Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Evaluation of factors for poor outcome in preterm newborns with posthemorrhagic hydrocephalus associated with late-onset neonatal sepsis
Authors Stevic M , Simic D , Ristic N , Budic I , Marjanovic V , Jovanovski-Srceva M , Repac N , Rankovic-Janevski M , Tasic G
Received 18 June 2018
Accepted for publication 17 September 2018
Published 10 October 2018 Volume 2018:14 Pages 1965—1973
DOI https://doi.org/10.2147/TCRM.S177535
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Marija Stevic,1 Dusica Simic,1 Nina Ristic,2 Ivana Budic,3 Vesna Marjanovic,3 Marija Jovanovski-Srceva,4 Nikola Repac,5 Milica Rankovic-Janevski,6 Goran Tasic5,7
1Department of Anesthesia, Medical Faculty University of Belgrade, Belgrade, Serbia; 2Department of Gastroenterology, University Children’s Hospital, Belgrade, Serbia; 3Department of Anesthesia, Medical Faculty University of Nis, Nis, Serbia; 4Department of Anesthesia, Medical Faculty University of Skopje, Skopje, Macedonia; 5Clinical Center of Serbia, Institute of Neurosurgery, Belgrade, Serbia; 6Department of the Specialized Care, Institute of Neonatology, Belgrade, Serbia; 7Department of Neurosurgery, Medical Faculty University of Belgrade, Belgrade, Serbia
Purpose: Preterm newborns, due to many factors, are at increased risk for poor neural development, intraventricular hemorrhages, infections, and higher rate of mortality. The aim of this study was to evaluate the risk factors associated with poor outcome in preterm neonates with late-onset neonatal sepsis (LONS) who had posthemorrhagic hydrocephalus and underwent neurosurgical procedures for treatment of the hydrocephalus.
Patients and methods: Preterm neonates who had undergone insertion of ventriculoperitoneal shunt or Ommaya reservoir, during the 10-year period at University Children’s Hospital, were retrospectively analyzed. According to the presence or absence of LONS, patients were divided into LONS group and non-LONS group. In both groups, we analyzed demographic and clinical data as well as nondependent factors. Additionally, we evaluated the patients who had lethal outcome in respect to all the analyzed factors.
Results: A total of 74 patients were included in the study, 35 in LONS group and 39 in control group. Patients in LONS group were born significantly earlier with lower birth weight, needed significantly higher O2 inspiratory concentration, and had longer duration of mechanical ventilation when compared to the nonseptic group. Five patients in LONS group had lethal outcome, and for these patients we identified a grade American Society of Anaesthesiologists score of 4 (P=0.000), ductus arteriosus persistens (P=0.000), bronchopulmonary dysplasia (P=0.003), and pneumothorax (P=0.003) as independent preoperative risk factors for lethal outcome.
Conclusion: Neurosurgical procedures are relatively safe in neonates with posthemorrhagic hydrocephalus without LONS after birth. However, if LONS is present, various conditions such as preoperative high grade American Society of Anaesthesiologists score, ductus arteriosus persistens, bronchopulmonary dysplasia, and pneumothorax markedly increase the risk for a lethal outcome after the operation.
Keywords: late-onset neonatal sepsis, risk factors, preterm infants, posthemorrhagic hydrocephalus, mortality
Introduction
Preterm newborns have increased morbidity and mortality rate due to a lot of factors. The literature describes various reasons including low birth weight, gestational age, and immaturity of the immune system, brain, and other organs, and has analyzed various prenatal conditions that make them more prone to sepsis, organ failure, increased need for intensive care, and even death. Even though many studies have studied each factor separately and in combination, the consensus and the debate are still ongoing.1–5
Many authors have studied the poor neurodevelopment in correlation to immaturity in preterm newborns focusing on factors that contribute to the occurrence of intraventricular hemorrhage (IVH),1 but, to our knowledge, evaluation of late-onset neonatal sepsis (LONS) as a predictor for poor outcome in preterm infants with posthemorrhagic hydrocephalus (PHH) has never been investigated, evaluated, or analyzed.
