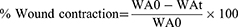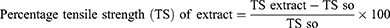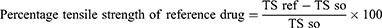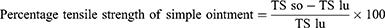Back to Journals » Journal of Experimental Pharmacology » Volume 14
Evaluation of Wound Healing Activity of 80% Methanol Root Crude Extract and Solvent Fractions of Stephania abyssinica (Dill. & A. Rich.) Walp. (Menispermaceae) in Mice
Authors Yiblet TG, Tsegaw A, Ahmed N, Dagnew SB, Tadesse TY , Kifle ZD
Received 28 February 2022
Accepted for publication 11 July 2022
Published 8 August 2022 Volume 2022:14 Pages 255—273
DOI https://doi.org/10.2147/JEP.S364282
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Paola Rogliani
Tesfagegn Gobezie Yiblet,1 Asegedech Tsegaw,2 Nejat Ahmed,3 Samuel Berihun Dagnew,1 Tesfaye Yimer Tadesse,1 Zemene Demelash Kifle2
1Department of Pharmacy, College of Medicine and Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia; 2University of Gondar, College of Medicine and Health Sciences, School of Pharmacy, Department of Pharmacology, Gondar, Ethiopia; 3Department of Pathology, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Tesfagegn Gobezie Yiblet, Tel +251935462779, Email [email protected]
Background: The root of Stephania abyssinica (Dill. and A. Rich.) Walp. (Menispermaceae) is traditionally used to treat wounds. Despite the fact that there have been in vitro studies and claims supporting wound healing, there has been no scientific data on the in vivo wound healing activities of the root of S. Abyssinica.
Objective: The aim of the study was to evaluate the wound healing activity of 80% methanol root extract and solvent fractions of S. Abyssinica in mice.
Methods: The roots of S. Abyssinica were air dried, ground and macerated by 80% methanol three times successively. The crude extract was fractionated by water, hexane and ethyl acetate separately. The acute dermal toxicity test was done by applying 2000 mg/kg of the 10% w/w crude extract. Wound healing activity of crude extract was evaluated on excision, incision and burn wound models, while the fractions were evaluated on excision wound model only.
Results: In mice, an acute dermal toxicity test of 2000 mg/kg of the 10% w/w crude extract was found to be safe. The 10% w/w crude extract ointment (CEO) produced significant (p < 0.001) wound contraction from 4th to 16th post wounding days, and the 5% w/w CEO were significant (p < 0.01) wound contraction on 10th post wounding day as compared to simple ointment (SO) treated group on excision wound. On burn wound models, the CEO showed highly significant (p < 0.001) from the 6th post wounding days onwards. The tensile strength was increased significantly (p < 0.001) by the CEO treated mice as compared to the untreated group and SO group.
Conclusion: The data obtained from this study showed 80% methanol crude extract, the aqueous and the 10% w/w ethyl acetate fraction possessed better wound healing activities, and decreased period of epithelialization.
Keywords: wound healing, Stephania abyssinica, wound models, Ethiopia
Introduction
The different organs of the body have different structures and functions.1 Mechanical, thermal, chemicals, or radiation therapy can induce wounds by destroying the integrity of skin epithelial tissue and disrupting the structure and function of underlying normal tissue.2 Depending on how long it takes for a wound to heal, it might be classified as chronic or acute. Wounds that demonstrate delayed healing 12 weeks after the initial insult are termed chronic wounds, often as a result of prolonged pathological inflammation.3 The wounding of tissue causes a coordinated physiological response that initiates the wound healing processes of hemostasis, inflammation, proliferation, and remodeling.2 The most common treatment options are infection prevention or treatment (eg, antibiotics), debridement, dressing, and irrigation.4
S. Abyssinica (Dill. and A. Rich.) Walp (Figure 1) belongs to the family Menispermaceae and the genus Stephania. It is distributed in tropical and subtropical Asia, parts of Africa and Oceania.5 This plant has different local names in Ethiopia as Yeayit Hareg, Etse Iyesus, chewchawit (in Amharic.), Hidda kalaalaa (in Oromic.).6 S. abyssinica is traditionally used to treat different human ailments in different areas of Ethiopia such as wounds, unwanted pregnancy, swelling, anthrax, common cold, blackleg, external tumor, and rabies.7–15 Hepatoprotective, antioxidant, antibacterial, antimalarial, anti-inflammatory and analgesic activities of the plant were done.16–20 The phytochemical screening of the root showed the presence of alkaloids, flavonoids, saponins, and phenols, but no tannins, coumarins, and anthraquinones.16 Despite this study and claims, no scientific data reported so far about its wound healing activity of the study plant on animals. The present study was, therefore, conducted to investigate the wound healing activities of S. Abyssinica root.
