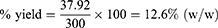Back to Journals » Hepatic Medicine: Evidence and Research » Volume 14
Evaluation of the Effect of Hydromethanolic Seed Extract of Lepidium sativum L. (Fetto) on Deep-fried Palm Oil Diet Induced Nonalcoholic Fatty Liver Disease on Male Swiss Albino Mice
Authors Tofik Ahmed E , Zawdie B , Nair SKP, Welde M , Mateos Husen T
Received 22 November 2021
Accepted for publication 29 January 2022
Published 22 February 2022 Volume 2022:14 Pages 1—12
DOI https://doi.org/10.2147/HMER.S350703
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Ebsa Tofik Ahmed,1 Belay Zawdie,2 Suresh Kumar P Nair,2 Mengistu Welde,2 Tigist Mateos Husen2
1Department of Biology, College of Natural and Computational Sciences, Mettu University, Mettu, Ethiopia; 2Division of Medical Biochemistry, Department of Biomedical Sciences, Institute of Health Sciences, Jimma University, Jimma, Ethiopia
Correspondence: Ebsa Tofik Ahmed, Tel +251921168204, Email [email protected]
Introduction: Nonalcoholic fatty liver disease (NAFLD) is the most prevalent disease due to a dramatic change in dietary habits, especially an increase in consumption of fat and carbohydrates in deep-fried foods.
Objective: The objective was to evaluate the effect of hydromethanolic seed extract of Lepidium sativum on deep-fried palm oil diet induced NAFLDon male mice.
Methods: An experimental study design was conducted. Twenty-four male mice aged 8 to 10 weeks, weighing 32– 42 g were divided into four groups. The four groups were divided into two controls and two treatments. Mice in normal control (C0) were administered only with the basal diet whereas negative control (C1) provided only with the deep-fried palm oil diet. The treatment groups T1, and T2 were administered with deep-fried palm oil diet and HMSELS at dose of 200 and 400 mg/kg/day, respectively for 28 days. Then on day 29, the mice were fasted overnight, anaesthetized and sacrificed by cervical dislocation after blood was taken by cardiac puncture for liver function tests while liver tissues were taken for histopathology investigation.
Results: The serum ALT and total bilirubin showed significant decrement whereas the serum albumin levels showed significant increment in T2 group. However, serum AST and ALP levels were decreased significantly in both T1 and T2 groups. Besides, the T2 group liver sections of mice were showed better effect of HMSELS on restoring the damaged liver histopathology almost toward normal.
Conclusion: The HMSELS at a dose of 400 mg/kg/day (T2) was more effective on the liver function tests and liver histopathology that altered by feeding deep-fried palm oil diet. The good protective effect of HMSELS against deep-fried palm oil diet-induced NAFLD might be due to its antioxidant content.
Keywords: Lepidium sativum, deep-fried, palm oil, NAFLD, mice
Graphical Abstract:
Introduction
The liver is one of the largest organs in the human body. It is an important site for intense metabolism and excretion. Some of these include carbohydrate, protein and fat metabolism, detoxification, secretion of bile and storage of vitamin.1,2 It is also involved with almost all the biochemical pathways to growth, fight against disease, nutrient supply, energy provision and reproduction. Generally, it has a surprising role in the maintenance, performance, and homeostasis of the body. This all makes it the first and most vulnerable organ to damage related to metabolism and intoxication as it is the first organ to encounter toxins and highly reactive metabolites than any other organs in the body.3
Chronic liver disease is a major cause of morbidity and mortality throughout the world. NAFLD is one of the most common examples of chronic liver injury.4 NAFLD is the presence of a significant (>5% of hepatocytes) fat accumulation in the liver, in the absence of an unsafe quantity of alcohol consumption, and any other cause of liver diseases. In the last two decades, it has become the most emerging liver disease worldwide following the spread of a western lifestyle.5 The increasing incidence of NAFLD has been related to a dramatic change in dietary habits, notably an increase in consumption of fat and simple carbohydrates.6
Rapidly increasing urbanization, mechanization and economic development of Africa and the world at large have resulted in a dietary transition from a traditional to a modernized diet (food in which the quality has been affected). Similarly, Ethiopia is also undergoing an epidemiologic transition mainly driven by demographic and lifestyle changes that promotes enormous changes in diets. Notably, fried foods are gaining more popularity which in turn resulted in extensive sales of a variety of fried foods and rapid expansion of deep frying practices.7–9
However, a major drawback is because the process of deep-frying often needs a large amount of oil people most often keep the used frying oil for reuse to ensure cost-effectiveness. In addition to cost, the underlying reason is a low level of awareness among the public about its negative effect on health.10