Despite controversies, progress in treatment of preterm newborns has been achieved. In line with the progress, the incidence of IVH and neurological morbidity are decreasing in preterm infants.1–3 PHH occurs in about 35% of the patients with IVH, with approximately 15% of them requiring permanent cerebrospinal fluid (CSF) diversion that can result in complications and death as the final outcome.2–8
On the other hand, LONS typically occurs after 3 postnatal days and represents an important cause of neurodevelopmental impairment with a mortality rate of 18% in premature infants hospitalized in the neonatal intensive care unit.9 Epidemiological data on newborns show that the predominant pathogens causing LONS are coagulase-negative staphylococci, followed by Gram-negative bacilli, and fungi. Apart from immaturity, other well-recognized risk factors for LONS include long-term use of invasive interventions, such as mechanical ventilation (MV), intravascular catheterization, prolonged parenteral nutrition, hospitalization, surgery, and underlying respiratory and cardiovascular diseases.9,10
Even though preterm newborns with LONS and PHH are at increased risk for morbidity, it is still not clear whether perinatal or postnatal, or combinations of both factors, influence their poor outcome.
The aim of this study was to analyze and evaluate the risk factors associated with poor outcome in preterm neonates with LONS who had PHH and underwent neurosurgical procedures for treatment of the hydrocephalus.
Materials and methods
Study material and outcome measurements
A retrospective review of the medical records of operated preterm newborns (<35 weeks of gestational age) was performed in the neonatal period (0–28 days old), who had been treated for IVH grade III or IV, at the University Children’s Hospital in Belgrade, between December 2005 and December 2015. All patients who were included in the study had been transferred from other medical centers and neonatology units. Patients were divided into two study groups: patients with LONS (diagnosed after birth) and control group which consisted of nonseptic patients (non-LONS group).
In both groups, we analyzed and compared baseline characteristics: gestational age (in weeks), sex, birth weight (in grams), head size (in centimeters), and Apgar score of 1 and 5 minutes. We also collected the information on the presence of preoperative conditions such as 1) preoperatively diagnosed IVH grade III or IV, 2) timing of late-onset sepsis (days from birth), 3) timing of neurosurgery (days from birth), 4) the type of neurosurgical procedure: ventriculoperitoneal (VP) shunt vs Ommaya reservoir, 5) the conversion of Ommaya reservoir to VP shunt, 6) multiple revision (≥2 revisions), 7) duration of hospitalization (days), 8) resuscitation after birth, 9) intubation in the first 3 days of life, 10) high inspiratory concentration of O2 in the first 24 hours of life ≥30%, 11) preoperative platelet count (PLT) <150.000/mL, 12) preoperative use of red blood cell (RBC transfusion), 13) preoperative use of inotropes such as dopamine or dobutamine, 14) MV, 15) MV longer than 7 days, 16) bronchopulmonary dysplasia (BPD), 17) pneumothorax, 18) respiratory distress syndrome (RDS), and 19) ductus arteriosus persistens (DAP).
Additionally, we analyzed the mortality rate in both groups, and subsequently (according to the lethal or nonlethal outcome), we also evaluated previously analyzed factors in respect to whether the lethal or nonlethal outcome was notified.
The exclusion criteria included presence of congenital anomalies, brain atrophy, diffuse cystic encephalomalacia, and spina bifida.
Definitions of procedures, conditions, and analyzed factors
Neurosurgical diagnosis and interventions: The indication for neurosurgery treatment was established after the development of convincing clinical symptoms and signs of elevated intracranial pressure. Clinical symptoms of raised intracranial pressure and PHH were rapidly increasing of head circumference, more than 1 cm/wk, full or tense anterior fontanel, and split sutures confirmed with serial ultrasounds as well as apnea/bradycardia occurrence. IVH was graded according to the Papile classification.11 Preoperative examination included computed tomography. The Ommaya reservoir or VP shunt were the operative neurosurgical techniques used in these patients. Indication for Ommaya reservoir was body weight of infants <1,500 g. Indication for VP shunt was body weight more than 1,500 g, tolerant of feeding, no possibility of developing necrotizing enterocolitis, repeated negative CSF microbiological cultures, and CSF protein <1.5 g/L.7,8 Surgery was performed under general anesthesia with the routine administration of prophylactic antibiotic ceftriaxone 100 mg/kg, and the same dose was continued for 5 days after the operation.