 |
Figure 1 Photograph of the Stephania abyssinica taken from site of collection. |
Materials and Methods
Drugs, Chemicals Supplies, and Equipment
Methanol absolute (Taflen industries, Ethiopia), n-hexane (Alpha Chemika, India), ethyl acetate (Alpha Chemika, India), white soft paraffin (Ethiopian pharmaceuticals manufacturing, SH.CO), wool fat (Blulux Laboratories Pvt. Ltd, India), hard paraffin (Lab tech chemicals), cecostearyl alcohol (Blulux Laboratories Pvt. Ltd, India), 2% nitrofurazone skin ointment (Shanghai General Pharmaceuticals Co., Ltd., China), ketamine hydrochloride injection USP (Neon Laboratories limited, India), diazepam injection (Gland pharm limited, India), 70% alcohol (Yilmana chemical production, Ethiopia), bee wax (Bo International, Delhi, India), hematoxylin and eosin (Alpha Chemika Maharashtra, India), Glacial Acetic Acid (Blulux laboratories Pvt., Ltd, India), Iodine (Riedel Laboratories Pvt., Ltd, Germany), sulphuric acid (Blulux Laboratories Pvt. Ltd, India), lead acetate (Guangdong Guanghua Chemicals, China), Potassium Iodide (Caliber Engineering, India), hydrochloric acid (Pentokay Laboratories Pvt., Ltd, India), Ammonia (Blulux Laboratories Pvt. Ltd, India), Ethanol (Hayman Laboratories Pvt. Ltd, England), chloroform (Blulux Laboratories Pvt. Ltd, India), ferric chloride (Fin Chem Industries, India), Wagner’s reagent (Research –Lab, Fin Chem Industries, India), and 10% formalin solution were procured from pharmacy, suppliers and all the reagents were of analytical grade.
Plant Material Collection and Preparation
Fresh roots of S. Abyssinica were collected from Aba Aregay village located in Farta District, South Gondar Zone of the Amhara Regional State, and North-central Ethiopia in February 2021. The plant material was identified and authenticated by a botanist from Biology Department, College of Natural and Computational Sciences, Debre Tabor University and deposited with a voucher specimen number (TG1) for future reference. The fresh roots were washed with tap water until the dirty part removed and air dried under shade in the house at room temperature. The dried roots were pounded into a coarse powder using a mortar and pestle.
Experimental Animals
For the evaluation of wound healing in the three models (incision, excision, and burn), healthy Swiss albino mice of either sex (20–30 g, 6–8 weeks old) were used for the study. The animals were given full access to food and water, and were kept in an individual cage with a regular 12 hour light/dark cycle at room temperature. The mice were acclimatized for five days to the laboratory environment before the experiment began. Mice were handled according to the international and East African care and usage criteria throughout the experiment.21,22
Preparation of the Crude Extract
In an Erlenmeyer conical flask, one kilogram of the coarsely powdered root was macerated in 80% methanol (1:6 ratio)23 for three days with regular shaking. After three days, the mixture was filtered with sterile gauze followed by Whatman filters paper No. 1. To increase the extract yield, the residue was again macerated twice with the same amount of methanol for a total of six days. The mixed filtrates were evaporated and concentrated by a rotatory evaporator and a drying oven at 40°C, respectively. Finally, the concentrated extract was frozen in a deep freezer and then freeze dried in a lyophilizer to remove the water from the extract. The final dried extract (131.20 gm) was stored in a refrigerator at −4°C until required.24,25
Solvent Fractionation of Crude Extract
Fractionation was performed by hexane, ethyl acetate, and water. In a given amount of distilled water, 75 mg of crude extract (1:6 ratio)26 was suspended in a separatory funnel. An equal amount of hexane was added to the mixture. Then, the mixture was allowed to mix well by shaking for 3–5 minutes at room temperature. Two phases, the hexane phase at the upper and the aqueous phase at the lower were formed. After a clear layer was formed between the two phases, the hexane fraction was separated from the mixture and collected in a separate container. This was done two times in the same manner. After the separation of the hexane fraction, an equal amount of ethyl acetate was mixed with the aqueous residue, and the ethyl acetate fraction was separated. Like the previous procedure, it was done two times and the ethyl acetate fractions then separated from the aqueous fractions. The two fractions (ethyl acetate and hexane) were dried and concentrated at 40°C through a dry oven, while the aqueous fraction was frozen in refrigerator and dried in with a lyophilizer. Finally, the dried fractions (5.81g hexane, 20.63g ethyl acetate, and 40.95g aqueous) were stored in a tight container at 4°C until use.27
Ointment Formulation
Simple ointment (Table 1), the 80% methanol crude extract and the three fractions (hexane, ethyl acetate and aqueous) were prepared according to the master formula taken from British pharmacopeia.28
 |
Table 1 Simple Ointment Formula |
The medicated ointments of crude extract and solvent fractions were prepared as 5% w/w and 10% w/w ointments. The 10 g and 5 g crude extract was added to 90 gm and 95 gm simple ointment, respectively, to prepare 10% w/w and 5% w/w methanol crude extract ointment. Similarly, the 5% w/w and 10% w/w solvent fraction ointments were prepared by mixing 1.5 g and 3 g of each fractions mixed into 28.5 g and 27 g of simple ointment base, respectively, to yield 30 g medicated ointment of the fractions.
