse P3 probe, there is no need for additional probe manipulation.
Finding 4: Probe Positioning and Adjustment
This consensus panel places the limbal-matching curvature ‘bunny ears’ of the revised MicroPulse P3 probe on the limbus (Figure 1). If the limbus is not clearly defined, it is acceptable to err up to 1 mm posterior from this structure. When in doubt of the location of the ciliary body, such as in very high myopes or hyperopes, transillumination or UBM can be used to adjust probe positioning. (With the revised MicroPulse P3 probe, the actual laser fiber from where the laser light emanates is located 3.0 mm posterior to the center of the anterior edge of the probe in between the bunny ears.12
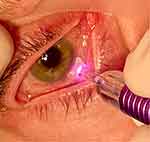 |
Figure 1 Revised MicroPulse P3 Probe placed on the limbus. Image courtesy of Brian Jerkins. |
Finding 5: Probe Sweeping
Most panel members make a sweep of 180 degrees, then reverse direction like a pendulum. The typical treatment pattern is to move the probe in a back-and-forth manner over the hemisphere. Treating by quadrant can also be done, according to the preference of the surgeon. Stopping and restarting during the treatment cycle is acceptable to optimize probe positioning.
Finding 6: Tissue Considerations
Surgeons should be careful not to traumatize areas of fragile tissue. One should not treat over thin, avascular areas of conjunctiva, including over blebs or tubes. However, the panel feels it is appropriate to treat over areas of prior surgery and scleral patches if the conjunctiva and sclera are normal in appearance and contour. This panel recommends sparing treatment over the 3 o’clock and 9 o’clock positions and agrees that localized areas of scleral thinning should be spared. If areas are skipped, the same overall sweep time is maintained, and the probe is applied over a smaller area. Panelists avoid treating over a dense, elevated subconjunctival hemorrhage.
Finding 7: Vasoconstriction
Topical vasoconstrictors (eg, phenylephrine 5% or brimonidine 0.15% to 0.2%) can be considered if the surgeon desires to decrease the risk of subconjunctival heme and minimize the potential absorption of energy by superficial vessels. No adjustment in treatment technique or parameters is required for an eye that is very hyperemic prior to surgery. Application of a vasoconstrictor is appropriate if desired by the treating physician.
Finding 8: Eyeball Manipulation
The use of an eyelid speculum is surgeon preference. Items used to manipulate the globe to facilitate exposure are also up to the preference of the surgeon. Examples are Q tips, muscle hooks, and indentation instruments. It is acceptable both to use or not use these devices.
Post-Operative Care
Finding 9: Post-op visits
Due to the safety profile of MicroPulse TLT and the time for maximal effect, the panel typically conducts post-op visits at one-week and then at one month, but sooner follow-up may be justified based on the individual patient characteristics.
Evidence: The studies that specifically reported follow-up times reported at either 1 day or 1 week following surgery, followed by 1 month, 3 months and 6 months thereafter.7,8,13–15
Finding 10: Post-op Inflammation Management, Pain Medications and Cycloplegia
The experts in this panel use topical NSAIDS and/or topical steroids with various dosage regimens ranging from 1 to 4 weeks for most cases. Routine post-op cycloplegia is not deemed necessary. If patients experience pain postoperatively, paracetamol/acetaminophen can be given. For a patient who has lower pain tolerance, providing longer acting IV pain medication during the procedure is appropriate. Some surgeons give IV steroids during the procedure as well. More complicated clinical scenarios such as those of post-keratoplasty eyes may require more aggressive steroid regimens.
Evidence: Most studies of MicroPulse TLT report the use of topical steroids at varying frequencies with a taper over time following surgery. The type of steroid used includes prednisolone, dexamethasone, difluprednate and loteprednol. Several studies also report the regular use of systemic NSAIDs or topical NSAIDs. Two studies reported the use of subconjunctival dexamethasone injection at the conclusion of the MicroPulse TLT.5,16
Studies of keratoplasty patients undergoing MicroPulse TLT used an aggressive steroid regimen including postoperative intravenous solumedrol and topical prednisolone every 1 −2 hours tapered over 3 months to prevent graft rejection,17 whereas a less intensive regimen of prednisolone 4 times a day for 1 week then tapering may result in a greater number of immunologic rejection episodes within a year of treatment.10 Only 3 studies mention the use of cycloplegics.5,18,19 Inflammation after MicroPulse TLT is usually mild and does not require cycloplegics, although exceptions can occur.43
Finding 11: Pre-op/Post-op Antibiotics
A postoperative antibiotic is typically not recommended for routine cases.
