Back to Journals » Clinical Ophthalmology » Volume 14
Evaluation of Retinal Detachment After Diabetic Vitrectomy: Causes and Ways of Management
Authors Abdelhadi AM , Helaly HA , Abuelkeir A
Received 23 October 2019
Accepted for publication 9 December 2019
Published 9 January 2020 Volume 2020:14 Pages 53—60
DOI https://doi.org/10.2147/OPTH.S235757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ahmed M Abdelhadi, Hany Ahmed Helaly, Amr Abuelkeir
Ophthalmology Department, Faculty of Medicine, Alexandria University, Alexandria, Egypt
Correspondence: Hany Ahmed Helaly
Ophthalmology Department, Faculty of Medicine, Alexandria University, 30 Roshdy Street, Roshdy, Alexandria, Egypt
Tel +20 1225466733
Email [email protected]
Purpose: To assess the causes and the ways of management of rhegmatogenous retinal detachment (RRD) after pars plana vitrectomy (PPV) performed in diabetic patients with advanced diabetic eye disease.
Methods: Retrospective review of the records of the patients who had undergone PPV for complicated proliferative diabetic retinopathy (PDR) was done. Cases with RRD after the PPV were analyzed in the study (n = 32). Preoperative, operative, and postoperative data of the patients were recorded. All patients were recruited for a final follow-up visit.
Results: This retrospective case-control study included 400 eyes of 345 patients. Prolonged surgical duration increased the risk of developing RRD (odds ratio = 1.6342, p = 0.0321). The presence of intraoperative retinal breaks increased the risk of developing postoperative RRD (odds ratio = 2.2308, p = 0.0380). Also, complex diabetic detachment that needed for bimanual dissection of the membranes during surgery were associated with a higher risk of developing postoperative RRD (odds ratio = 2.7311, p = 0.0401).
Conclusion: Rhegmatogenous retinal detachment following diabetic vitrectomy needs a further vitrectomy for the management and usually has poor visual outcome. Prolonged surgical duration, the presence of intraoperative retinal breaks, and the need for bimanual dissection of the membranes (major complex cases) during surgery were associated with higher risk of developing RRD postoperatively.
Keywords: vitrectomy, proliferative diabetic retinopathy, recurrent retinal detachment, complications, proliferative vitreoretinopathy, retinal break
Introduction
Pars plana vitrectomy is used to manage cases suffering from proliferative diabetic retinopathy complications mainly vitreous hemorrhage or tractional retinal detachment.1–4
Microincision vitrectomy surgery (MIVS) has the advantage of sutureless transconjunctival route.5–7 It has the advantage of decreased operation time, increased patient comfort, and decreased corneal distortion.8 It is now the preferred technique for managing cases with complicated proliferative diabetic retinopathy.5–7
Rhegmatogenous retinal detachment after pars plana vitrectomy for proliferative diabetic retinopathy is a serious complication. It usually results from peripheral or less commonly posterior retinal breaks which can result into proliferative vitreoretinopathy and/or anterior segment neovascularization in a short time. The reported incidence in the literature is around 5%. Rhegmatogenous retinal detachment needs a further pars plana vitrectomy for the management and usually has poor visual outcome.9,10
The aim of the current study was to assess the causes and the ways of management of rhegmatogenous retinal detachment (RRD) after pars plana vitrectomy (PPV) performed in diabetic patients with advanced diabetic eye disease.
Subjects and Methods
Retrospective review of the records of the patients who had undergone microincision primary pars plana vitrectomy for complicated proliferative diabetic retinopathy (PDR) with at least 6 months postoperative follow up was done. We included cases in which the primary PPV surgery was performed from January 2015 till January 2017. Cases with rhegmatogenous retinal detachment after the PPV were analyzed in the current study. The indication for primary PPV was persistent vitreous hemorrhage, progressive or persistent fibrovascular proliferations despite sufficient laser treatment, and/or tractional retinal detachment involving or threatening the macula. Cases with previous ocular surgery other than cataract surgery, insufficient follow-up period, or incomplete data were excluded. This research was approved by the local ethics committee of Faculty of Medicine, Alexandria University, Egypt. The tenets of the declaration of Helsinki were followed. All included patients signed an informed consent.