The palm oil, like other edible oil, undergoes various physical and chemical reactions during the deep-frying process. A physical reaction involves formation of foam, increase in viscosity, darkening of color, and deterioration of flavor, which affect the sensory qualities of fried foods. Chemical reactions include hydrolysis, isomerization, polymerization, and oxidation.11,12 These results in the formation of increased harmful products such as, monoglycerides, diglycerides, free fatty acids, trans fatty acids, triglyceride dimmer (TGD), triglyceride oligomer (TGO), peroxides, hydroperoxides, carbonyls, dimeric, and trimeric, which deposit in frying oils and are absorbed by the fried food. Many of these products are responsible for inducing NAFLD and other advanced liver diseases. For example, trans fatty acids are able to increase lipid peroxidation, and the lipid peroxides formed will damage the rough endoplasmic reticulum so that the synthesis of lipoprotein-forming proteins is impaired. Low lipoprotein levels cause some of the fat in the liver to not bind to lipoproteins to be transported to other parts of the body. The accumulation of fat in the liver causes fatty degeneration of the liver cells and NAFLD.13
NAFLD is associated with diabetes, obesity, and hyperlipidemia. Thus, it is considered to be the hepatic manifestation of metabolic syndrome and its prevalence increase in parallel with the prevalence of obesity, diabetes, and other metabolic syndrome. Besides, the trans fatty acids can also induce an increase in LDL levels which is the main parameters of dyslipidemia that are in turn highly associated with NAFLD.14,15 Nowadays, because of the increased prevalence of metabolic syndrome and obesity, NAFLD have expanded to a substantial extent. The prevalence of NAFLD in the US has risen from 18% in 1988–1991 to 31% in 2011–2012.14 However, conventional medical therapy for many common liver disorders, including nonalcoholic fatty liver disease and viral hepatitis, has limited efficacy and potentially life-threatening side effects.15
Therefore, this increased the need to look for alternative methods of improving such health problems with fewer adverse effects, be more cost effective, locally available, and easily consumable to provide better safety and efficacy. Currently, the increased dependence on complementary and alternative medicine (CAM), especially herbal medicine, has witnessed an upsurge of interest in developing and developed countries. In Ethiopia also about 80% of the human population and 90% of livestock depend on herbal medicines.16–18
One of the most important herbal medicines is Lepidium sativum L. seed. In Ethiopia, the Lepidium sativum L. seed traditionally used for treating skin problems, eye diseases, amoebic dysentery, abortion, asthma, intestinal complaints, gastritis, ringworm, malaria, tonsillitis, and stomach ache.19,20 In addition, it is also used to treat hypertension, liver diseases, and jaundice. Furthermore, its seed is one of the functional foods that contains ingredient such as saponin, flavonoids, alkaloids, terpenoids and steroids which have antioxidant, antiatherosclerotic, and hepatoprotective capacity as supported by different literature.21,22
However, even though there is reported scientific data pertaining to Lepidium sativum seed effect in other countries with different experimental designs such studies are lacking in our countries. Therefore, the aim of this study was to evaluate the effect of HMSELS (collected from local markets) on male Swiss albino mice fed on deep-fried palm oil diet.
Materials and Methods
Study Setting and Study Design
The random posttest only control group design was performed from July 28 to August 27,2020 on male Swiss albino mice at the Veterinary Medicine Postgraduate Laboratory of Jimma University.
Experimental Animals
The experimental animals used in this study were 24 male mice weighing 32–42 g and aged 8 to 10 weeks. Female mice were excluded from the study because of their estrogen hormone that can affect the liver biochemical parameter levels. All the mice were obtained from the Tropical and Infectious Disease Research Center (TIDRC), Sokoru, Jimma, South Western Ethiopia. Accordingly, they were brought to Veterinary Medicine Postgraduate Laboratory and had free access to standard mice pellets and distilled water in accordance with the National Institutes of Health (NIH) Guidelines for Care and Use of Laboratory Animals.23 The mice were housed in a transparent plastic cage with dimensions of length 40 cm, width 20 cm, height 15 cm and with SS sipper 250 mL water bottle at room temperature of 20–26°C, relative humidity of 40–50% and 12-h light/dark cycle.
Wood shavings were used as bedding and were replaced every morning after the cage was cleaned. The mice were allowed to acclimatize to the laboratory environment for 14 days before being subjected to the experiments.