LONS was defined as a positive blood or CSF culture after the 3rd postnatal day, accompanied by treatment with antibiotics for at least 5 days (unless death occurred before 5th day of antibiotic treatment).3,9 Before the surgical procedure, systemic and intracerebral infections were ruled out by blood tests and using CSF samples.
BPD was defined as oxygen use at 36 weeks postmenstrual age or at discharge/transfer before 36 weeks in infants who survived to 36 weeks.3
Pneumothorax was defined as the presence of air in the pleural cavity, diagnosed by chest radiography.
RDS was diagnosed according to the presence of respiratory distress and a characteristic chest radiography.6
DAP was defined as the condition in which the ductus arteriosus fails to close after birth, diagnosed by echocardiography.
Metabolic acidosis was defined as a capillary pH value lower than 7.25 with normal pCO2 and capillary HCO3 values lover than 20.0 mM/L.
The American Society of Anesthesiologists-Physical Status score (ASA-PS) was defined as follows: class I, healthy patients; class II, patients with a mild systemic disease; class III, patients with a severe systemic disease; class IV, patients with severe systemic disease that is a constant threat to life; and class V, a moribund patient not expected to survive 24 hours with or without operation; and class VI, declared brain-dead and organs are being removed for donor purposes (Figure 1).
  | Figure 1 ASA-PS score. |
Lethal outcome/postoperative death was defined as the lethal outcome that occurred at any time during the hospitalization after completion of the neurosurgical procedure.
Statistical analysis
Descriptive measures were presented according to the variable characteristics, ie, the mean and SD for continuous variable and percentage for a categorical variable. Data distribution normality was tested using the coefficient of variation, Q–Q plots, and Kolmogorov–Smirnov test. The differences in means were tested using Student’s t-test for parametric variables and Mann–Whitney U test for nonparametric data. Differences in proportions were tested using chi-squared test or Fisher’s exact test. Descriptive measures were presented according to the variable characteristics, ie, mean and SD for parametric variable, the median and interval for nonparametric numeric variable, and percentage for a categorical variable. Statistical analysis was performed using SPSS for Windows (version 17.0, SPSS Inc., Chicago, IL, USA).
Ethical considerations
The study was approved by the Medical Faculty University of Belgrade Ethics Committee (No 29/V-14). All procedures performed in the study involving human participants were in accordance with the 1964 Declaration of Helsinki and its later amendments covering patient data confidentiality. The Ethics Committee of the Medical Faculty University of Belgrade waived the need for written informed consent from the patients because the study was retrospective. Data were deidentified to protect the privacy and maintain confidentiality of patient information.
Results
Patient population
During the 10-year period between December 2005 and December 2015, 97 infants with IVH grade III or IV were operated on at the University Children’s Hospital in Belgrade. Out of them, 74 patients met the criteria to be included in the study, with 39 patients with LONS being included in the LONS group and 35 patients in the non-LONS group. Demographic and clinical characteristics of both groups and comparison between the groups are shown in Figure 2.
  | Figure 2 Clinical study design and approaches. |
Patients and clinical characteristics
There was a significant difference between the groups in terms of gender (19 [70.4%] girls had LONS vs 20 [42.6%] boys, χ2=5.32, P=0.03), gestational age (30.46±3.63 in LONS group vs 32.29±3.1 in the non-LONS group, t=2.31, P=0.024), head size (28.98±3.1 in LONS group vs 31.37±3.4 in the non-LONS group, t=2.78, P=0.007), body weight (1,652.56±778.25 in LONS group vs 2,132.14±823.15 in the non-LONS group Mann–Whitney test, P=0.005), Apgar score at 5 minutes (5.46±2.1 in LONS group vs 6.66±1.74 in the non-LONS group Mann–Whitney test, P=0.070), ASA-PS score (3.26±0.72 in LONS group vs 2.26±0.56 in non-LONS group, t=6.63, P=0.000), and duration of hospitalization (59.15±45.88 in LONS group vs 33.06±14.55 in non-LONS group Mann–Whitney test, P=0.003). The mean time when the LONS was diagnosed was 7.15±2.40 days.