Phytochemical Screening
Qualitative preliminary phytochemical screening tests were done for Stephania abyssinica root crude extract and aqueous, ethyl acetate and hexane fractions as per the standard methods so as to determine the presence or absence of secondary metabolites23,29,30 (Annex 1)
Acute Dermal Toxicity Test
The acute dermal toxicity test was done according to OECD guideline 404.31 Three adult female Swiss albino mice with normal skin texture were individually caged and acclimatized to laboratory conditions for 5 days before the test began. Before 24 hours of conducting the study, 10% of the body surface of mice from the dorsal area of the trunk was shaved after proper anesthesia with ketamine 50 mg/kg intrapertonially. In the first test, a single dose of 2000 mg/kg (maximum limit dose) of S. Abyssinica 10% w/w crude extract was applied evenly over the shaved region in a single mouse. Within 60 minutes of application of the ointment, the mice’s responses were examined, and recorded. A confirmatory test was performed on other two mice after 24 hours of observation of the initial test. After the residual ointment was removed, the mice were observed daily for the development of any adverse skin reaction in terms of edema and erythema for 14 days.32
Grouping and Dosing of Experimental Animals
A total of 126 mice were used to evaluate wound healing activity of the root crude extract and solvent fractions of S. Abyssinica on excision, incision, and burn wound models. Each wound model had four groups and one additional group on incision wound model,24 and six mice per group. In all three wound models, group I treated with simple ointment (served as a negative control), whereas groups II, III, and IV treated with 5% w/w, 10% w/w crude extract ointment (CEO), and nitrofurazone 0.2% ointment (served as a positive control), respectively. Group V in the incision wound model was left untreated.24,25
Solvent fractions (ethyl acetate, hexane and aqueous) were evaluated on excision wound model with eight groups (each group had 6 mice) I–VIII. Groups I, II, III, IV, V, VI, VII and VIII were treated with simple ointment, nitrofurazone, 5% w/w hexane fraction (HF), 10% w/w HF, 5% w/w ethyl acetate fraction (EAF), 10% w/w EAF, 5% w/w aqueous fraction (AQF), and 10% w/w AQF, respectively.33 The mice were sacrificed with high dose of ketamine (four times the anesthetic dose) and buried in a controlled environment at the end of the experiment.
Wound Healing Evaluation
Circular Excision Wound Model
The wound healing activity of crude extract and solvent fractions were evaluated on circular excision wound. The wound was created on the dorsothoracic region of mice after proper anesthesia with diazepam (5 mg/kg) and ketamine (50 mg/kg) intrapertonially.34 After labeling with a permanent marker, a circular excision of 300 mm235 was excised using forceps and sterilized scissors. The day when the wound was created was considered as wounding day 0. The mice were treated on a daily basis beginning on the first post wounding day, and continuing until the test group was healed. The healing activity was examined using parameters such epithelialization time, histopathological study, and percentage of wound contraction (which was measured every two post wounding days using 1mm2 graph paper and a transparent sheet).36 Percent wound contraction was calculated by the following formula:
Where WA0- wound area at day 0, WAt- wound area at day t
t = number of days, ie, 2nd, 4th, 6th, 8th, 10th, etc.
Epithelialization refers to the number of days it takes for dead tissue remains to fall off without leaving a raw wound, as evaluated at the end of the study.37
Histopathological investigation: 3µm thickness samples of tissues were obtained from each group after mice were scarified with high dose of ketamine (four times the anesthetic dose) and stored in 10% buffered formalin. It was then processed and stained by Hematoxylin and Eosin. Finally, the tissue was analyzed by pathologist and graded for the level of inflammatory cells, collagen fibers, fibroblast, mononuclear/polymorph nuclear cells and angiogenesis as none (-), low (+), moderate (++) and high (+++) concentration.38
Linear Incision Wound Model
The incision wound was created in 30 mice after suitable anesthesia with diazepam and ketamine. The dorsal fur of each mouse was shaved and a 3 cm long, longitudinal paravertebral incision was done24 (Figure 2). The edges of the wound were closed together with silk no. 00 round and curved needle (no. 11). Mice were treated with extract (group 1 with 5% and group 2 with 10%), simple ointment (group 3), and nitrofurazone (group 4) once daily for 9 days starting from the 1st post-wounding day and one group was left untreated (group 5) as described on the grouping and dosing section. The suture was removed on the 8th day of post wounding and the degree of healing was assessed by measuring the tensile strength on the 10th day by using continuous water flow technique.35,39 The percentage of tensile strength for each group with their respective treatment was measured as follows:
 |
Figure 2 Incision wound tensile strength measurement by water flow technique. |
Where so-simple ointment, lu- left untreated, ref-reference
Burn Wound Model
A total of 24 mice were anesthetized and shaved in the same manner as in excision and incision wound models. A partial-thickness burn wound was created by pouring hot molten wax at 80°C into a metal cylinder of 300 mm2 circular openings placed on the shaven area of the mice and stayed until the wax gets solidified for 10 minutes. After the wax solidified, the metal cylinder with wax that adhered to the skin was removed. The mice were treated daily with the extracts, simple ointment, and nitrofurazone with their respective groups. Wound healing was assessed by the parameters like the circular excision wound model.35
Statistical Analysis
The data obtained from this experiment were expressed as mean ± SEM. Then, it was analyzed by one way ANOVA, followed by post hoc Tukey’s test with SPSS version 25 software. At confidence level 95% and p-value <0.05, the difference was considered as statistically significant.