Animals Grouping and Dose Administration
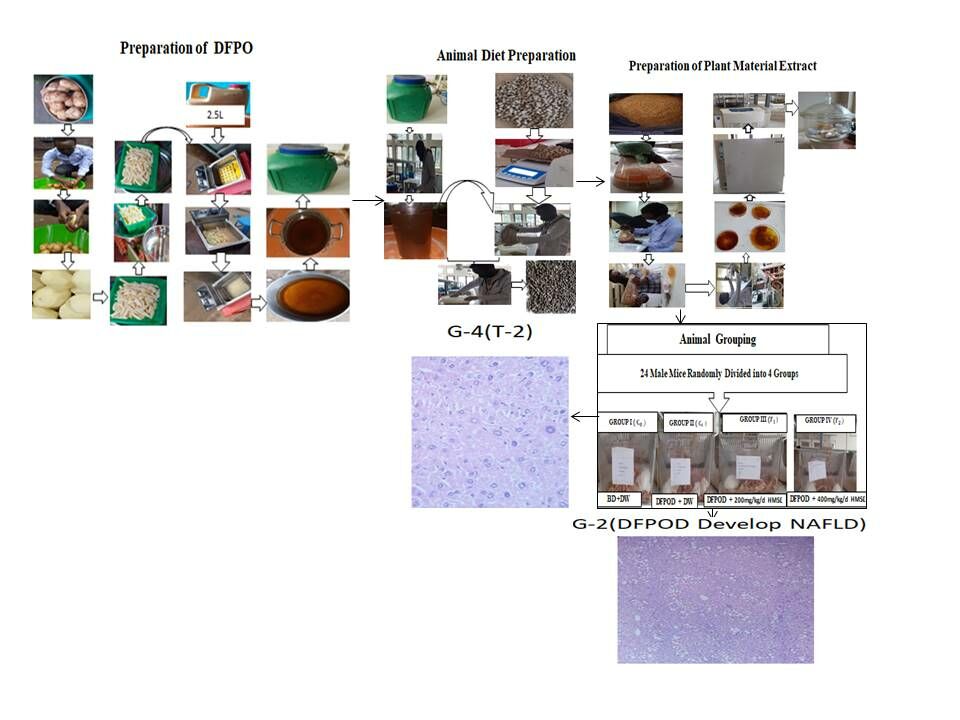
Animal grouping and dose administration was conducted in Veterinary Medicine Postgraduate Laboratory, College of Agriculture and Veterinary Medicine, Jimma University. The mice were divided randomly into four groups that contain six in each cage. Each mouse in the given group was differentiated by a number on its tail in permanent marker. At the beginning of the experiment and then weekly, mouse body weight was measured by electronic balance to adjust the HMSELS dose administration. Administration of extract dose was based on the methanolic extract of the Lepidium sativum seed that was safe up to 2000 mg/kg.24 Then, based on these the initial dose was taken 10% (200 mg/kg/day) of the tested safe dose (2000 mg/kg/day) and the second dose was taken by doubling of the initial dose 20% (400 mg/kg/day) according to OCED guidelines to scale-up the HMSELS dose administration and administered per oral (PO) by gavage. The control group was treated with distilled water to experience similar stress with other groups administered of either extract (for treatment groups) or deep fried oil (negative control group) for 28 days of the entire experimental study period (as shown in Table 1).
 |
Table 1 Animal Grouping |
Plant Material Collection and Preparation
Lepidium sativum L. seed was purchased from a local market, near Jimma University, Jimma, Ethiopia in April 2020. The small amount of purchased seed was sown, and grown for the purpose of authentication. Then, the leaf part of the plant was authenticated by a plant taxonomist at the National Herbarium of Addis Ababa University. The voucher number of the plant (ET-01/2020) was given. The plant material preparation was conducted in the Organic Chemistry Postgraduate Laboratory, Natural Science College, Jimma University. The seeds were winnowed, washed, shade dried and ground into coarse seed powder using mortar and pestle. The coarse seed powder was weighed and then packed well in a clean plastic container to avoid the entrance of air and other surrounding material until extracted.
Preparation of Plant Material Extract
The coarse seed powder (300 g) was extracted by maceration in 80% methanol (hydromethanol) for 72 h (three days) at room temperature by shaking three times per day throughout the maceration time. The mixture was first filtered using cotton wool and then with Whatman no. 1 filter paper. The filtrate was dried (methanol evaporated) by rotary evaporator at 50°C and 90 rpm. Then, the filtrate was evaporated in thermostatic oven at 40°C to remove the remaining methanol. The final gummy HMSELS was lyophilized, weighed, put in tight glass containers and kept in a refrigerator at 4°C until it was used for further experimental purpose.
Percentage Yield of HMSELS
The amount of crude extracts which was obtained from 300 g coarse powder of Lepidium sativum L. seed was 37.92 g. Therefore, the percentage yield was calculated and given as:
Preliminary Phytochemical Screening
The preliminary phytochemical screening was conducted in the Organic Chemistry Postgraduate Laboratory, Natural Science College, Jimma University. HMSELSS was used to screen the phytochemicals such as alkaloids, phenolic compounds, flavonoids, saponins, steroids, terpenoids, and quinones. The methods of screening employed were those described by25,26 for the presence of various active components.