Evidence: Since MicroPulse TLT is a non-incisional procedure, there should be no risk of infection following surgery. Prophylactic antibiotic use is not necessary unless there is a condition that increases the risk of infection such as keratoprosthesis or recent penetrating keratoplasty, severe ocular surface disease, poor epithelial integrity or prior trabeculectomy with avascular bleb. There is one case in the literature of severe bacterial endophthalmitis developing 5 days following the procedure in a patient with glaucoma and a Boston keratoprosthesis type I placed the year prior.20 It was hypothesized that irregularity of the globe and the disruption of epithelial integrity due to the keratoprosthesis allowed the ingress of bacteria. Several published studies used routine topical antibiotics for 1 week.14,16,18,21
Finding 12: Postoperative Glaucoma Medications
With the exception of acetazolamide, which in many cases can be discontinued immediately after treatment based on individual patient characteristics, topical hypotensive medications are routinely continued until the response to MicroPulse TLT permits the tapering of medications. Meaningful IOP lowering effect of MicroPulse TLT is usually seen at one-week follow-up, with the full effect typically observed at one month.
Evidence: The majority of the published literature on MicroPulse TLT reports the continued use of IOP-lowering medications following treatment, with use tapered according to IOP response.5–9,14,19–24
Outcomes
Finding 13: Expected Outcomes with the Original MicroPulse P3 Probe
As referenced in the consensus panel’s first published paper, treatment power, total treatment time and sweep velocity all play a role in treatment efficacy in IOP-lowering.25 Published results of MicroPulse TLT using the original MicroPulse P3 probe demonstrated IOP lowering in the range of approximately 30–50% using treatment parameters in the higher range of the literature (see Table 1). The role of MicroPulse TLT in medication reduction is not clear, as it was not a primary outcome measure in most studies. Lens status does not appear to affect outcomes. Additional research into this area is needed and will help to optimize results even further.
 |
Table 1 Summary of MicroPulse TLT Treatment Settings and Outcomes |
Evidence: Given the paucity of research on MicroPulse TLT with higher energy dosing, the lack of consistent surgical techniques or description of standardized parameters like sweep velocity, reliably predicting outcomes is somewhat difficult. Furthermore, the lack of consistent definitions of success makes this analysis more difficult (see Table 2). Overall, the research clearly indicates that increasing power, treatment time and/or slowing sweep velocity improves outcomes in terms of pressure lowering as a percentage of the baseline IOP.
 |
Table 2 How Various Authors Defined Successful MicroPulse TLT Treatment and Percentages of Patients Achieving It |
A given patient’s response to MicroPulse TLT is somewhat difficult to predict given the variability in the settings previously used and the fact that many publications did not list or record treatment sweep velocity. Fluence is a parameter that takes into account sweep velocity. Fluence equals power × duty cycle × dwell time divided by area, thus fluence increases with increased power and slower sweep velocity. Grippo et al25 performed a meta-analysis of all available studies in a literature review and calculated the fluence for each of the studies in which sweep speed was documented. Overall, studies performed with fluences at or below 52.4 J/cm2 showed an average decrease in IOP of 36.1% ± 6.1, while studies performed with fluences above 52.4 J/cm2 had an average decrease in IOP of 52.9% ± 2.9. Given these findings, one can expect better pressure reduction with higher fluence.
Marchand et al20 looked prospectively at MicroPulse TLT performed with longer treatment times and found that patients treated with longer treatment times had a greater reduction in IOP. Patients underwent the procedure with 2000 mW power, 31.3% duty cycle and 10s sweep velocity. The treatment times varied based upon iris pigmentation and severity of glaucoma. Patients with mild, moderate and severe glaucoma received treatment times of 160s, 240s and 240–320s, respectively. The authors saw a reduction in IOP of 29.5% at 12 months and 35.6% at 18 months from the baseline across all groups. Patients treated for 240s had a reduction of 30.8%, while those treated with 320s had a reduction of 40.1%, despite similar preoperative pressures. There was no statistically significant reduction in medications after treatment. Success rate at 12 and 18 months was 61.5% and 59.6% with success defined as IOP between 6 and 21 mmHg with a reduction of 25% from baseline without additional medications or procedures. Retreatment rate was 19.6%.
At the other end of the treatment spectrum, Tong et al26 analyzed primary open-angle glaucoma (POAG) eyes treated with 2000 mW, 31.3% duty cycle and lower treatment times. Most patients received 100s total treatment per eye (50s per hemisphere), but both power and duration of the treatment were sometimes further decreased at the discretion of the surgeon. In addition, the authors stated that in some instances, up to 50% of the limbal circumference of the eye was left untreated to avoid areas of previous surgery. This translated into a 12.1% reduction in IOP from baseline at 12 months. There was no reduction in topical hypotensive medications, but there was a reduction in oral antihypertensive medications. With these parameters, the authors found that MicroPulse TLT was very safe with no persistent complications and stable visual acuities. However, the overall energy applied in Tong et al was at the low end of the range reported in the literature, and these low settings (low amounts of energy delivered and area treated) may have been subtherapeutic.27
The Tong paper also highlights some of the problems that using prior literature poses in predicting outcomes. The authors did not record sweep velocity and stated that the surgeons decreased the treatment parameters at their own discretion. Without consistent and well-documented treatment power, time and sweep velocity, it is difficult to interpret results and to use them as predictors for future outcomes.27 In a subsequent reply to Grippo et al’s letter to the editor, Tong et al stated that low settings were used, given the higher pigmentation of their cohort of patients that could translate into higher energy absorption and higher risk for potential side effects.28 The impact of higher pigmentation on energy absorption during MicroPulse TLT is unproven.