Result
Phytochemical Screening
The different secondary metabolites produced from 80% methanol crude extract and solvent fractions revealed by phytochemical screening. Among the solvent fractions, the aqueous fraction showed the secondary metabolites present in the crude extract (Table 2).
 |
Table 2 Secondary Metabolites from Phytochemical Screening |
Acute Dermal Toxicity
The topical application of 2000 mg/kg of the 10% w/w methanol crude extract ointment was safe. There was no edema or erythema, as well as no evidence of any toxicity or death, during the 14 days of monitoring and observation, as indicated in Figure 3.
 |
Figure 3 Acute dermal toxicity test. Before (A) and after (B) application of crude extract ointment. |
Excision Wound Model
Wound contraction
The topical application of the crude extract ointments (5% w/w and 10% w/w) showed a higher percentage of wound contraction (Table 3). The 10% w/w crude extract-treated group and the positive control group showed substantial wound contraction (p < 0.05) starting from the 2nd post-wounding day onwards compared to the simple ointment treated group. However, the 5% crude extract ointment showed significant wound contraction (p < 0.001) from the 4th day onwards. No apparent difference was observed between the treatment groups on all the treatment day, except on the 10th day, whereby the contraction induced by the 5% was significantly lower (p < 0.05) than the 10% and the standard.
 |
Table 3 Effect of Topical Application of the Crude Root Extract Ointment on Excision Wound |
Wound contraction percentage of 5% w/w and 10% w/w treated mice was higher on the 14th post wounding day that is 96.33% and 97.78%, respectively, and on the 16th day 99.22% for simple ointment and 100% of crude extract. This wound contraction percentage on the 14th and 16th post-wounding days were significant (p < 0.001) as compared to the simple ointment treated group (Figures 4 and 5).
 |
Figure 4 Percent wound contraction induced by crude extract on excision wound. Abbreviations: CEO, crude extract ointment; NF, nitrofurazone; SO, simple ointment. |
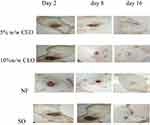 |
Figure 5 Photograph of excision wound on day 2, 8, and 16. |
Period of Epithelialization
Both crude extracts and nitrofurazone treatment produced a significant reduction in the period of epithelialization (p < 0.001) as compared to the group treated with simple ointment. There was no significant decrease in the period of epithelialization between the crude extracts and standard drug-treated groups. The percentage reduction in epithelialization period was 16.65%, 21.65%, and 21.84% for 5% w/w, 10% w/w crude extract ointment, and 0.2% nitrofurazone, respectively (Table 4).
 |
Table 4 Effect of Topical Application of the Crude Root Extract on Epithelialization Period of Excision Wound |
Histopathological Analysis
Histopathological analysis was done on the excision wound on day 16th. The crude extract-treated groups had a moderate to the high concentration of fibroblasts, collagen fibers, blood capillaries (angiogenesis), and few inflammatory cells, whereas the negative control-treated groups had a higher concentration of inflammatory cells (Table 5 and Figure 6).
 |
Table 5 Histopathological Qualitative Determination of Wound Healing Processes of Crude Extract on Excision Wound |
Incision Wound Model
The 5% w/w, 10% w/w crude extract, and 0.2% nitrofurazone treated groups produced statistically significant (p < 0.001) increase in tensile strength measured by water flow technique as compared to the simple ointment and untreated groups. In comparison to the untreated group, the simple ointment treatment group showed a significant (p < 0.05) increase in mean breakage of the repaired tissue. Percentage of tensile strength of 5% w/w, 10% w/w and 0.2% nitrofurazone was 53.35%, 61.01%, and 61.11%, respectively (Table 6).
 |
Table 6 Effect of 80% Crude Extract on Tensile Strength on Incision Wound |
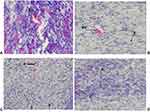 |
Figure 6 Photograph of histological section on excision wound tissues treated by crude extract and controls A-nitrofurazone, B-10% crude extract, C-5% crude extract and D-simple ointment. |
Burn Wound Model
Wound Contraction
The 10% w/w CEO produced significant wound contraction (p < 0.05) on 2nd, (p < 0.01) on 4th, and (p<0.001) from 6th post wounding days onwards when compared to the negative control group (Table 7). Similarly, it produced significant wound contraction (p < 0.05) on 6th and 8th, (p < 0.01) on 10th post wounding days as compared to 5% w/w crude extract treated groups.
 |
Table 7 Effect of Crude Extract on Burn Wound |
Groups treated with nitrofurazone showed statistically significant contraction (p < 0.01) on 8th post-wounding day as compared to 5% w/w crude extract treated groups and 10% w/w treated groups showed significant (p < 0.05) on 6th and 8th, (p < 0.01) on 10th post wounding days as compared to 5% w/w crude extract treated groups.
On the other hand, 5% w/w crude extract showed significant wound contraction (p < 0.05) on 4th and 6th, (p < 0.01) on 8th, and (p < 0.001) on 10th post wounding days onwards as compared to simple ointment treated mice.
The wound was completely closed on day 20 for groups treated with 10% w/w CEO and nitrofurazone and on day 22 for groups treated with 5% w/w CEO (Figures 7 and 8).
 |
Figure 7 Percent wound contraction induced by crude extract on burn wound. |
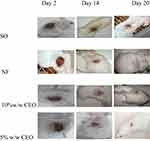 |
Figure 8 Wound area of burn wound on day 2, 14, and 20. |
Period of Epithelialization
The complete epithelialization period was significantly shorter (p < 0.001) for crude extracts and nitrofurazone treated mice as compared to negative control in partial thickness burn wound. There was no significant difference of epithelialization period between crude extracts and the standard drug (Table 8).
 |
Table 8 Methanol Crude Extract Effect on Period of Epithelialization of Burn Wound |
Histopathological Analysis
The histopathological analysis on burn wound was done on 20th post wounding day on each treatment group. The result showed that the crude extract treated groups had more fibroblasts, collagen fibers, blood capillaries (angiogenesis), and few inflammatory cells, whereas the negative control treated groups had a higher concentration of inflammatory cells and a lower concentration of collagen fibers, fibroblasts, and blood capillary cells (Table 9 and Figure 9).