Preparation of Potato
The potatoes were purchased from the market, Jimma, South Western Ethiopia. The potatoes were prepared by the principal investigator in collaboration with street food vendors. Potato was washed, peeled and cut into slices of uniform size using a vegetable slicer. The sliced potatoes were kept in water, blotted with tissue paper and then, 500 g was weighed for the frying process.
Preparation of Deep-fried Palm Oil
The preparation of deep-fried palm oil was carried out according to previously described methods13,28 with minor modification based on information gathered from street food vendors around Jimma University, Jimma. Palm oil was purchased from the market, Jimma, Ethiopia. Then, 2.5 L of fresh palm oil was added into a deep fat fryer and frying was carried out at a temperature of 200°C (detected using cooking thermometer). A batch of 500 g raw sliced potatoes was fried for 20 min and then the fried potato batch was removed from the fryer. Then the frying operation was carried out for a new potato batch. The frying procedure was done once daily for five consecutive days. The same oil was used repeatedly to fry the next batch of potatoes without adding any fresh palm oil to top up the lost oil during frying process. At the end of the frying, the oil was taken out, filtered, kept in a bottle until used for animal diet preparation.
Animal Diet Preparation
Animal diet preparation was conducted in Veterinary Medicine Postgraduate Laboratory, College of Agriculture and Veterinary Medicine, Jimma University. The animal diet was prepared by mixing deep-fried palm oil with normal mice pellets to contain 15% deep-fried palm oil.
The normal mice pellet diet of 85% w/w was mixed manually with prepared deep-fried palm oil of 15% w/w. The mixtures were left to absorb the fried oils at room temperature overnight before the feeding was conducted.28,29
Data Collection
The body weight of the mice was taken at the interval of a week to observe body weight change in all groups of animals during the study period. At the end of the study period, mice in all groups were fasted overnight and anesthetized by 100 mg/kg ketamine/12.5 mg/kg xylazine injection. The mice were euthanized by cervical dislocation following 2–2.5 mL of the blood collection from each mouse through cardiac puncture. Then, the blood was collected with the serum separator tube (SST) and left for 30 min at room temperature to clot. The serum was separated through centrifugation with speed of 3000 rpm at room temperature for 10 min, then, it was pipetted off using a micropipette and transferred into other clean serum vials. Finally, the serum vials were put in the refrigerator until they were analyzed for the liver function tests.
Data Analysis
The data were entered to the Epi-Data version 3.1 and exported to statistical package for social science (SPSS) version 25 for analysis after it was checked and cleaned. The results were expressed as mean ±SEM. One-way ANOVA was done to determine statistical differences among all groups of the study. This was followed by Tukey's post hoc test using SPSS software version 25 and (P<0.05) considered as statistically significant.
Ethical Considerations
The research was conducted after getting an ethical approval letter from the Jimma University Institutional Review Board with reference No. IHRPGD/714/2020 and the support letter was written to the Tropical and Infectious Disease Research Center, Chemistry, Veterinary Medicine, and Pathology Department from Biomedical Science Department. All experimental activities were carried out following the ethics of experimental animal which comply with scientific and ethical guidelines.
Results
Preliminary Phytochemical Screening
The preliminary phytochemical screening of HMSELS was revealed the status of phytochemical constituents such as alkaloid, flavonoid, phenol, steroid, saponin, and quinone as shown in (Table 2).
 |
Table 2 Preliminary Phytochemical Screening Results of HMSELS |
Effect of HMSELS on Body Weight Change
Group II showed nonsignificant decrement in body weight at the end of day seven but started to increase in the remaining days, 14, 21, and 28 days when compared to normal control Group I. Groups III and IV was showed nonsignificant decrement in body weight on days 14, 21, and 28 when compared to Group II as shown in (Figure 1).
 |
Figure 1 Effect of HMSELS on the body weight change at different days (expressed in terms of mean ±SEM). |
In the present study, the body weight change of Group II that were fed on the deep-fried palm oil diet was increased nonsignificantly compared to Group I normal control. The body weight change of Groups III and IV were decreased nonsignificantly compared to Group II that were fed on the deep-fried palm oil diet. As shown in Table 3, the absolute liver weight and liver index of Group II increased significantly compared to level of Group I normal control group. The Groups III and IV levels of absolute liver weight and liver index were decreased, but only Group IV was decreased significantly compared to the levels of Group II that were fed on deep-fried palm oil diet only.
 |
Table 3 Comparison of Mean ±SEM Value of Body Weight and Liver Weight Among the Four Groups |
Effects of HMSELS on Liver Enzymes
As shown in Table 4, the Group II serum ALT, AST, and ALP levels increased significantly when compared to Group I normal control. The Group III serum ALT level showed nonsignificant decrement when compared to Group II serum ALT level. The Group IV serum ALT level decreased significantly when compared to Group II serum ALT level as shown in Table 4. The Groups III and IV serum AST and ALP levels showed significant decrement, when compared to Group II.