Sarrafpour et al22 looked at the effect of varying treatment power with MicroPulse TLT (using the original MicroPulse P3 probe) on IOP-lowering outcomes. All patients were treated for 100s total (50s per hemisphere) and with a 31.3% duty cycle. The authors stated that 4–6 sweeps were performed per hemisphere, which translates to a sweep velocity of 8.3s to 12.5s. Treatment power was chosen based upon preoperative visual acuity; 2000 mW for vision of 20/20-20/70, 2250 mW for vision of 20/80-20/400, 2400 mW for vision of counting fingers or hand motion and 2500 mW for a vision of light perception or no light perception. There was a significant improvement in IOP lowering from baseline as the power increased. Patients treated with 2000 mW had a 30.1% reduction in IOP at one year. Those treated with higher powers had a larger reduction from baseline; 2250 mW, 2400 mW and 2500 mW saw reductions in IOP at one year of 51.2%, 51.3% and 57.1%, respectively. The study also reported a mean reduction in antihypertensive medication burden of 19% at one year, with 73.3% of patients on oral carbonic anhydrase inhibitors being able to stop them post-operatively. The patients treated with 2000 mW, 30.1% duty cycle and 100 seconds of total treatment time had similar settings to Tong et al but experienced different efficacy over time (30% IOP reduction versus 12%). Differences in not treating the entire circumference, patient characteristics, faster sweeps, decreasing power or decreasing total treatment time in many of the cohort in the Tong et al paper may account for the different outcomes.
Al Habash et al29 performed MicroPulse TLT (using the original MicroPulse P3 probe) with more robust settings of 2200 mW, 240s treatment time and 12s sweep velocity. The authors found a 52% reduction in IOP from the preoperative measurement with these settings. There was an overall reduction of one medication from the preoperative burden. These parameters also proved to be relatively safe with no incidence of phthisis bulbi, hypotony, hyphema, loss of light perception, macular edema, severe pain or corneal edema.
Finding 14: Expected outcomes with the revised MicroPulse P3 Probe and Consensus Recommended Settings
At the consensus-based recommended starting dose of 2500 mW, 31.3% duty cycle, and 4 sweeps at a sweep velocity of 20 seconds each per hemisphere, the clinician can expect intraocular pressure reduction of approximately 25–35%.
Evidence: The revised MicroPulse P3 probe has evolved to improve stability, visualization and energy coupling during treatment. However, there is limited information to date on MicroPulse TLT efficacy with the revised MicroPulse P3 probe. Checo et al2 conducted a prospective, non-comparative case study on 61 eyes of 40 glaucoma patients treated with the revised MicroPulse P3 probe using 2500 mW and 31% duty cycle. The authors compared 50-second-per-hemisphere and 60-second-per-hemisphere settings using 3 sweeps (16.5-second sweep or 20-second sweep), 4 sweeps (12.5-second sweep or 15-second sweep), and 5 sweeps (10-second sweeps or 12-second sweeps). At 12 months, the mean IOP decrease across all treatment groups was 44.7%, and more significant IOP reductions were generally associated with slower sweep velocity.
The recommended settings from the panel show a total energy of 126 J, which is around the total energy that has proven effective with the original probe and the slower sweep velocity increases the fluence values significantly. These settings coincide with what most panel members have been using as a starting set of parameters with good efficacy and a good safety profile.
Finding 15: Overall Energy Adjustments Based on Patient Characteristics
The literature at this point is limited when it comes to recommendations in energy adjustment based on individual patient characteristics. More research is needed. Depending on the individual clinical scenario, the panel would start to consider treating with higher than the recommended starting settings in the following situations when already on maximal tolerated medical treatment:
- Advanced disease with low target pressure.
- Refractory neovascular glaucoma.
- High baseline IOP.
The increase in dosage can be achieved by changing a given parameter by approximately 25%: 1) increasing power, 2) increasing number of sweeps or 3) decreasing sweep velocity.
Clinical Responses Pattern Definition and Retreatment/Enhancement
Finding 16: Pattern of Response Classification
This panel chose to create a common vocabulary for clinicians and for future research. In addition, these definitions have implications for retreatment.
- A non-responder is a patient who does not achieve and maintain at least 20% reduction in IOP within the first three months or a consistent reduction in at least 1 medicine.
- Early attrition is a patient who achieves at least 20% IOP reduction or the decrease in at least one medicine within the first 3 months but does not maintain the reduction for 12 months.