 |
Table 9 Histopathological Qualitative Determination of Wound Healing Processes of Crude Extract on Burn Wound |
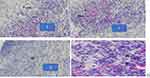 |
Figure 9 Photograph of histological section on burn wound tissues treated by crude extract. |
Wound Healing Activity of Solvent Fractions on Excision Wound Model
Wound Contraction
Mice treated with 10% w/w AQF showed statistically significant wound contraction (p < 0.001) from the 4th post wounding days onwards as compared to the negative control, again significant wound contraction (p < 0.01) on the 2nd and 4th, (p < 0.001) from the 6th post wounding day onwards when compared to groups treated with 5% w/w HF. There was also statistical significant as compared to 10% w/w HF treated mice which was (p < 0.05) on the 4th and 6th, (p < 0.01) on 8th post wounding days onwards.
Significant wound contraction (p < 0.05) on 2nd to 6th, (p < 0.01) from 8th post wounding days onwards was seen on groups treated with nitrofurazone as compared to groups treated by 5% w/w HF, also significant (p < 0.05) on 8th and 10th, (p < 0.01) from 12th to 16th post wounding days as compared to 10% w/w HF treated groups. There was significant wound contraction (p < 0.05) on 8th and 10th, (p < 0.01) from 12th to 16th post wounding days compared to 5% w/w EAF treated mice.
Ethyl acetate 10% w/w treated groups showed highly significant (p < 0.001) from 6th to 16th and (p < 0.001) from 12th and 16th post wounding days as compared to negative control and 5%w/w HF treated mice, respectively.
The wound was completely closed on the 16th day in groups treated with 10% AQF, 10% EAF and nitrofurazone (Table 10, Figure 10).
 |
Table 10 Stephania Abyssinica Root Extract Solvent Fractions Effect on Excision Wound |
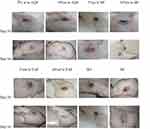 |
Figure 10 Wound area of solvent fractions and the control groups at day 10 and 16. |
Period of Epithelialization
The AQF, 10% w/w EAF, and nitrofurazone produced significant (p < 0.001) and 5% w/w EAF and 10% HF (p < 0.01) decreased period of epithelialization as compared to simple ointment treated groups (Table 11). Mice treated with 10% w/w AQF, 10% w/w EAF and nitrofurazone were significant (p < 0.001) and 5% w/w AQF significant (p < 0.01) as compared to 5% w/w HF treated mice. There were no significance difference in period of epithelialization between AQF, 10% EAF, and nitrofurazone.
 |
Table 11 Effect of Solvent Fractions on Period of Epithelialization |
The 10% w/w AQF had the shorter period of epithelialization (15.17±0.40 days); on the other hand, the 5% w/w HF treated mice had longer period of epithelialization (18.50±0.56 days).
Histopathological Analysis
The histopathological analysis of solvent fraction treated groups taken from granulation tissue revealed that the 5% w/w and 10% w/w AQF, 10% w/w EAF had more fibroblast, collagen, and blood capillaries, and few inflammatory cells, while the HF and the simple ointment treated groups showed a high level of inflammatory cells (Table 12).
 |
Table 12 Histopathological Qualitative Determination of Wound Healing Processes of Solvent Fractions on Excision Wound Model |
Discussion
The medicinally active component of S. Abyssinica root was isolated from the insoluble cellular marc by the maceration technique with 80% methanol. From different extraction techniques, maceration is convenient, cost-effective, widely used, and more applicable for the extraction of medicinal plants. Maceration with 80% methanol was the most widely used solvent and yield the maximum flavonoid and phenolic content.40,41
To ensure the prolonged and constant release of active ingredients in the wound area, both the crude extract and fractions were prepared as ointments. Treating wound by topical application is preferable due to ease of removal, sufficient concentration in the applied area, and low systemic side effect. This promotes wound healing. The ointment ingredients, wool fat, and cecostearyl alcohol provide thickness and stabilization of the ointment, while soft and hard paraffin prevents the skin from drying.24
Acute dermal toxicity test showed there were no signs of toxicity during the 14 days of monitoring and observation. Therefore, the S. Abyssinica root extract might be used safely as a topical application to treat wounds.
In this study, wound healing of crude extracts and solvent fractions on excision and burn wound models were assessed by the rate of wound contraction, period of epithelialization, and histopathological analysis, while tensile strength of the incision wound model. Rapid wound closure, a shorter time for a wound to heal, and wound breaking strength are all factors that contribute to adequate and timely wound healing.42
The decreased period of epithelialization, increased percentage of wound contraction, increase tensile strength, more collagen, fibroblast, new blood cells and low level of inflammatory cells by crude extracts might be associated with the individual or additive effect of bioactive constituents or secondary metabolites including, alkaloids, terpenoids, steroids, flavonoids, saponins, and phenols present in plant extract, which are obviously promoting wound healing.