 |
Table 4 Comparison of the Mean ±SEM Value of Liver Enzymes Among the Four Groups of Male Swiss Albino Mice |
Effects of HMSELS on Albumin and Total Bilirubin
The Group II serum albumin level decreased significantly when compared to Group I normal control serum albumin level. The Group III serum albumin level showed nonsignificant increment when compared to Group II serum albumin level. The Group IV serum albumin levels were significantly increased when compared to Group II serum albumin level as shown in Table 5. The Group II serum total bilirubin level showed significant increment when compared to the Group I serum total bilirubin level. The Group IV serum total bilirubin levels showed significant decrement when compared to Group II serum total bilirubin level.
 |
Table 5 Comparison of the Mean ±SEM Value of Albumin and Total Bilirubin Among the Four Groups of Male Swiss Albino Mice |
Liver Histopathology
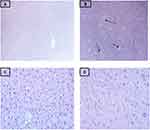
Histopathological examination of the liver of Group I normal control group revealed normal hepatic parenchyma-composed plates of hepatocytes, central veins, and hepatic vessels as shown in (Figure 2A). Histopathological alterations were observed in the deep-fried palm oil diet fed Group II included hepatic parenchyma with severe vacuolar and fatty change as shown in (Figure 2B). Liver sections of mice treated with deep-fried palm oil diet + 200 mg/kg/day of HMSELS showed the hepatocyte with mild vacuolar degeneration as shown in (Figure 2C). Liver sections of mice treated with deep-fried palm oil diet + 400 mg/kg/day of HMSELS appeared more or less similar to the normal control group liver sections as shown in (Figure 2D).
Discussion
Medicinal plants are getting great attention as valuable sources of food additives and bioactive substances with beneficial health effects. The incorporation of the products of medicinal plants in the daily food intake may be one of the highly used methods to modify the sensory characteristics of the diet and this may, in turn, also resolve the problems of underutilization of medicinal plants. Previous studies reported, intake of food which is rich in plant bioactive compounds such as polyphenols, flavonoids, saponin, and others, in particular, may exert beneficial effects towards human health.30
Effects of HMSELS on Liver Index
In the present study the liver index of Group II was increased significantly when compared with Group I normal control. This was in line with a previous study.31 This might be due to different chemical products which were produced during the frying process of oil that inhibit the activation of different proteins involved in the fatty acid catabolism and lipid efflux from the liver. However, the HMSELS in Groups III and IV had decreased liver index even though only Group IV was decreased significantly when compared to Group II. This might be due to phytochemical constituents of the HMSELS that enhance the restoration of the liver normal function such as proper fat oxidation and efflux of lipid from the liver.
Effects of HMSELS on Liver Enzymes
The serum ALT, AST, and ALP are among serum liver function tests, with their increase in the serum indicating liver damage including NAFLD.32 In the present study also, Group II serum ALT, AST, and ALP were increased significantly (P<0.05) when compared to Group I serum levels. The present study findings were in agreement with the study results of Jaarin et al33 on repeatedly heated palm oil fed experimental rats; study results of Hussein et al34 on reused palm oil fed albino mice and study results of Amany et al35 on thermally oxidized oil fed rabbits. The probable explanation might be due to lipid peroxidation and its products that damaged structural integrity of the liver, produce a breakdown, loss of permeability, rupture of the cellular membrane, intrahepatic, and extrahepatic biliary obstruction of the liver, which combined and results in releases of liver enzymes into blood circulation.35–37
In the current study, the HMSELS serum ALT level significantly (P<0.05) decreased only in Group IV that was administered at 400 mg/kg/day when compared to Group II serum ALT level. However, it showed a significant (P<0.05) decrement in both serum AST and ALP levels at 200 and 400 mg/kg/day when compared to Group II serum levels. The results of the present study were in line with study finding of Fawiziah et al37 on diabetic rats fed on basal diets mixed with Lepidium sativum seed; Mamdoh et al38 on methanolic extract of Lepidium sativum seeds on mice infected with Trypanosoma evansi and Zamzami et al39 on the effect of Lepidium sativum seed on hepatotoxicity-induced rabbits.