- Late attrition is a patient who achieves and maintains at least a 20% IOP reduction or a decrease in at least one medicine for the first 12 months but loses efficacy beyond 12 months.
Finding 17: Risk Factors for Need for Retreatment
Lower amount of energy used and higher baseline IOP show a higher likelihood of need for retreatment, while lens status and prior glaucoma surgery do not. It remains unclear if glaucoma sub-type has an impact on outcomes. While the IOP-lowering effects of treatment may diminish over time, retreatment has a good chance of success, especially in those cases of late attrition.
Evidence: Retreatment rates in adults were reported in 18 clinical studies and varied significantly from 0.5% to 68.5%. Tekeli et al,34 Yelenskiy et al19 and Logioco et al33 showed that a higher baseline IOP correlated with a higher likelihood of retreatment. While de Crom14 found no difference in rate of retreatment or success due to diagnosis or primary versus secondary glaucoma; Tekeli et al34 found a lower retreatment rate in POAG and pseudoexfoliative glaucoma versus other types of glaucoma.
The total energy used varied greatly among these studies and likely contributed to the variable outcomes. Treatment time was the parameter that differed the most among these studies, while power also varied to some extent and sweep velocity was frequently unknown. Al Habash et al29 prospectively analyzed the outcomes of MicroPulse TLT in 71 eyes of 68 patients where the mean total energy used was 165.2 J and fluence was 69.2 J/cm2. Only 5.6% of eyes received a second treatment, with an average of 22.9 months (95% CI 21.9–24 months) to a second procedure. Yelenskiy et al19 used a total energy of 111.6–148.8 J and reported only 8.6% of eyes needing retreatment.
In comparison, Aquino et al1 treated 23 patients with a total energy of 62.6 J, and 11 patients (48%) were retreated due to uncontrolled IOP after a mean of 6.8 months (range 2 to 17 months). A third session was performed on 4 patients, all with neovascular glaucoma, and all failed to achieve target pressures. Tan et al4 also used lower energy settings with 62.6 J of total energy and retreated 14 of 40 eyes (35%). Of these, five were successful, nine failed and were not treated a third time.
In a study evaluating retreatment based on the initial treatment response, Chamard et al13 reported results of MicroPulse TLT in 94 eyes of 94 patients, with a definition of success of IOP 5–21 mmHg, reduction of 20% from baseline, with no retreatment and visual acuity of at least light perception. Early retreatment (within 6 months) was performed in 16% at a median time of 4.4 (1.3) months. Late retreatment (greater than 6 months) was performed in 20.2% of patients, at a median time of 10.2 (3.6) months. The success rate was significantly higher in the late retreatment group, with 63.6% meeting the criteria 6 months following retreatment, versus 16.7% in the early group. de Crom et al14 reported the primary failure to be 13.5% compared to the secondary failure of 16.3%. Overall, the eyes that needed retreatment had a 64% successful retreatment rate. The success rate was much lower in the eyes with primary failure with only 21% achieving success after retreatment.
Finding 18: Additional IOP Lowering and Timing for Enhancements
MicroPulse TLT can be used as an enhancement procedure 1 to 3 months following the initial procedure if the optimal IOP is not obtained with the initial treatment.
Evidence: Kaba et al9 reported on 342 eyes of 214 patients that received a total of 399 MicroPulse TLT procedures and found a 23.7% mean reduction in IOP at 12 months. Retreatment was considered after a minimum of one month if IOP reduction was not at target. Retreatment was performed in 57 eyes (14.3%), and the authors were able to obtain an additional mean 16.4% reduction in IOP (from 18.9 to 15.8 mmHg) with each retreatment. They also noted that additional treatment did not increase the risk of vision loss.
Nguyen et al15 reported on 95 eyes of 95 patients and defined success as 20% or greater IOP reduction from baseline beyond one month after the first treatment and qualified success after retreatment. Treatment success was achieved after a single treatment in 73 patients (76.8%). Any patient who did not maintain success at any point in follow-up was considered for retreatment, with the surgeon typically raising only the power level. Twenty-two patients had at least one retreatment, another eight had three treatments, four had four treatments and one patient had five treatments. Including those with multiple retreatments, 100% of patients achieved the success of IOP lowering 20% from baseline.
A similar multiple retreatment method was done by Magacho et al6 in 89 eyes of 76 patients. They achieved success (IOP 6–18 mmHg or 20% reduction, retreatment not considered a failure) in 87% of eyes at 12 months. To achieve success, 68.5% of patients needed one treatment, 15.7% needed two, 11.2% needed three and 4.5% needed four treatments. All procedures were performed within 2 months of the previous one.
Finding 19: Retreatment/Enhancement Consensus Recommendations
If a patient has an initial satisfactory response to MicroPulse TLT but has not reached their target IOP, MicroPulse TLT can be repeated with the same or an increase in laser energy delivery to enhance the effect. The enhancement/retreatment is usually done 3 months or more after the initial treatment.