Preoperative and postoperative data of the patients as well as the type of diabetes and the state of diabetic control (using HbA1c level) were recorded. Operative videos for the cases were revised, and cases were divided according to the surgical skill used into minor, moderate or major surgical procedures. Best-corrected distance Snellen visual acuity (BCVA) which was converted into logarithm of the minimum angle of resolution (logMAR) units, lens status, intraocular pressure, preoperative panretinal photocoagulation, indication of surgery (type of PDR complication), preoperative injection of anti-vascular endothelial growth factor, use of postoperative tamponade, surgical clock time, the presence of intraoperative break, postoperative complications, and the time interval between PPV and postoperative RRD were recorded.
Surgical Technique
After reviewing the surgical videos of all cases included in the study: All primary PPV surgeries were performed by an experienced surgeon. Microincision vitrectomy transconjunctival sutureless PPV was performed under general anesthesia using the Constellation® vision system (Alcon Japan Ltd., Tokyo, Japan). After performing initial core vitrectomy (cutting rate 3500 cuts per minute, vacuum 150–300 mmHg), posterior hyaloid detachment was attempted if not already present by pulling away from the optic nerve head with the vitrectomy cutter in the suction mode (this applies for cases with simple vitreous hemorrhage). This was followed by peripheral vitrectomy and vitreous base shaving aided by scleral indentation.
In minor complexity procedures, which usually comprised cases with vitreous hemorrhage and a complete posterior vitreous detachment, no fibrovascular membrane dissection was required. Only core vitrectomy with detachment of the posterior hyaloid was required. BBG (brilliant peel from DORC) was administered to highlight any residual posterior vitreous. These cases did not need a tamponade.
Medium complexity procedures comprised cases with focal vitreoretinal attachments (single or multiple) or posterior pole broad vitreoretinal attachments without underlying retinal folds, in which there are multiple small neovascular pegs. In these cases, fibrovascular tractional membranes were managed by the retrograde high-speed cutter in the shaving mode set on the machine (cutting rate 5000 cuts per minute, vacuum 100–150 mmHg). These cases usually ended in gas and sometimes with silicone tamponade, which was left in place for 3 months before silicone oil removal was attempted.
Major complexity procedures generally comprise cases with broad vitreoretinal attachments reaching the equator or beyond with underlying retinal folds, in which the surgical plane was less clearly defined because of diffuse fibrovascular membrane attachment. A chandelier assisted bimanual delamination was the method of choice to decrease iatrogenic breaks. In most of these cases, silicone was the tamponade of choice.
Thorough endolaser pan-retinal photocoagulation was performed up to the peripheral retina. Meticulous search for any retinal breaks especially peripherally using scleral indentation was carefully done. Any retinal break (previously present or iatrogenic) was treated with surrounding laser photocoagulation (150–200 mw, 200 ms) after fluid–air exchange, and if needed, a non-expansible mixture (14%) of perfluoropropane (C3F8) gas or 10,000 centistokes (cST) silicone oil was injected into the vitreous cavity. In cases with iatrogenic breaks or those with evident retinal ischemia diagnosed during surgery, silicone oil was preferred. The microcannulas were gently removed and the conjunctival tissue over the sclerotomy was displaced to avoid fistula formation. At the end of the surgery, the sclerotomy site was checked for any leak. If any leak was found, the sclerotomy site was sutured using 8/0 vicryl sutures to prevent postoperative transient hypotony. When patients had a significant cataract, phacoemulsification with the insertion of a foldable hydrophobic intraocular lens in the bag through 2.8 mm clear corneal incision was done before performing the incisions of the vitrectomy. Post operative Fortymox plus eye drops a steroid, antibiotic combination was used in all patients 5 times daily for 5 days (contains Moxifloxacin hydrochloride corresponding to 5 mg moxifloxacin and 1.1 mg of dexamethasone Sodium phosphate corresponding to 1 mg dexamethasone phosphate; Orchidia pharmaceuticals, Egypt).