Terpenoids facilitate wound healing and decrease the period of epithelialization due to their astringent, antimicrobial effect by disrupting bacterial membranes, antioxidant activity by preventing DNA damage, lipid peroxidation, and inactivation of free-radical scavenging enzymes caused by free radicals.43 Alkaloids have wound healing activity of their antibacterial and anti-inflammatory effect by blocking the synthesis of inflammatory mediators such as prostaglandins from arachidonic acid.34 The lowering of the synthesis of inflammatory mediators facilitates the wound phase enter into the maturation phase and the wound healed rapidly. The anti-inflammatory, free radical scavenging activity thus prevents cell damage, enhances cellular vascularity which decreases cell death and antimicrobial activity of flavonoids are important effect that promotes wound healing and shortens the period of epithelialization.1,32 The wound-healing effect of steroids and phenolic compounds could be due to their antimicrobial and anti-inflammatory activity.44
The fast wound contraction in excision and burn wound models by the extract could be related to greater collagen fibers, fibroblasts, and capillary blood cells as indicated by histological investigation. The fibroblasts are activated and differentiated to myofibroblasts which regulate the formation and growth of other cells and new epithelial tissue over the wound area. The myofibroblast increases the pulling force between the cells on the opposite side of the wound which increases the rate of wound contraction. Neovascularization is necessary to promote wound healing by supplying oxygen to the wound site, which is essential for cell metabolism, particularly the synthesis of energy and necessary nutrients required for wound repair.45
The parameter used to assess the effect of crude extract on the incision wound model is tensile strength. Tensile strength measures the resistance of breakage of repaired tissue against tension, as well as evaluates how good the mended tissue is.46 This increased resistance to breaking could be related to the synthesis of collagen by fibroblast at the wound site. S. Abyssinica root crude extract’s increased tensile strength might be due to collagen synthesis and stabilization achieved by plant extract.47
Period of epithelialization was another parameter to assess the wound healing activity of S. Abyssinica root on excision and burn wound models. The significantly decreased period of epithelialization on these models by crude extracts might be related to keratinocyte proliferating and migration across the wound area. Wound contraction decreases the distance of epithelial cells to migrate toward each other, thus decreasing the period of epithelialization.1
From a previous study, the 80% methanol root extract of the study plant had antibacterial and antioxidant activity.16,17 The wound healing activity of the study plant might be enhanced by the antibacterial and antioxidant activity of the plant.
The plant antioxidant activity scavenges free radicals, preventing damage to cellular membrane lipids, enzymes, proteins, and nucleic acids of the healing wound, therefore promoting the wound healing process.48 Higher wound contraction, a shorter period of epithelialization, and an increase in resistance to breakage of the repaired tissue against tension were observed in crude extract-treated groups, which could be associated with the plant antioxidant effect.16
Antibacterial activity of 80% methanol extract of S. Abyssinica root was found in a previous study on Staphylococcus aureus,17 this antimicrobial activity cleans the wound from infection and cellular debris. Bacterial infection and endotoxins boost proinflammatory cytokines (interleukin-1 and TNF-α). The inflammatory phase is prolonged when bacteria and bacterial endotoxins are present. If this phase is prolonged, the wound will become chronic and fail to heal.49 The antimicrobial activity of S. Abyssinica root could be related to a reduction in bacterial endotoxin load around the wound area, which improved fibroblast and epithelial cell proliferative activity, thus decreasing the epithelialization period.34
The solvent fractions had a different wound healing activity measured by the period of epithelialization, histopathological analysis, and wound contraction. Aqueous fractions showed better wound contraction, a shorter period of epithelialization, lower inflammatory cells, more collagen fibers, and fibroblast and blood capillaries as compared to simple ointment and hexane fractions. These effects of AQF might be due to the presence of more secondary metabolites or most of the bioactive components are water-soluble. These bioactive constituents enhance wound healing by their anti-inflammatory, astringent, antibacterial, and antioxidant activity. Furthermore, the presence of polar components in the aqueous portion causes the non-polar base ointment to release faster and more effectively. This release promotes the absorption of the AQF contents, resulting in a faster rate of wound healing and a shorter epithelialization period.44
The minimal wound contraction, longer period of epithelialization, more inflammatory cells, and low level of collagen, fibroblast, and new blood cell (angiogenesis) in the hexane fraction, possibly due to differences or low concentration of secondary metabolites or low amount of non-polar constituents present. Besides the slower release of its non-polar content from the non-polar ointment ingredients might be the reason for its minimal effect on wound healing.42
The wound healing activity of the 10% w/w ointments of all fractions was better than the wound healing of the 5% w/w fractions, which could be related to a dose-dependent antibacterial, astringent, anti-inflammatory, antioxidant, collagen synthesis, fibroblast proliferation, and angiogenesis effect of secondary metabolites.
Conclusion
From the present study, the topical application of the limit test dose 2000 mg/kg of the 10% w/w 80% methanol crude extract of Stephania abyssinica root was safe. The crude extract of Stephania abyssinica root and the aqueous and ethyl acetate fraction activity by increasing wound contraction, had better wound healing tensile strength, and shortening period of epithelialization. In the aqueous fraction, wound contraction was higher and the epithelialization period was shorter than other two fractions.
Abbreviations
FGF, Fibroblast Growth Factor; MMPs, Matrix Metalloproteases; NO, Nitric Oxide; NSAIDs, Non-steroidal Anti-inflammatory Drugs; OECD, Organization for Economic Cooperation and Development; PDGF, Platelet-Derived Growth Factor; PGE2, Prostaglandin E2; ROS, Reactive Oxygen Species; TGF, Transforming Growth Factor; TGF-β1, Transfer Growth Factor β1; TNF-α, Tumor Necrosis Factor α; TS, Tensile Strength; VEGF, Vascular Endothelial Growth Factor.
Data Sharing Statement
The datasets used and/or analyzed during the current work are available from the corresponding author up on a reasonable request.