- In a patient who DOES NOT have an initial satisfactory response within 3 months (non-responder), some surgeons would consider retreatment with higher settings, and others would move on to a different procedure.
- In a patient who has an initial good response beyond 3 months, but it loses efficacy over time, enhancement/retreatment with or without a 25% increase in laser energy delivery should be considered.
Side Effects
Finding 20: Many complications described in the early literature can be attributed to an excess amount of energy applied or inappropriate surgical techniques
With a current better understanding of dosimetry and energy limits,3 and refinements in surgical techniques and probe design, many side effects can be prevented or minimized. The revised MicroPulse P3 delivery device provides more stability during treatment and because of its angulation, it results in a more posterior treatment away from the anterior segment structures such as the iris root or the lens. Initial clinical evidence supports the concept that these features translate into an improved safety profile.2
Evidence: In the early literature, complications most commonly included anterior chamber inflammation and decreased vision. Other less common complications included cystoid macular edema (CME), mydriasis, pain, transient hypotony, hyphema, very rarely phthisis bulbi along with rare case reports of suprachoroidal hemorrhage, vitreous hemorrhage, conjunctival laceration, and neurotrophic keratitis.13,14,22,24,31,38–42,44 The prevalence of side effects appeared to be proportional to the amount of energy used.3,24,25,31 For example, when treatment is performed with settings that are closer to the average reported in the literature, minimal inflammation is expected as recorded by de Crom et al and Chamard et al. When more aggressive parameters are used, closer to or at the upper limit reported in the literature, up to 46% of eyes were noted to have inflammation at 3 months.31 The energy settings used by Emanuel et al represent the upper limit of energy used during MicroPulse TLT in clinical practice for both Fluence (up to 400 J/cm2) and total energy (up to 225J).
Another complication reported in the early literature with the original probe was decreased visual acuity, typically defined as loss of >2 lines of vision. Low treatment time and typical settings are associated with long-term decreased visual acuity rates of zero to less than 5%. At higher overall energy settings and more atypical treatment parameters, a decrease of at least 2 lines of vision was seen in 26.2% and 41% of patients by Habash et al29 and Emanuel et al,31 respectively. Many instances of decreased vision can be attributed to other causes not directly related to the procedure itself or, if related to MicroPulse TLT, are reversible. Cataract progression has been reported following MicroPulse TLT and contributes to the numbers reported for decrease in VA, although it can be resolved with surgery.13–15,19,22,24,29,32,33 A representative example to further exemplify this concept, is the work by Varikuti et al16 as they focused specifically on changes in visual acuity. They used a total energy of 100.2 J and a fluence of 52.4 J/cm2 using the original probe and followed their patients for 12 months. Out of 49 eyes, ten (20.8%) were found to have lost ≥2 lines of vision. Among the 10 eyes, 5 eyes had cataract progression that was addressed with subsequent cataract extraction, one eye had a history of CME before receiving MicroPulse TLT and developed CME after MicroPulse TLT, and two eyes had unexplainable vision loss, which the authors attributed to likely glaucoma progression. The remaining two eyes had a history of iritis and mild postoperative inflammation that resolved at subsequent follow-up visits after study completion.16
The incidence of CME from MicroPulse TLT ranges from 0% to 5% with expected resolution with anti-inflammatory treatment within a month and has been reported in patients with a previous history of diabetic macular edema.24 Hypotony (IOP <5 mmHg) is typically transient with incidences from 0% to 8%13,24,31 with resolution by 3 months. Prolonged hypotony was seen in rare instances in eyes with a history of multiple procedures or complex secondary glaucomas such as neovascular glaucoma.
Other reported side effects, such as mydriasis, have a low incidence (4 patients in 71,29 2 patients in 110,33 and 11 patients in 1005) and have been reported to resolve in many instances by 1 month. Likewise, corneal edema is a rare complication with a reported case of corneal hydrops following cataract surgery by Chan et al39 This was attributed to laser energy application too close to a recent clear corneal wound, and therefore, avoidance of treatment at, or adjacent to, a clear corneal wound is recommended.
Likewise, neurotrophic keratitis has been reported by Perez et al and may be related to treatment too anterior to the limbus and/or at 3 and 9 o’clock.38 Case reports of rare complications of choroidal and vitreous hemorrhage, conjunctival laceration, hyphema and phthisis bulbi have also been described.24,40,41 These complications are much less common in comparison to traditional continuous-wave cyclophotocoagulation1 and are usually seen when treatment is outside of the recommended dosing parameters.24,31
Dry eye has been reported in approximately 10% of cases of MicroPulse TLT.15 Theoretically, MicroPulse TLT treatment with the original probe over the perilimbal area may affect goblet cells and stem cells and lead to a transient corneal epitheliopathy and dry eye. A multi-center study with the original probe found that the second most common complication following treatment was severe superficial punctate keratitis seen in 11 out of 167 (7%) of eyes.5 Perhaps with this in mind, two studies reported routine use of artificial tears after surgery for an unspecified duration.13,37
The prospective study using the revised MicroPulse P3 probe by Checo et al reported no vision threatening complications over a 12-month period though 2 out of 60 patients (3.3%) had a decrease in vision of 2 lines or less after one year of follow-up.2
Conclusions
Physician experience and literature available during the second consensus Delphi panel have made it possible to re-evaluate and revise surgical technique, post-operative care, expected outcomes as well as indications and need for enhancements or retreatments. Technological improvements in laser delivery and a better understanding of all aspects of the MicroPulse TLT treatment have improved the procedure, optimized outcomes, and made it more user-friendly. In turn, this has expanded the indications for MicroPulse TLT compared to traditional transscleral cyclophotocoagulation.