Patients were followed up after the primary PPV. We specifically watched out for recurrent or a new onset of RD after the primary operation. Recurrent RD was considered to be early if it was detected during the first 4 weeks after the primary PPV and late if it was detected after 4 weeks post-primary PPV. We evaluated cases with for recurrent RD before silicone oil was removed. We felt that including cases with RRD after silicone oil was removed was not going to add much strength to the results as the occurrence of a recurrent RD after 3 months of stability before Silicone oil removal is rare. The management of the cases with complicated RRD is discussed in detail (vide infra). Detailed data of those patients were recorded. All patients were recruited for a final follow-up visit. Visual acuity was recorded in logMAR units. Cases with a BCVA of counting fingers (CF), hand motions (HM), perception of light (PL), and no perception of light (no PL) were counted as 2.1, 2.4, 2.7, and 3.0 logMAR units, respectively.11–13
Data analysis was performed using the software SPSS for Windows version 20.0 (SPSS Inc., Chicago, USA). Quantitative data were described using range, mean and standard deviation. Normality of data samples was evaluated using the Kolmogorov–Smirnov test. Student t tests (independent sample t-test and paired t-test) were used for comparisons between different means. Chi-square test and Fisher exact tests were used to compare between different percentages and ratios. Differences were considered statistically significant when the associated p-value was less than 0.05.
Results
This retrospective case-control study included 400 eyes of 345 patients with PDR that had undergone primary PPV. The patients were divided into two groups; the first group (group a) included eyes that developed postoperative RRD (n = 32 eyes of 32 patients) and the second group (group b) included eyes that did not develop postoperative RRD (n= 368 eyes of 313 patients).
Demographic Data
The mean age of the patients, it was 54.2 ± 12.1 years (range from 35 to 70 years) and 56.5 ± 14.2 years (range from 30 to 75 years) among group a and group b, respectively (p = 0.312). Males represented 56% (n = 18) and 60% (n = 220) among group a and group b, respectively (X2 = 0.152, p = 0.696). As regards type of diabetes (type 1 vs type 2), type 2 diabetes was present in 84.4% (n = 27) and in 83.7% (n = 308) among group a and group b, respectively (X2 = 0.009984, p = 0.92). The mean hemoglobin A1c (Hb A1c), as an indication for the diabetic control, was 7.5 ± 1.6% and 7.7 ± 1.8% among group a and group b, respectively (p = 0.388). The mean follow-up period for all included eyes was 9.2 months (ranged from 6 to 19 months).
Preoperative Data Comparison
As for, the preoperative data (BCVA, IOP, previous panretinal photocoagulation, anti-vascular endothelial growth factor injection prior to the surgery by 1 week), there was no statistical difference between the two groups. On the other hand, there was a statistically significant difference between the two groups regarding the intraoperative presence of retinal breaks (whether already present or iatrogenic breaks), the surgical time, and the ratio between cutter shaving for the membranes versus bimanual dissection. The use of a tamponade (gas or silicone oil) did not show a statistically significant difference between the 2 groups (p = 0.483). Table 1 shows the preoperative and intraoperative characteristics of eyes with RRD versus eyes that did not develop RRD after primary vitrectomy.
 |
Table 1 Characteristics of Eyes with Rhegmatogenous Retinal Detachment (RRD) versus Eyes That Did Not Develop RRD After Primary Vitrectomy |
Logistic regression model for detecting the risk for the development of postoperative rhegmatogenous retinal detachment was done (Table 2). Prolonged surgical duration increased the risk of developing RRD (odds ratio = 1.6342, p = 0.0321). The presence of intraoperative retinal breaks increased the risk of developing postoperative RRD (odds ratio = 2.2308, p = 0.0380). Also, major complexity cases needing bimanual dissection of the diffuse adherent membranes during surgery increased the risk of developing postoperative RRD (odds ratio = 2.7311, p = 0.0401).
 |
Table 2 Logistic Regression Model for Detecting the Risk for Development of Postoperative Rhegmatogenous Retinal Detachment |
The incidence of post-diabetic vitrectomy RRD: It was 8% (n = 32 out of 400 included eyes). Figure 1 presents a simplified chart that shows the type of tamponade used in cases with rhegmatogenous retinal detachment after vitrectomy and the timing of the detachment. Eighty-four percent of the RRD cases (n = 27) occurred during the first month postoperative (early RRD). Sixteen percent of the RRD cases (n = 5) occurred during after 4 weeks postoperative (late RRD).