There was no statistically significant difference between the groups in the Apgar score at 1 minute, IVH grade III or IV, timing of neurosurgery, and the type of neurosurgery procedures, ie, Ommaya reservoir or VP shunt. Also, there was no significant difference in the case of conversion of Ommaya reservoir to VP shunt and in the terms of multiple revisions. None of the patients who were included in the study had four or more operations.
A summary of the patients’ clinical characteristics is shown in Table 1.
Preoperative clinical, laboratory finding, and conditions within the groups and between the groups
There were statistically significant differences between the groups in terms of high inspiratory concentration of O2 in the first 24 hours of life (35 [89.7%] in LONS group vs 16 [45.7%] in the non-LONS group, χ2=16.69, P=0.000), resuscitation after birth (30 [76.9%] in LONS group vs 6 [17.1%] in the non-LONS group, χ2=26.39, P=0.000), and intubation in the first 3 days of life (31 [79.5%] in LONS group vs 8 [22.9%] in the non-LONS group, χ2=23.73, P=0.000) with respect to the groups.
Laboratory and metabolic findings like preoperative low PLT count (28 [71.8%] in LONS group vs 6 [17.1%] in non-LONS group, χ2=22.18, P=0.000), preoperative metabolic acidosis (30 [76.9%] vs 10 [28.6%] in non-LONS group, χ2=17.36, P=0.000), and preoperative use of RBC transfusion (30 [76.9%] vs 11 [31.4%], χ2=15.45, P=0.000), also showed statistically significant differences when assessing the LONS and non-LONS groups.
There were statistically significant differences between the groups concerning preoperative use of inotropes (26 [66.7%] in LONS vs 4 [11.4%] in non-LONS group, χ2=23.35, P=0.000), MV (31 [79.5%] in LONS group vs 12 [34.3%] in non-LOS group, χ2=15.48, P=0.000), and MV longer than 7 days (26/31 [83.9%] in LONS group vs 3/12 [25%] in non-LONS group, χ2=13.56, P=0.001).
Pneumothorax occurred significantly more frequently in the LONS group (6 [15.4%] vs 0 [0%] than in the non-LONS group, Fisher’s exact test, P=0.026). There were statistically significant differences between the groups concerning RDS between groups (25 [64.1%] in LONS group vs 8 [22.9%] in the non-LONS group, χ2=12.7, P=0.000), BPD (6 [15.4%] in LONS group vs 0 [0%] in the non-LONS group, Fisher’s exact test, P=0.026), and DAP (14 [35.9%] in LONS group vs 0 [0%] in the non-LONS group, Fisher’s exact test, P=0.000). The summarized data reporting the preoperative clinical and laboratory findings and conditions within the groups and between the groups are shown in Table 2. The modes of preoperative MV are shown in Table 3.
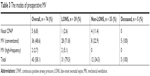  | Table 3 The modes of preoperative MV |
Analyses and evaluation in respect to the outcome (lethal or nonlethal)
A lethal outcome was found only in 5 (6.76%) patients in the LONS group. Sixty-nine (93.24%) patients had a nonlethal outcome. When we subsequently analyzed and evaluated data according to the lethal or nonlethal outcome, we found out that ASA-PC score 4 (t=−3.75, P=0.000), pneumothorax (Fisher’s exact test, P=0.003), BPD (Fisher’s exact test, P=0.003), and DAP (Fisher’s exact test, P=0.000) were significantly higher in patients that had lethal outcome.