Used with proper energy settings, techniques and treatment indications, MicroPulse TLT is a safe and effective treatment for many glaucomas and is a useful addition to the glaucoma treatment armamentarium. Future updates on the evolution of this technique will be provided based on ongoing studies and experience.
Ethics Approval
This paper did not require any original research, and thus, no ethics approval was needed.
Consent to Publication
Brian Jerkins consents to have the image of the MicroPulse P3 Probe on the limbus published.
Acknowledgments
Writing and editing support was provided by Adrianne Resek, MA. Ms. Resek participated in the development and analysis of the Delphi method questionnaires in addition to preparation of the manuscript. Paul Chew, MD, and Ziad Khoueir, MD, reviewed this manuscript and provided comments.
Funding
Funding for research on this paper was provided by Iridex Corporation. Funding for writing support for this paper was provided by Iridex Corporation to Adrianne Resek, MA. Iridex was not privy to questionnaires, responses or data collection during the Delphi Consensus process nor were they privy to the manuscript during its preparation, writing and submission.
Disclosure
The following authors have received grant support (S), lecture fees (L), or consulting fees (C) from Iridex Corporation: Jella An (S, L); Jacob W Brubaker (S, L); Ronald MPC de Crom (S, L); Brian A Francis (S, L); Michael Giovingo (S, L); Tomas M Grippo (C, S, L); Brian Jerkins (S); Robert Noecker (C, S, L); Nathan Radcliffe (S); Marc Toeteberg-Harms (S, L). Prof. Dr. Marc Töteberg-Harms also reports speaker’s honorarium from Allergan, MLase AG, ELIOS, ELT Sight, Novartis/Alcon, Santen, Heidelberg Engineering, and Glaukos; honorarium for Advisory Board Activity from Reichert, outside the submitted work. Dr Michael Giovingo also reports grants from Santen, outside the submitted work. Dr Jella An also reports personal fees from Alcon, New World Medical, Aerie, Sight Sciences; grants from Abbvie, outside the submitted work. Dr Nathan Radcliffe also reports personal fees from Alderya, Avellino, Aerie Pharmaceuticals; Alcon Vision, Alimera Sciences, Allergan/AbbVie, Bausch + Lomb, Beaver-Visitec International, Belkin, CATS, Carl Zeiss Meditec, CATS, Dompe, Ellex, ELIOS Vision Inc, Equinox, Eyepoint Pharmaceuticals, Glaukos, Iridex, IrisVision, Kala Pharmaceuticals, Lumenis, New World Medical, Novartis, Ocular Science, Ocular Therapeutix, Omeros, Reichert, Santen, Shire, Sight Sciences, Spyglass, Tarsus, Thea, TearClear and ViaLase, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Aquino MC, Barton K, Tan AM, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Exp Ophthalmol. 2015;43(1):40–46. doi:10.1111/ceo.12360
2. Checo L, Wagner I, Ahuja A, et al. Prospective comparison of different energy parameters of micropulse transscleral laser therapy.