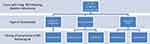 |
Figure 1 Type of tamponade used in cases with rhegmatogenous retinal detachment after vitrectomy and timing of the detachment. |
After Subgroup Analysis
A) As for, the tamponade used in group a vs group b cases, it was silicone oil in 3 vs 28 eyes, long-acting gas in 20 vs 214 eyes, and no or air tamponade was used in 9 vs 126 eyes (p = 0.763). Analysis of the RRD cases (group a) time of occurrence and the original tamponade, all cases with no or air tamponade (n = 9) developed early RRD. Cases with gas tamponade (n = 20) developed early RRD in 17 eyes and late RRD in 3 eyes. Cases with silicone oil tamponade (n = 3) developed early RRD in 1 eye and late RRD in 2 eyes.
B) The indication of the primary surgery in relation to the RRD: As regards the indication for primary PPV in group a vs group b cases, it was persistent vitreous hemorrhage in 12 vs 158 eyes. From the 12 cases with RRD, 9 cases were left with no or air tamponade while 3 cases required gas tamponade. The other indication for primary PPV, progressive or persistent fibrovascular proliferations and/or tractional retinal detachment involving or threatening the macula was the indication in 20 eyes from group a vs 210 eyes from group b (p = 0.551). These eyes ended with gas tamponade in 17 eyes and silicone oil tamponade in 3 eyes.
C) Analysis of the occurrence RRD (group a) with the technique performed in the original surgery: We found that in minor complexity cases where no fibrovascular membrane dissection was required. The RRD in these cases (9 eyes) appeared to be due to a peripheral break in 3 cases and a posterior break in 6 cases, from which 4 were related to laser scars performed during the original surgery.
Medium complexity cases usually ended in gas (RRD in 20 eyes from which 17 had early recurrence and 3 late recurrences) and sometimes with Silicone tamponade (1 case had late recurrence). Major complexity procedures generally comprise cases in which Silicone was the tamponade of choice. From this group, only two cases suffered recurrence one early within 4 weeks from surgery and another case had late recurrence 5 months postoperative.
Early Rhegmatogenous retinal detachment following vitrectomy in diabetic eyes in the current study appeared within the first 3–4 weeks post-operative. And usually earlier than this time frame in cases without tamponade. Late recurrence is defined when at least a period of 4–6 weeks has elapsed since surgery, with attached retina and probably some visual improvement.
D) The cause of recurrence in relation to the time of occurrence of RRD: we found that in all cases of early rhegmatogenous detachment, the cause was identified to be a posterior retinal break in 24 eyes, and a peripheral break in three eyes. New iatrogenic retinal breaks were identified as the cause in 22 eyes with posterior breaks and 1 eye with peripheral break. While a break related to laser marks was the cause in the remaining 4 eyes. Late recurrence (5 eyes) was all due to proliferative diabetic vitreoretinopathy with or without a concomitant break which occurred inferiorly and peripherally in three eyes, but was noticed to occur around posterior pole breaks in 2 cases with TRD in a young type I (less than 35 years) diabetic patients.
Management Plan for Eyes with RRD
The management plan for early RRD was to re-intervene as soon as the diagnosis of RD was made. In all these cases 3 port vitrectomy was done, the previous tamponade – if present – was removed. After Fluid air exchange, BBG (brilliant peel from DORC) was administered, left for 1 min. Then, flushing the vitreous cavity with saline to regain a better view was next done. Single-handed dissection to remove pre retinal and subretinal membranes was resorted to in early cases in the study but was sometimes complicated with more iatrogenic breaks, so chandelier assisted bimanual peeling was the technique of choice if proliferative membranes were identified. We then used the low setting of the diathermy (30–50%) to mark the causative breaks, shifting to air to test the retinal mobility and to perform air-fluid exchange to flatten the retina once more. Laser treatment to all the causative breaks was performed. Silicone oil (1000 cst) was injected at the end of the surgery.
Late RRD occurred in three eyes with gas tamponade and in 2 eyes with Silicone tamponade. The management plan differs according to the original tamponade used. In the eyes where gas was used, RRD complicating the vitrectomy usually progresses rapidly to proliferative diabetic vitreoretinopathy with the macula being involved, and loss of central vision in all these eyes. Consequently, rapid intervention is necessary as mentioned in cases with early recurrence with an obvious difference of excessive fibrous proliferation that required meticulous and careful bimanual dissection so as not to leave behind any residual tractional element. Any posterior breaks created in these cases were complicated later by re-proliferation of membranes on to the posterior pole and macula which affected the final visual outcome significantly. Judicious laser therapy to any breaks creaked as the excessive laser was noticed to invite more fibrous proliferation.