On the other hand, statistically significant differences for gestational age, gender, head size, body weight, Apgar score in the 1st and 5th min, IVH grade III or IV, the high inspiratory concentration of O2 in the first 24 hours, intubation in the first 3 days of life, resuscitation after birth, LONS, the time when the LONS was diagnosed, time of neurosurgery, MV, MV longer than 7 days, preoperative use of RBC transfusion, preoperative low PLT count, preoperative metabolic acidosis, preoperative use of inotropes, and RDS were not found between the patients with or without lethal outcome. The summarized characteristics of analyzed factors with respect to the outcome are shown in Tables 4 and 5.
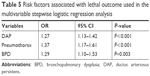  | Table 5 Risk factors associated with lethal outcome used in the multivariable stepwise logistic regression analysis |
Individual analyses of the patients with lethal outcome
There were a total of five postoperative deaths (6.76% of the entire cohort) in our study population, classified as being sepsis-related. Deaths occurring in infants with Gram-negative recurrent LONS accounted for 3/5 (60%). In one patient, death was associated with fungal infection, 1/5 (20%), and in one patient, the cause of lethal outcome was associated with polymicrobial infection, 1/5 (20%). One patient died 14 days after percutaneous endoscopic gastrostomy implantation associated with Gram-negative recurrent LONS. Two patients died 20 days after revision of VP shunt infection and obstruction. In the first patient, VP shunt was initially placed, and in the second patient Ommaya reservoir was indicated as initial treatment. In two patients, postoperative recovery was complicated with recurrent LONS associated with ventilator-associated pneumonia; they died 27 and 42 days after the operation, respectively. All patients who died were mechanically ventilated.
Gram-negative, polymicrobial, and fungal microorganisms that had caused recurrent LONS episodes, shunt infections, and obstructions, combined with prolonged MV, significantly and independently increased the likelihood of postoperative death.
Discussion
Our retrospective study evaluated and analyzed different factors that may be correlated with the poor outcome in patients with LONS who underwent neurosurgical treatment for PHH.
Literature is decisive that the early neonatal period, which lasts from birth to the 7th day of life, is the most dangerous period for a neonate, with an increased risk of morbidity and mortality, including prematurity, IVH, and complications of infections.3–5 In previous studies, authors reported a decrease in mortality rate in preterm neonates, but they emphasized the high risk of subsequent adverse neurodevelopmental outcomes in the survivors of IVH, and sequelae that frequently involved reduced developmental quotient, epilepsy, cerebral palsy, and cognitive impairment.5,12–14
Based on our 10-year experience, we did not have lethal outcomes in nonseptic patients who had undergone neurosurgery for PHH. The results in our study considering mortality seem to be logical since sepsis by itself is an enormous factor that leads to a lethal outcome. Several studies have also elaborated the epidemiological, clinical, and microbiological characteristics of patients with LONS and have confirmed that the low birth weight, gestational age, and immaturity of the immune system, may be additional factors that contribute to the lethal outcome in patients with LONS.1–5,9,10,15,16
Similarly, but using the different methodology in the present study, we showed that the patients with LONS were sicker, which could explain association of LONS in preterm neonates with lethal outcome. In the LONS group, patients had lower gestational age, head size, body weight, lower Apgar score at 5 minutes, longer hospital stay, and higher preoperative ASA-PS score than patient without sepsis, similar to the previous studies.3,5,6,13–16
Also in the present study, infants with LONS had higher incidence of preoperative comorbidities like BPD, RDS, DAP, metabolic acidosis, low PLT counts, and pneumothorax; this is in line with the results described in the previous studies which found a relationship with lethal outcome.3,5,6,15 Preterm infants with LONS required longer MV support and more often received preoperative transfusion and inotropes.5,13,16
The present study does not suggest that the type or timing of surgical intervention and grade III or IV are significant predictors of lethal outcome. Also, in the present study there was no statistically significant difference in case of conversion of Ommaya reservoir to VP shunt and in the terms of multiple revisions in both groups, which is in correlation with the results of other authors.7,8
We showed that preoperative ASA-PS score 4, pneumothorax, DAP, and BPD were strongly associated with lethal outcome in patients with LONS.