3. Grippo TM, de Crom RM, Giovingo MC, et al. Evidence-based consensus guidelines for micropulse transscleral laser therapy: dosimetry and patient selection. Clin Ophthalmol. 2022;16:1837–1846. doi:10.2147/OPTH.S365647
4. Tan AM, Chockalingam M, Aquino MC, et al. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol. 2010;38(3):266–272. doi:10.1111/j.1442-9071.2010.02238.x
5. Radhakrishnan S, Wan J, Tran B, et al. Micropulse cyclophotocoagulation: a multicenter study of efficacy, safety, and factors associated with increased risk of complications. J Glaucoma. 2020;29(12):1126–1131. doi:10.1097/IJG.0000000000001644
6. Magacho L, Lima FE, Ávila MP. Double-session micropulse transscleral laser (CYCLO G6) as a primary surgical procedure for glaucoma. J Glaucoma. 2020;29(3):205–210. doi:10.1097/IJG.0000000000001426
7. Elhefney EM, Mokbel TH, Hagras SM, et al. Micropulsed diode laser cyclophotocoagulation in recurrent pediatric glaucoma. Eur J Ophthalmol. 2020;30(5):1149–1155. doi:10.1177/1120672119858226
8. Abdelrahman AM, El Sayed YM. Micropulse versus continuous wave transscleral cyclophotocoagulation in refractory pediatric glaucoma. J Glaucoma. 2018;27(10):900–905. doi:10.1097/IJG.0000000000001053
9. Kaba Q, Somani S, Tam E, Yuen D. The effectiveness and safety of micropulse cyclophotocoagulation in the treatment of ocular hypertension and glaucoma. Ophthalmol Glaucoma. 2020;3(3):181–189. doi:10.1016/j.ogla.2020.02.005
10. Subramaniam K, Price MO, Feng MT, Price FW
11. Patel S, Kakouri A, Chaudhary S, et al. The effect of various media and probe angles on the power output of the cyclo G6 glaucoma laser system. Lasers Med Sci. 2021;36(3):605–609. PMID: 32681220. doi:10.1007/s10103-020-03089-w
12. Iridex. MicroPulse P3 Device. Engineering Specifications. Available from: https://iridex.com/Portals/0/downloads/MicroPulse_P3_Device_Brochure.pdf
13. Chamard C, Bachouchi A, Daien V, Villain M. Efficacy, safety and re-treatment benefit of micropulse transscleral cyclophotocoagulation in glaucoma. J Glaucoma. 2021;30:781–788. doi:10.1097/IJG.0000000000001900
14. de Crom RMPC, Slangen CGMM, Kujovic-Aleksov S, et al. Micropulse trans-scleral cyclophotocoagulation in patients with glaucoma: 1- and 2-year treatment outcomes. J Glaucoma. 2020;29(9):794–798. doi:10.1097/IJG.0000000000001552
15. Nguyen AT, Maslin J, Noecker RJ. Early results of micropulse transscleral cyclophotocoagulation for the treatment of glaucoma. Eur J Ophthalmol. 2020;30(4):700–705. doi:10.1177/1120672119839303
16. Varikuti VNV, Shah P, Rai O, et al. Outcomes of micropulse transscleral cyclophotocoagulation in eyes with good central vision. J Glaucoma. 2019;28(10):901–905. doi:10.1097/IJG.000000000000133
17. Lee JH, Vu V, Lazcano-Gomez G, et al. Clinical outcomes of micropulse transscleral cyclophotocoagulation in patients with a history of keratoplasty. J Ophthalmol. 2020;9(2020):6147248.
18. Preda MA, Karancsi OL, Munteanu M, Stanca HT. Clinical outcomes of micropulse transscleral cyclophotocoagulation in refractory glaucoma-18 months follow-up. Lasers Med Sci. 2020;35(7):1487–1491. doi:10.1007/s10103-019-02934-x
19. Yelenskiy A, Gillette TB, Arosemena A, et al. Patient outcomes following micropulse transscleral cyclophotocoagulation: intermediate-term results. J Glaucoma. 2018;27(10):920–925. doi:10.1097/IJG.0000000000001023
20. Marchand M, Singh H, Agoumi Y. Micropulse trans-scleral laser therapy outcomes for uncontrolled glaucoma: a prospective 18-month study. Can J Ophthalmol. 2021;56(6):371–378. PMID: 33577756. doi:10.1016/j.jcjo.2021.01.015
21. Egorov VV, Samokhvalov NV, Marchenko AN. Clinical evaluation of the results of micropulse laser cyclophotocoagulation in the treatment of refractory glaucoma on the first day after surgery. Mod Technol Ophthalmol. 2020;82–86. doi:10.25276/2312-4911-2020-1-82-86
22. Sarrafpour S, Saleh D, Ayoub S, Radcliffe NM. Micropulse transscleral cyclophotocoagulation: a look at long-term effectiveness and outcomes. Ophthalmol Glaucoma. 2019;2(3):167–171. doi:10.1016/j.ogla.2019.02.002
23. Souissi S, Baudouin C, Labbé A, Hamard P. Micropulse transscleral cyclophotocoagulation using a standard protocol in patients with refractory glaucoma naive of cyclodestruction. Eur J Ophthalmol. 2021;31(1):112–119. doi:10.1177/1120672119877586
24. Williams AL, Moster MR, Rahmatnejad K, et al. Clinical efficacy and safety profile of micropulse transscleral cyclophotocoagulation in refractory glaucoma. J Glaucoma. 2018;27(5):445–449. doi:10.1097/IJG.0000000000000934