The Lepidium sativum seed extract contains important flavonoids such as naringenin, naringin, kampferol, apigenin, and luteolin as confirmed from previous studies. These compounds, either in combination or alone, have antioxidant and antiradical capacity that protect cell membranes from radical damage. The decreased serum liver enzymes levels in the present study might be due to radical scavenging activity of flavonoids that prevent lipid peroxidation that in turn normalize the lipid profile of hepatocyte membrane;39,40 and high polyphenol content in the seed of the Lepidium sativum that protect the liver from the damage induced by oxidants.42
Effects of HMSELS on Albumin and Total Bilirubin
In the present study, the Group II serum albumin level decreased significantly (P<0.05) when compared to Group I normal control serum albumin level. The present study finding was in agreement with the results of Adedayo et al43 on thermally oxidized palm oil fed rats. The lowered serum albumin level in the present study might be due to damaged liver that lost protein synthesis activity. Furthermore, it might be due to nutritional loss through decreased protein digestibility and absorption due to cross-linking reactions of secondary lipid oxidation products with proteins.44 In the present study, Group IV that were administered HMSELS at 400 mg/kg/day, increased significantly (P<0.05) in serum albumin level when compared to Group II serum albumin level. The current study finding was consistent with the study result of Zamzami et al38 on the amelioration of Lepidium sativum seeds in CCl-4-induced hepatotoxicity in rabbits. This might be due quercetin content of the seeds that scavenge free radicals and bind transition metal ions.45
The Group II serum total bilirubin level showed significant (P<0.05) increment when compared to the Group I serum total bilirubin level. The present study finding was in agreement with the results of Amany et al35 on thermally oxidized oil fed rabbits. The elevated level of serum total bilirubin might be due its leakage from hepatocytes to plasma as a result of hepatic obstruction to bile outflow. The Group IV that were administered HMSELS at 400 mg/kg/day serum total bilirubin level had shown significant (P<0.05) decrement when compared to Group II serum total bilirubin level. The current study finding was consistent with the study results of Zamzami et al39 on the amelioration of Lepidium sativum seeds in CCl-4-induced hepatotoxicity in rabbits. These might be attributed due to the improvement of the liver functions because of the presence of important flavonoids such as naringin and naringenin.41
Liver Histopathology
In the present study, the liver histopathology examination confirmed that the male Swiss albino mice liver sections exposed to deep-fried palm oil diets only showed hepatic parenchyma with severe vacuolar and fatty change which is the main manifestation of NAFLD as shown in (Figure 2B). The results of the present study are consistent with those of Ahmed et al34 that reported severe degenerative changes in hepatocytes including fatty changes in which hepatocytes showed ring appearance in thermoxidized palm oil fed rabbits. The present study revealed that concurrent treatment of male Swiss albino mice that were administered deep-fried palm oil diet with HMSELS improved the liver histopathology architecture in the form of mild vacuolar degeneration (at 200 mg/kg/day of HMSELS), normal hepatic parenchyma composed of central veins and portal tracts with portal veins (at 400 mg/kg/day of HMSELS), thus approving ameliorative effect of HMSELS. The present study results are in line with Zamzami38 that reported the improvement of the histological structure in rabbits treated with Lepidium sativum seed extract concurrent to CCl-4 administration.
Conclusions
The HMSELS (at 400 mg/kg/day) showed more significant decreasing effect on the important liver function tests which are main biomarker of NAFLD such as ALT, AST, ALP, and total bilirubin levels while increasing effect on serum albumin level. This confirmed the restoring capacity of HMSELS on the deranged liver function tests (induced NAFLD) in male Swiss albino mice fed on deep-fried palm oil diet. Moreover, the HMSELS at 400 mg/kg/day restored the liver histopathology of mice almost similar toward the normal control group. Hence, it could be concluded that 400 mg/kg/day doses of HMSELS had a better effect on liver function tests and liver histopathology that deranged or developed NAFLD than 200 mg/kg/day dose. Therefore, the HMSELS might be helpful as the dose increase in preventing future damages caused by deep-fried palm oil diet such as liver damage which is the main triggering factor for different chronic disease.
Acknowledgments
We would like to thank both Mettu University and Jimma University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nasir A, Abubakar MG, Shehu RA, Aliyu U, Toge BK. Hepatoprotective effect of the aqueous leaf extract of Andrographis paniculata nees against carbon tetrachloride – induced hepatotoxicity in rats. Niger J Basic Appl Sci. 2013;21(1):45–54.
2. Velmurugan V, Arunachalam G. Hepatoprotective activity of methanol extract of stem bark of prosopis. Int J Pharm Pharm Sci. 2014;6(2):5–7.