Although a number of authors have indicated that pneumothorax increased the degree of IVH, an association of pneumothorax with the frequency of IVH was not found.8,17–25 We found out that pneumothorax was strongly associated with mortality in operated newborns with PHH, which was not been described in the literature.
BPD is the most common chronic lung disease affecting preterm infants, and the incidence of BPD is inversely related to gestational age. It is associated with growth, health, and neurodevelopmental problems during childhood. According to the literature data, many authors have indicated BPD as a risk factor for IVH.2,26–30 The results of our study showed that BPD was a significant risk factor associated with lethal outcome in preterm infants, which, to our knowledge, has not yet been reported in the literature.
A patent ductus arteriosus in the first 3 days of life is a physiological shunt in healthy infants, but in preterm infants, it can have significant clinical consequences. More than 50% of preterm infants <28 weeks gestation receive medical or surgical therapy for DAP to prevent respiratory decompensation, heart failure, IVH, and brain injury.30 Women who take nonselective cyclooxygenase inhibitors such as indometacin prenatally, as well as tocolysis for preterm labor, are more likely to have an infant with DAP. In previous reports, the association between DAP and IVH was described, but the association between DAP and lethal outcome was not reported.2,27,31–34 In contrast, the positive correlation between DAP and lethal outcome was found in our study.
Multiple factors contribute to the risk of developing LONS, including invasive procedures and devices, lack of enteral feeding, and prolonged hospitalization. Different strategies for preventing hospital-acquired LONS include rigorous hand hygiene procedures, skin care, human milk feeding, discontinuing invasive devices when not needed, and standardization of processes related to central line care with central bundles. All preventive measures described above are important cost-effective strategies for reducing the frequency of LONS.3–6,9,10,35–39
In the present study, the association of recurrent LONS with lethal outcome after the operation may be explained as a result of existing postoperative comorbidities associated with unfavorable outcomes. Gram-negative, polymicrobial, and fungal infections, shunt infections, and obstructions could lead to prolonged MV and development of complications.3–8,40–44
Limitations of the study
The most significant limitation of this study is that it was a retrospective study from a single medical center, and not a prospective multicenter trial. Another significant limitation is that the small changes of investigated parameters of patients who died significantly changed the statistical result, which decreased the power of this study. Death was a multifactorial endpoint, and despite best efforts, any statistical analysis of postoperative contributing factors cannot be applied in such a small sample.
Conclusion
Neurosurgical procedures are relatively safe in neonates with PHH without LONS after birth. However, if LONS is present, various conditions such as preoperative high grade of ASA score, DAP, BPD, and pneumothorax markedly increase the risk for a lethal outcome after operation. Further studies are warranted to establish the causality of this association.
Author contributions
All authors contributed toward data analysis, drafting, and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Horbar JD, Edwards EM, Greenberg LT, et al. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatr. 2017;171(3):e164396. | ||
Robinson S. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr. 2012;9(3):242–258. | ||
Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314(10):1039–1051. | ||
du Plessis AJ. The role of systemic hemodynamic disturbances in prematurity-related brain injury. J Child Neurol. 2009;24(9):1127–1140. | ||
Stoll BJ, Hansen NI, Bell EF. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. | ||
Levit O, Bhandari V, Li FY, Shabanova V, Gallagher PG, Bizzarro MJ. Clinical and laboratory factors that predict death in very low birth weight infants presenting with late-onset sepsis. Pediatr Infect Dis J. 2014;33(2):143–146. | ||