25. Grippo TM, Sanchez FG, Stauffer J, Marcellino G. MicroPulse transscleral laser therapy – fluence may explain variability in clinical outcomes: a literature review and analysis. Clin Ophthalmol. 2021;15:2411–2419. doi:10.2147/OPTH.S313875
26. Tong W, Shen TYT, Wong WC, et al. One-year outcomes of micropulse cyclophototherapy for primary open-angle glaucoma. J Glaucoma. 2021;30:911–920. doi:10.1097/IJG.0000000000001925
27. Grippo TM, Brossard Barbosa N, Noecker R, et al. Letter to the editor: one-year outcomes of micropulse cyclophototherapy for primary open-angle glaucoma. J Glaucoma. 2022;31(6):e41–e42. doi:10.1097/IJG.0000000000002040
28. Tong W, Yu Ting Shen T, Wong HC, et al. Response to letter to the editor: one-year outcomes of micropulse cyclophototherapy for primary open-angle glaucoma. J Glaucoma. 2022;31(6):e42. doi:10.1097/IJG.0000000000002041
29. Al Habash A, Al Ahmadi AS. Outcome of micropulse transscleral photocoagulation in different types of glaucoma. Clin Ophthalmol. 2019;13:2353–2360. doi:10.2147/OPTH.S226554
30. Sanchez FG, Lerner F, Sampaolesi J, et al. Efficacy and safety of micropulse transscleral cyclophotocoagulation in glaucoma. Arch Soc Esp Oftalmol. 2018;93(12):573–579. doi:10.1016/j.oftal.2018.08.003
31. Emanuel ME, Grover DS, Fellman RL, et al. Micropulse cyclophotocoagulation: initial results in refractory glaucoma. J Glaucoma. 2017;26(8):726–729. doi:10.1097/IJG.0000000000000715
32. Jammal AA, Costa DC, Vasconcellos JPC, Costa VP. Prospective evaluation of micropulse transscleral diode cyclophotocoagulation in refractory glaucoma: 1 year results. Arq Bras Oftalmol. 2019;82(5):381–388. doi:10.5935/0004-2749.20190076
33. Logioco C, Perrone LD, Caruso D, et al. Assessment of efficacy and safety of micropulse diode laser treatment in glaucoma: one year follow-up. Arch Soc Esp Oftalmol. 2020;95(7):327–333. doi:10.1016/j.oftal.2020.03.002
34. Tekeli O, Kose HC. Outcomes of micropulse transscleral cyclophotocoagulation in primary open angle glaucoma, pseudoexfoliation glaucoma, and secondary glaucoma. Eur J Ophthalmol. 2021;31(3):1113–1121. doi:10.1177/1120672120914231
35. Waibel S, Herber R, Pillunat LE, Pillunat KR. One-year follow-up of pars plicata versus pars plana application of transscleral micropulse cyclophotocoagulation. J Glaucoma. 2021;30(4):340–346. doi:10.1097/IJG.0000000000001775
36. Wong KYT, Aquino CM, MAcasaet AM, et al. MP3Plus: a modified micropulse transscleral cyclophototherapy technique for the treatment of refractory glaucoma. J Glaucoma. 2020;29(4):264–270. doi:10.1097/IJG.0000000000001443
37. Zaarour K, Abdelmassih Y, Arej N, et al. Outcomes of micropulse transscleral cyclophotocoagulation in uncontrolled glaucoma patients. J Glaucoma. 2019;28(3):270–275. doi:10.1097/IJG.0000000000001174
38. Perez C, Han Y, Rose-Nussbaumer J, Ou Y, Hsia YC. Neurotrophic keratitis after micropulse transscleral diode laser cyclophotocoagulation. Am J Ophthalmology Case Rep. 2019;15:100459. doi:10.1016/j.ajoc.2019.100469
39. Chan P, Lam M, Baig N. Case report – acute corneal subepithelial hydrops during micropulse transscleral cyclophotocoagulation. BMC Ophthalmol. 2020;20:409. doi:10.1186/s12886-020-01669-6
40. Aldaas K, Brasington C, Zhang A, et al. A case of choroidal and vitreous hemorrhage following micropulse transscleral cyclophotocoagulation. J Glaucoma. 2020;29(7):e57–e59. doi:10.1097/IJG.0000000000001529
41. Kiyama Y, Nakashima K, Inour T. A case of primary open angle glaucoma with conjunctival laceration after micropulse transscleral cyclophotocoagulation. J Glaucoma. 2020;29(12):e135–e137. doi:10.1097/IJG.0000000000001658
42. Prager A, Anchala A. Suprachoroidal hemorrhage after micropulse cyclophotocoagulation diode therapy. AM J Ophthalmol Case Rep. 2020;18:100659. doi:10.1016/j.ajoc.2020.100659
43. Dhanireddy S, Yin HY, Dosakayala N, et al. Severe inflammation and hyphema after micropulse diode transscleral cyclophotocoagulation. J Glaucoma. 2020;29(6):e50–e52. PMID: 32287149. doi:10.1097/IJG.0000000000001508
44. Garcia GA, Nguyen CV, Yelenskiy A, et al. Micropulse transscleral diode laser cyclophotocoagulation in refractory glaucoma: Short-term efficacy, safety, and impact of surgical history on outcomes. Ophthalmol Glaucoma. 2019;2(6):402–412.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