In cases with late recurrence where the original tamponade was silicone (2 eyes in the study), the management plan differs according to the extent and cause of detachment. In one patient, which belonged to the medium complex cases, the detachment was peripheral in position, inferior with no obvious breaks or progression, the cause in these cases was incomplete posterior vitreous removal. We preferred to postpone the re-intervention till the time of silicone oil removal between 3 and 4 months from the original surgery. At that time through staining and removal of the posterior hyaloid and any proliferative membranes was done. The other patient with late recurrence and silicone tamponade, which originally was categorized as a major complex case, the recurrent detachment occurred 2 months after the original surgery was done and involved the macula, consequently, immediate intervention as above was adopted.
As shown above, a second pars plana vitrectomy was needed for the 32 eyes with RRD complicating diabetic vitrectomy. Retinal reattachment was successful in 28 eyes (87.5%). Four eyes needed a third intervention in which usually bimanual dissection of membranes was not enough and retinectomy had to be resorted to. Silicone oil was re-injected. Final BCVA of the 32 eyes was 1.12 ± 0.88 logMAR. This level of visual acuity did not show a statistically significant difference from pre-primary PPV levels (p = 0.265). For the non-RRD group, the final BCVA was 0.71 ± 0.51 log MAR. This level of visual acuity was significantly better than that of RRD group (p = 0.031).
Discussion
Rhegmatogenous retinal detachment is a serious complication that can occur after diabetic vitrectomy. It usually results from a peripheral or less commonly a posterior break. As mentioned above, the literature reported an incidence of around 5%.9,10 In the current study, the incidence was 8% which is slightly higher than the reported incidence. This may be explained by differences in the preoperative retinal findings as regards tractional detachment and/or fibrovascular proliferations as our patients showed more advanced condition which was reflected on the surgical time and the complexity of surgical manipulations.
There was no statistically significant difference between group a and group b as regards age, sex, type of diabetes, and level of diabetic control. This rules out the effect of those variables when comparing the data between the two groups. The shortest duration of postoperative follow-up period was 6 months. Again, there was no statistically significant difference between the two groups as regards pre-primary PPV parameters. Preoperative injection of bevacizumab was done 1week before primary PPV in 10 cases of RRD group and in 90 cases of no RRD group. There was no statistically significant difference between the two groups (0.395). It has the advantage of pharmacologic involution of new vessels, simplifying the removal of the membranes, and decreasing the intraoperative bleeding. However, there is a concern that it might exacerbate tractional retinal detachment.14
Prolonged surgical time appeared to increase the risk of developing postoperative RRD (odds ratio = 1.6342, p = 0.0321). Cases with postoperative RRD had prolonged surgical time (95.2 ± 29.4 mins). This may be associated with more manipulations which may lead to an iatrogenic break and may also reflect a more complicated nature of the retinal condition requiring more surgical maneuvers. The same idea applies in cases requiring major complexity procedures. There is a higher risk of developing postoperative RRD when there is a need for bimanual dissection of the membranes during surgery or a presence of intraoperative retinal breaks (odds ratio = 2.7311 and 2.2308, p = 0.0401 and 0.0380, respectively).
Ramezani et al15 in their study showed that the development of iatrogenic break (AOR, 0.25), and use of heavy SO (AOR, 0.13) were independently associated with a higher risk of recurrent detachment. This is similar to our study that more difficult cases, required longer time, had higher incidence of retinal breaks and probably needed silicone oil at the conclusion of surgery. Consequently, all these factors represent different characteristics of an advanced diabetic eye condition, managed by whichever technique.
High-speed cutting rate of 5000 cuts per minute (cpm) has the advantage of reducing the vitreous turbulence by allowing only the small pieces of vitreous to enter the ports which results in less retinal traction and decreases the need for bimanual dissection technique using the endo-illuminator in less advanced cases. Yet, iatrogenic retinal breaks do occur as the complexity of the dissection and the friable retinal tissues in advanced cases can lead to inevitable breaks.16,17 Yakota et al18 stated that the incidence of intraoperative iatrogenic retinal breaks was significantly lower in 23-gauge vitrectomy in comparison to the older 20-gauge vitrectomy (21% vs 35%, p = 0.024).