3. Duncan SA. Transcriptional regulation of liver development. Dev Dyn. 2000;142:131–142. doi:10.1002/1097-0177(2000)9999:9999<::AID-DVDY1051>3.3.CO;2-E
4. Morita M, Ishida N, Uchiyama K, Yamaguchi K, Itoh Y, Shichiri M. Fatty liver induced by free radicals and lipid peroxidation. Free Radic Res. 2012;6(46). doi:10.3109/10715762.2012.677840
5. Masarone M, Rosato V, Dallio M, et al. Review article role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018;7(4). doi:10.1155/2018/9547613
6. Panahi Y, Atkin SL, Butler AE. Efficacy of artichoke leaf extract in non ‐ alcoholic fatty liver disease: a pilot double ‐ blind randomized controlled trial. WiILEY. 2018;2(7):1382–1387. doi:10.1002/ptr.6073
7. Swallah MS, Sun H, Affoh R, Fu H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int J Food Sci. 2020;1(1):2–8.
8. Solomon Ali LN, Misganaw A, Worku A, Destaw Z. The burden of cardiovascular diseases in Ethiopia from 1990 to 2017: evidence from the global burden of disease study. Int Health. 2020;2(3):1–9. doi:10.1093/inthealth/ihaa069
9. Teruel MD, Gordon M, Linares MB, Garrido MD, Ahromrit A, Niranjan K. A comparative study of the characteristics of french fries produced by deep fat frying and air frying. J Food Sci. 2015;80(2):1750–3841. doi:10.1111/1750-3841.12753
10. Biliaed AM, Ahmed MA, Okasha MM, Alwakdi OM. The effects of frying on the thermal behaviour of some vegetable oils. Int J Agric Res Rev. 2016;4(7):529–537.
11. Prakash K, Uma D, Kalpana R. Physio ‐ chemical changes during repeated frying of cooked oil: a review. J Food Biochem. 2015;1(1):2–9. doi:10.1111/jfbc.12215
12. Goswami G, Rajni B, Mahipat R. Oxidation of cooking oils due repeated frying and human health. Int J Sci Technol Manag. 2015;4(1):2–8.
13. Sunarti S, Fachrial E, Harahap U, Delyuzar D, Widyawati T, Lubis LD. Hepatoprotective effect of red ginger rhizome extract in deep frying oil-fed male wistar rats. Universal Med. 2017;36(3):2407. doi:10.18051/univmed.2017.v36.228-235
14. Le MH, Devaki P, Ha NB, et al. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One. 2017;3(6):1–13.
15. Umer S, Asres K, Veeresham C, Umer S, Asres K, Veeresham C. Hepatoprotective activities of two Ethiopian medicinal plants Hepatoprotective activities of two Ethiopian medicinal plants. Pharm Biol. 2010;2(3). doi:10.3109/13880200903173593
16. Beyi MW. Traditional medicinal plants in Ethiopia. Int J Biol Phys Mat. 2019;1(1):80–87.
17. Tilahun Tolossa Jima MM. Ethnobotanical study of medicinal plants used to treat human diseases in Berbere District, Bale Zone of Oromia Regional State, South East Ethiopia. Evid Based Complement Altern Med. 2018;18(5):2–15.
18. Gabriel T, Guji T. Ethnopharmacological survey of medicinal plants in Agaro District, Jimma Zone, South West Ethiopia. Int J Pharm Sci Res. 2014;5(8):3551–3559. doi:10.13040/IJPSR.0975-8232.5(8).3551-59
19. Jansen PCM, Spices M. Condiments and Medicinal Plants in Ethiopia, Their Taxonomy and Agricultural Significance. Wageningen, The Netherlands: Bernan Press (PA); 1981.
20. Attia ES, Amer AH, Hasanein MA. The hypoglycemic and antioxidant activities of garden cress (Lepidium sativum L.) seed on alloxan-induced diabetic male rats. Nat Prod Res. 2017;18(55):1–5. doi:10.1080/14786419.2017.1413564
21. Yahya F, Sfouq F, Mahmoud AZ, Nayyar N. Chemical composition and antimicrobial, antioxidant, and anti-inflammatory activities of Lepidium sativum seed oil. Saudi J Biol Sci. 2018;3(1):2–5. doi:10.1016/j.sjbs.2018.05.007
22. Balgoon MJ. Assessment of the protective effect of Lepidium sativum against aluminum-induced liver and kidney effects in albino rat. Biomed Res Int. 2019;19(1):3–8. doi:10.1155/2019/4516730
23. Barbee Garber RW, Janet C. Guide for the care and use of laboratory animals. 2011.
24. Divanji M, Shylaja H, Rajesh S. Antidiarrheal activity of methanolic extracts of seeds of Lepidium sativum. J Nat Remedies. 2009;9(2):197–201. doi:10.18311/jnr/2009/240
25. Neeta MP, Mukta N, Bilwa K. Comparative qualitative phytochemical analysis of Sesamum indicum L. Int J Curr Microbiol App Sci. 2015;2(2):172–181.
26. Zohra FT. Extraction of secondary metabolites, phytochemical screening and the analysis of antibacterial activity in Stevia rebaudiana. Indian J Appl Res. 2015;3(4):4–35.