Ethical Approval
Ethical clearance was requested and obtained from Department of Pharmacology, College of Medicine and Health Science, University of Gondar with reference number SOP4/104/13. Throughout the experiment, the mice were handled in line with the guide for the care and use of laboratory animals (22).
Acknowledgment
Authors are grateful to University of Gondar for material support and for allowing to use the laboratory facility and Debre Tabor University for providing some financial support.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. TG conceived the idea, drafted the proposal, collected the plant materials and conducted the actual laboratory work. TG, ZDK and TYT prepared and critically reviewed the final manuscript for publication. AT, SBD, and NA were involved in the design and implementation stage of the study, and revising the manuscript critically for important intellectual content. All authors read and approved the final version of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maver T, Kurečič M, Smrke DM, Kleinschek KS, Maver U. Plant-derived medicines with potential use in wound treatment. Herbal Med. 2018. doi:10.5772/intechopen.72813
2. Enoch S, Leaper DJ. Basic science of wound healing. Surgery. 2008;26(2):31–37. doi:10.1016/j.mpsur.2007.11.005
3. Young A, McNaught C-E. The physiology of wound healing. Surgery. 2011;29(10):475–479. doi:10.1016/j.mpsur.2011.06.011
4. Ghanem E, Heppert V, Spangehl M, et al. Wound management. J Arthroplasty. 2014;29(2):84–92. doi:10.1016/j.arth.2013.09.041
5. De Wet H, Struwig M, Van Wyk B-E. Taxonomic notes on the genus Stephania (Menispermaceae) in Southern Africa. S Afr J Bot. 2014;95:146–151. doi:10.1016/j.sajb.2014.09.006
6. Lemma AA. College Of Natural And Computational Sciences [doctoral dissertation]. Ethiopia: University of Gondar; 2017.
7. Kefalew A, Asfaw Z, Kelbessa E. Ethnobotany of medicinal plants in Ada’a District, East Shewa Zone of Oromia regional state, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):1–28. doi:10.1186/s13002-015-0014-6
8. Bussa N, Belayneh A. Traditional medicinal plants used to treat cancer, tumors and inflammatory ailments in Harari Region, Eastern Ethiopia. S Afr J Bot. 2019;122:360–368. doi:10.1016/j.sajb.2019.03.025
9. Kassa Z, Asfaw Z, Demissew S. Ethnobotanical study of medicinal plants used by the local people in Tulu Korma and its surrounding areas of Ejere District, Western Shewa Zone of Oromia Regional State, Ethiopia. J Med Plants Stud. 2016;4(2):24–47.
10. Megersa M. Ethnobotanical Study of Medicinal plants in Wayu Tuka Wereda, East Wollega zone of Oromia Region, Ethiopia [MSc Thesis]. Addis Ababa University; 2010.
11. Atnafu H, Awas T, Alemu S, Wube S. Ethnobotanical study of medicinal plants in selale mountain ridges, North Shoa, Ethiopia. Int J Biodivers. 2018;2(6):567–577.
12. Getaneh S, Girma Z. An ethnobotanical study of medicinal plants in Debre Libanos Wereda, Central Ethiopia. Afr J Plant Sci. 2014;8(7):366–379. doi:10.5897/AJPS2013.1041
13. Amde Lemma A. Ethnobotanical Study Of Traditional Medicinal Plants In Debark District, North Gondar, Ethiopia [doctoral dissertation]. 2017.
14. Kebebew M. Diversity, knowledge and use of medicinal plants in Abay Chomen district, Horo Guduru Wollega zone, Oromia region of Ethiopia. J Med Plant Res. 2017;11(31):480–500. doi:10.5897/JMPR2016.6274
15. Amenu E. Use And Management Of Medicinal Plants By Indigenous People Of Ejaji Area (Chelya Woreda) West Shoa, Ethiopia: An Ethnobotanical Approach [M Sc Thesis]; 2007.
16. Washe AP, Fanta D. Hepatoprotective activities and bioactive constituents of Stephania abyssinica. Int J Pharm Res. 2016;10:1–9.
17. Chakraborty A, Asres K, Stipsits S, Eibl U, Brantner A. Biological properties of Stephania abyssinica roots. Pharm Pharmacol Lett. 2000;10(1):19–22.
18. Anyango OR. Anti-Malarial Activity And Phytochemical Studies Of Cissampelos Mucronata And Stephania Abyssinica [master thesis]. Kenyatta University; 2011.
19. Birru EM, editor. In vivo antimalarial activity of the 80% methanol leaves extract and solvent fractions of Stephania abyssinica Quart.-Dill. & A. Rich. (Menispermaceae) against Plasmodium berghei infection in mice.
20. Leyikun T. Evaluation of the Analgesic and Anti-Inflammatory Activities of 80% Methanol Leaf Extract of Stephania abyssinica (Quart.-Dill. & A. Rich.) Walp. (Menispermaceae) in Mice [doctoral dissertation]. Addis Ababa University; 2015.
21. Rigalli A, Di Loreto V. Experimental Surgical Models in the Laboratory Rat. CRC Press; 2016.
22. National Research Council. Guide for the Care and Use of Laboratory Animals. National Academies Press; 2010.
23. Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem. 2014;2(5):115–119.