In the current study, there was no statistical difference regarding the type of tamponade at the end of the original surgery between the two groups a and b. This differs from the work of Storey et al19 where they found that eyes receiving silicone oil tamponade had lower single-surgery reattachment rates (77.6% vs 87.6%; P = 0.013) as well as lower reattachment rates at the final follow-up (85.7% vs 92.6%; P = 0.048), this finding might be explained by the fact that they chose silicone as tamponade in difficult, more ischemic TRD and in cases with iatrogenic breaks during the original surgery, especially if the traction on the edges of theses breaks was not relieved completely.
As regards the indication of the primary vitrectomy, the current study showed a trend towards the appearance of RRD (20 eyes) more with TRD or progressive fibrovascular proliferation being present as the original indication for the primary intervention. On the other hand, only 12 cases complicated eyes in which vitreous hemorrhage was originally present. Similar statistically significant results were found by Huang et al20 where they found that their study group (age younger than 40 years) had significantly higher proportions of active fibrovascular proliferation and traction retinal detachment, and with significantly higher-severity grading than those in the control group (age more than 40 years). After the operation, the recurrent detachment rates were 13.2% in the study group and 1.3% in the control group (p = 0.006).
In the study by Shen et al21 breaks with adjacent unreleased traction was identified as the only variable associated with final anatomic success (p=0.024). In all their studied, cases silicone oil was the final tamponade with an anatomic success of 85.2% in the third month postoperatively and 83.3% at last follow up (≥12 months). This difference in the causative factor of recurrence between the current study – iatrogenic breaks, proliferative diabetic vitreoretinopathy, or both- and the latter one may be because they did not state the cause of the break whether iatrogenic, laser related or due to proliferative diabetic vitreoretinopathy as the latter might cause recurrence even in well supported superior breaks.
In relation to tamponade type at the conclusion of the original surgery, and its relation to the timing of recurrence. Similar difficult diabetic tractional cases were described by Han et al22 In their study, where they concluded that vitrectomy for severe equatorial fibrovascular proliferation differs from conventional approaches to diabetic retinopathy in that relief of retinal traction must be attained by scleral buckling and adequate dissection of peripheral fibrovascular tissue. The different approach in their eyes was probably due to the lake of chandelier assisted surgery in 1995 when they published their data. Also, all their cases were unoperated eyes, with more resilient retinal tissue.
Limitation of the current study is the retrospective nature in reviewing the primary surgery; consequently, the original technique was not standardized. On the reverse, it has the advantages of relatively large number of included eyes with an adequate postoperative follow-up period and the comparative nature of the study with a control group. This study also outlined a simple algorithm for the first time on how to deal with different possibilities for post-diabetic vitrectomy RRD in a simple manner covering most of the actual clinical situations that a vitreoretinal surgeon might face.
In conclusion, rhegmatogenous retinal detachment (RRD) following diabetic vitrectomy needs a further vitrectomy for the management in which silicone oil is the tamponade of choice and usually has poor visual outcome. Prolonged surgical duration, the presence of intraoperative retinal breaks, and major complexity cases in which bimanual dissection of the membranes during surgery was utilized were more prone to be complicated with RRD.
Ethics and Consent to Participate
The study was approved by the local ethics committee at the Faculty of Medicine, Alexandria University, Egypt. Tenets of the Declaration of Helsinki were followed. All included patients were recalled for a final follow-up visit and signing of an informed consent. Informed consent was obtained from all individual participants included in the study.
Data Sharing Statement
Data are available upon reasonable request to the authors.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Banerjee PJ, Moya R, Bunce C, Charteris DG, Yorston D, Wickham L. Long-term survival rates of patients undergoing vitrectomy for proliferative diabetic retinopathy. Ophthalmic Epidemiol. 2016;23(2):94–98. doi:10.3109/09286586.2015.1089578
2. Sulak M, Urbancic M, Petrovic MG. Predicting visual outcomes of second eye vitrectomy for proliferative diabetic retinopathy. Retina. 2017;7.
3. Fujii T, Akashi M, Morishita S, et al. Vitrectomy for proliferative diabetic retinopathy in a patient with heparin-induced thrombocytopenia. Case Rep Ophthalmol. 2016;7(1):67–73. doi:10.1159/000443720
4. Sato T, Tsuboi K, Nakashima H, Emi K. Characteristics of cases with postoperative vitreous hemorrhage after 25-gauge vitrectomy for repair of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(4):665–671. doi:10.1007/s00417-016-3522-8
5. Qin L, Jian-Hong W, Ming-Mei Z, Ying W. Effect of ranibizumab intravitreal injection before 23 G-vitrectomy surgery in the treatment of patients with proliferative diabetic retinopathy. Int Eye Science. 2016;16(10):1959–1961.