27. Famurewa A, Folawiyo AM, Ekiti A. Repeatedly heated palm kernel oil induces hyperlipidemia, atherogenic indices and hepatorenal toxicity in rats: the beneficial role of virgin coconut oil supplementation. Acta Sci Pol Technol Aliment. 2017;16(4):4–11. doi:10.17306/JAFS.2017.0513
28. Ani EJ, Nna V, Akpan OU, Ekpenyong C. Effect of aloe vera gel on thermoxidized palm oil-induced derangements in some haematological and biochemical parameters. Sch Res Libr. 2014;6(6):448–452.
29. Mekonnen Z, Gebreselema A, Abere Y. Effect of locally manufactured Niger seed oil on lipid profile compared to imported palm and sunflower oils on rat models. J Lipids. 2018;3(2):3–8.
30. Visioli F, Lastra CA, Andres-Lacueva C, et al. Polyphenols and human health: a prospectus. Crit Rev Food Sci Nutr. 2011;51(6):5–24. doi:10.1080/10408391003698677
31. Chong CLG, Hussan F, Othman F. Hepatoprotective effects of Morinda citrifolia leaf extract on ovariectomized rats fed with thermoxidized palm oil diet: evidence at histological and ultrastructural level. Oxid Med Cell Longev. 2019;20(2):2–10.
32. Wasihun Y, Makonnen E, Afewerk M, Ergete W. Hepatoprotective activity of aqueous and ethanol extract of Lippia adoensis leaf against carbon tetrachloride-induced hepatotoxicity in mice. Pathol Lab Med. 2017;1(1):5–13. doi:10.11648/j.plm.20170101.12
33. Jaarin K, Aini UN, Aishah MAS, Das S. Palm oil fat diet consumption and its effects on serum enzymes and microscopic changes in experimental rats. Pakistan J Nutr. 2015;14(9):575–580. doi:10.3923/pjn.2015.575.580
34. Gumaih HS. Effect of reused palm oil on biochemical and hematological parameters of mice. Egypt Acad J Biol Sci. 2015;7(1):13–21.
35. Abdallah AA, El-Deen NA, Neamat-Allah AN, Abd El-Aziz HI. Evaluation of the hematoprotective and hepato-renal protective effects of thymus vulgaris aqueous extract on thermally oxidized oil-induced hematotoxicity and hepato-renal toxicity. Comp Clin Path. 2019;29(9):3–11.
36. Urrutia-Hernández TA, Santos-López JA, Benedí J, et al.Antioxidant and hepatoprotective effects of croton hypoleucus extract in an induced-necrosis model in rats. Molecules. 2019;24(2):8–13.
37. Alharbi FK, Sobhy M. Influence of dietary supplementation of garden cress (Lepidium sativum L.) on histopathology and serum biochemistry in diabetic rats. J Chem Environ Health. 2017;3(1):1–19.
38. Al-Otaibi MSA, Al-Quraishy S, Al-Malki ES, Abdel-Baki AAS. Therapeutic potential of the methanolic extract of Lepidium sativum seeds on mice infected with Trypanosoma evansi. Saudi J Biol Sci. 2019;26(7):1473–1477. doi:10.1016/j.sjbs.2018.08.031
39. Zamzami MA, Baothman OAS, Samy F, Abo-golayel MK. Amelioration of CCl 4 -induced hepatotoxicity in rabbits by Lepidium sativum seeds. Evid Based Complement Altern Med. 2019;1(2):1–12. doi:10.1155/2019/5947234
40. Pari L, Amudha K. Hepatoprotective role of naringin on nickel-induced toxicity in male Wistar rats. Eur J Pharmacol. 2011;650(1):364–370. doi:10.1016/j.ejphar.2010.09.068
41. Azzi R, Lahfa F, Ahmed E, Naima K. The nutraceutical potential of Lepidium sativum L. seed flavonoid ‐ rich extract in managing metabolic syndrome components. J Food Biochem. 2018;45(5):1–11. doi:10.1111/jfbc.12725
42. Al-Snafi AE. Chemical constituents and pharmacological effects of lepidium sativum-a review. Int J Curr Pharm Res. 2019;11(6):1–10. doi:10.22159/ijcpr.2019v11i6.36338
43. Falade AO, Oboh G, Ademiluyi AO, Odubanjo OV. Consumption of thermally oxidized palm oil diets alters biochemical indices in rats. J Basic Appl Sci. 2015;4(2):150–156. doi:10.1016/j.bjbas.2015.05.009
44. Abou-Elkhair TS, Abou-Elkhair M. Potential hazards of feeding albino rats on diet containing repeatedly boiled cooking oil: clinicopathological and toxicological studies. Int J Adv Res. 2015;3(3):134–147.
45. Alrawaiq N. A review of flavonoid quercetin: metabolism, bioactivity and antioxidant properties. IJPRIF. 2019;6:933–941.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.