24. Demilew W, Adinew GM, Asrade S. Evaluation of the wound healing activity of the crude extract of leaves of Acanthus polystachyus Delile (Acanthaceae). Evid-Based Complementary Altern. 2018;2018:1–9. doi:10.1155/2018/2047896
25. Belachew TF, Asrade S, Geta M, Fentahun E. In vivo evaluation of wound healing and anti-inflammatory activity of 80% methanol crude flower extract of Hagenia abyssinica (Bruce) JF Gmel in mice. Evid-Based Complementary Altern. 2020;2020. doi:10.1155/2020/9645792
26. Upadhayay V. Plant extraction; 2016.
27. Kumar V, Khan AA, Nagarajan K. Animal models for the evaluation of wound healing activity. Int Bull Drug Res. 2013;3(5):93–107.
28. Tallo D. The British National Formulary. Nursing Stand. 2016;31(10):64–65. doi:10.7748/ns.31.10.64.s47
29. Mengie T, Mequanente S, Nigussie D, Legesse B, Makonnen E. Investigation of wound healing and anti-inflammatory activities of solvent fractions of 80% methanol leaf extract of Achyranthes aspera L. (Amaranthaceae) in Rats. J Inflammation Res. 2021;14:1775. doi:10.2147/JIR.S298244
30. Yadav R, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol. 2011;3(12):10–14.
31. No OT. 404: Acute Dermal Irritation/Corrosion. OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing; 2015.
32. Tekleyes B, Huluka SA, Wondu K, Wondmkun YT. Wound healing activity of 80% methanol leaf extract of Zehneria scabra (Lf) Sond (Cucurbitaceae) in mice. J Exp Pharmacol. 2021;13:537. doi:10.2147/JEP.S303808
33. Belachew TF, Asrade S. In vivo evaluation of wound healing and anti-inflammatory activity of 80% methanol crude flower extract of Hagenia abyssinica (Bruce). J F Gmel in Mice. 2020;2020:9645792.
34. Ayal G, Belay A, Kahaliw W. Evaluation of wound healing and anti-inflammatory activity of the leaves of Calpurnia aurea (Ait.) Benth (fabaceae) in mice. Wound Med. 2019;25(1):100151. doi:10.1016/j.wndm.2019.100151
35. Thakur R, Jain N, Pathak R, Sandhu SS. Practices in wound healing studies of plants. Evid-Based Complementary Altern. 2011;2011:1–17. doi:10.1155/2011/438056
36. Srivastava P, Durgaprasad S. Burn wound healing property of Cocos nucifera: an appraisal. Indian J Pharmacol. 2008;40(4):144. doi:10.4103/0253-7613.43159
37. Begashaw B, Mishra B, Tsegaw A, Shewamene Z. Methanol leaves extract Hibiscus micranthus Linn exhibited antibacterial and wound healing activities. BMC Complement Altern Med. 2017;17(1):1–11. doi:10.1186/s12906-017-1841-x
38. Fahimi S, Abdollahi M, Mortazavi SA, Hajimehdipoor H, Abdolghaffari AH, Rezvanfar MA. Wound healing activity of a traditionally used poly herbal product in a burn wound model in rats. Iran Red Crescent Med J. 2015;17(9). doi:10.5812/ircmj.19960
39. Krishnaveni B, Neeharika V, Venkatesh S, Padmavathy R, Reddy BM. Wound healing activity of Carallia brachiata bark. Indian J Pharm Sci. 2009;71(5):576. doi:10.4103/0250-474X.58184
40. Butsat S, Siriamornpun S. Effect of solvent types and extraction times on phenolic and flavonoid contents and antioxidant activity in leaf extracts of Amomum chinense C. Int Food Res J. 2016;23(1):180–187.
41. Azwanida N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants. 2015;4(196):2167–2412.
42. Lambebo MK, Kifle ZD, Gurji TB, Yesuf JS. Evaluation of wound healing activity of methanolic crude extract and solvent fractions of the leaves of Vernonia auriculifera Hiern (Asteraceae) in mice. J Exp Pharmacol. 2021;13:677. doi:10.2147/JEP.S308303
43. Wang C-Y, Chen Y-W, Hou C-Y. Antioxidant and antibacterial activity of seven predominant terpenoids. Int J Food Prop. 2019;22(1):230–238. doi:10.1080/10942912.2019.1582541
44. Taddese SM, Gurji TB, Abdulwuhab M, Aragaw TJ. Wound healing activities of hydromethanolic crude extract and solvent fractions of Bersama abyssinica Leaves in mice. Evid-Based Complementary Altern. 2021;2021:1–20. doi:10.1155/2021/9991146
45. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi:10.1177/0022034509359125
46. Lodhi S, Jain AP, Rai G, Yadav AK. Preliminary investigation for wound healing and anti-inflammatory effects of Bambusa vulgaris leaves in rats. J Ayurveda Integr Med. 2016;7(1):14–22. doi:10.1016/j.jaim.2015.07.001
47. Liu H, Lin S, Xiao D, Zheng X, Gu Y, Guo S. Evaluation of the wound healing potential of Resina Draconis (Dracaena cochinchinensis) in animal models. Evid-Based Complementary Altern. 2013;2013. doi:10.1155/2013/709865
48. Singh M, Govindarajan R, Nath V, Rawat AKS, Mehrotra S. Antimicrobial, wound healing and antioxidant activity of Plagiochasma appendiculatum Lehmet Lind. J Ethnopharmacol. 2006;107(1):67–72. doi:10.1016/j.jep.2006.02.007
49. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17(2):91–96. doi:10.1097/00001432-200404000-00004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.