6. Li F, Chen J, Sun L. Does 23-gauge vitrectomy decrease complications and operation time in treating retinal diseases? A meta-analysis. Int J Clin Exp Med. 2016;9(6):10424–10433.
7. Chiquet C, Aptel F, Combey-de Lambert A, et al. Occurrence and risk factors for retinal detachment after pars plana vitrectomy in acute postcataract bacterial endophthalmitis. Br J Ophthalmol. 2016;100(10):1388–1392. doi:10.1136/bjophthalmol-2015-307359
8. Narayanan R, Tibra N, Mathai A, Chhablani J, Kuppermann BD. Sutureless 23-gauge versus 20-gauge vitrectomy with silicone oil injection in rhegmatogenous retinal detachment. Retina. 2012;32:1013–1016. doi:10.1097/IAE.0b013e3182327cf9
9. Yorston D, Wickham L, Benson S, Bunce C, Sheard R, Charteris D. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:365–368. doi:10.1136/bjo.2007.124495
10. Schrey S, Krepler K, Wedrich A. Incidence of rhegmatogenous retinal detachment after vitrectomy in eyes of diabetic patients. Retina. 2006;26:149–152. doi:10.1097/00006982-200602000-00004
11. Jackson TL, Donachie PH, Sparrow JM, Johnston RL. United Kingdom national ophthalmology database study of vitreoretinal surgery: report 1; case mix, complications, and cataract. Eye (Lond). 2013;27(5):644–651. doi:10.1038/eye.2013.12
12. Jackson TL, Donachie PH, Sparrow JM, Johnston RL. United Kingdom national ophthalmology database study of vitreoretinal surgery: report 2, macular hole. Ophthalmology. 2013;120(3):629–634.
13. Jackson TL, Donachie PH, Sallam A, Sparrow JM, Johnston RL. United Kingdom National ophthalmology database study of vitreoretinal surgery: report 3, retinal detachment. Ophthalmology. 2014;121(3):643–648. doi:10.1016/j.ophtha.2013.07.015
14. Zhang ZH, Liu HY, Hernandez-Da Mota SE, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta-analysis of randomized controlled trials. Am J Ophthalmol. 2013;156(1):106–115. doi:10.1016/j.ajo.2013.02.008
15. Ramezani A, Ahmadieh H, Rozegar A, et al. Predictors and outcomes of vitrectomy and silicone oil injection in advanced diabetic retinopathy. Korean J Ophthalmol. 2017;31(3):217–229. doi:10.3341/kjo.2016.0018
16. Stalmans P. 23-gauge vitrectomy. In: Microincision Surgery. Vol. 54. Karger Publishers; 2014:38–44.
17. Celik E, Sever O, Horozoglu F, Yanyali A. Segmentation and removal of fibrovascular membranes with high-speed 23 G transconjunctival sutureless vitrectomy, in severe proliferative diabetic retinopathy. Clin Ophthalmol. 2016;10:903. doi:10.2147/OPTH.S95145
18. Yakota R, Inoue M, Itoh Y, Rii T, Hirota K, Hirakata A. Comparison of microincision vitrectomy and conventional 20-gauge vitrectomy for severe proliferative diabetic retinopathy. Jpn J Ophthalmol. 2015;59(5):288–294. doi:10.1007/s10384-015-0396-y
19. Storey PP
20. Huang CH, Hsieh Y, Yang CM. Vitrectomy for complications of proliferative diabetic retinopathy in young adults: clinical features and surgical outcomes. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):863–871. doi:10.1007/s00417-016-3579-4
21. Shen YD, Yang CM. Extended silicone oil tamponade in primary vitrectomy for complex retinal detachment in proliferative diabetic retinopathy: a long-term follow-up study. Eur J Ophthalmol. 2007;17:954–960. doi:10.1177/112067210701700614
22. Han DP, Pulido JS, Mieler WF, Johnson MW. Vitrectomy for proliferative diabetic retinopathy with severe equatorial fibrovascular proliferation. Am J Ophthalmol. 1995;119(5):563–570. doi:10.1016/S0002-9394(14)70213-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