Tröbs RB, Sander V. Posthemorrhagic hydrocephalus in extremely low birth weight infants: Ommaya reservoir vs. ventriculoperitoneal shunt. Childs Nerv Syst. 2015;31(8):1261–1266. | ||
Christian EA, Melamed EF, Peck E, Krieger MD, McComb JG. Surgical management of hydrocephalus secondary to intraventricular hemorrhage in the preterm infant. J Neurosurg Pediatr. 2016;17(3):278–284. | ||
Greenberg RG, Kandefer S, Do BT, et al. Late-onset Sepsis in Extremely Premature Infants: 2000–2011. Pediatr Infect Dis J. 2017;36(8):774–779. | ||
Tsai MH, Hsu JF, Chu SM, et al. Incidence, clinical characteristics and risk factors for adverse outcome in neonates with late-onset sepsis. Pediatr Infect Dis J. 2014;33(1):e7–e13. | ||
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. | ||
Scavarda D, Bednarek N, Litre F, et al. Acquired aqueductal stenosis in preterm infants: an indication for neuroendoscopic third ventriculostomy. Childs Nerv Syst. 2003;19(10–11):756–759. | ||
Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1–8. | ||
Cooke RW. Factors associated with periventricular haemorrhage in very low birthweight infants. Arch Dis Child. 1981;56(6):425–431. | ||
Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical, and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics. 2002;109(1):34–39. | ||
Boghossian NS, Page GP, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. J Pediatr. 2013;162(6):1120–1124. | ||
Lian C, Xie Z, Wang Z, et al. Pediatric preoperative risk factors to predict postoperative ICU admission and death from a multicenter retrospective study. Paediatr Anaesth. 2016;26(6):637–643. | ||
Cooke RW. Factors associated with periventricular haemorrhage in very low birthweight infants. Arch Dis Child. 1981;56(6):425–431. | ||
Hill A, Perlman JM, Volpe JJ. Relationship of pneumothorax to occurrence of intraventricular hemorrhage in the premature newborn. Pediatrics. 1982;69(2):144–149. | ||
Lipscomb AP, Thorburn RJ, Reynolds EO, et al. Pneumothorax and cerebral haemorrhage in preterm infants. Lancet. 1981;1(8217):414–416. | ||
Pishva N, Parsa G, Saki F, Saki M, Saki MR. Intraventricular hemorrhage in premature infants and its association with pneumothorax. Acta Med Iran. 2012;50(7):473–476. | ||
Sarkar S, Bhagat I, Dechert R, Schumacher RE, Donn SM. Severe intraventricular hemorrhage in preterm infants: comparison of risk factors and short-term neonatal morbidities between grade 3 and grade 4 intraventricular hemorrhage. Am J Perinatol. 2009;26(6):419–424. | ||
Van de Bor M, Van Bel F, Lineman R, Ruys JH. Perinatal factors and periventricular-intraventricular hemorrhage in preterm infants. Am J Dis Child. 1986;140(11):1125–1130. | ||
Wells JT, Ment LR. Prevention of intraventricular hemorrhage in the preterm infant. Early Hum Dev. 1995;42(3):209–233. | ||
Wallin LA, Rosenfeld CR, Laptook AR, et al. Neonatal intracranial hemorrhage: II. Risk factor analysis in an inborn population. Early Hum Dev. 1990;23(2):129–137. | ||
Cekmez F, Tanju IA, Canpolat FE, et al. Mean platelet volume in very preterm infants: a predictor of morbidities? Eur Rev Med Pharmacol Sci. 2013;17(1):134–137. | ||
Dani C, Poggi C, Barp J, Berti E, Fontanelli G. Mean platelet volume and risk of bronchopulmonary dysplasia and intraventricular hemorrhage in extremely preterm infants. Am J Perinatol. 2011;28(7):551–556. | ||
Hussein NF, Helaly NSE, Abdel Ghany EA, Anis SK. Relationship between mean plated volume and bronchopulmonary dysplasia and intraventricular hemorrhage in very low birth weight neonates. J Am Sci. 2012;8(5):544–560. | ||
Polin RA. Systemic infection and brain injury in the preterm infant. J Pediatr (Rio J). 2008;84(3):188–191. | ||
Clyman RI. Ibuprofen and patent ductus arteriosus. N Engl J Med. 2000;343(10):728–730. | ||
Behjati S, Emami-Naeini P, Nejat F, El Khashab M. Incidence of hydrocephalus and the need to ventriculoperitoneal shunting in premature infants with intraventricular hemorrhage: risk factors and outcome. Childs Nerv Syst. 2011;27(6):985–989. | ||
Lee JY, Kim HS, Jung E, et al. Risk factors for periventricular-intraventricular hemorrhage in premature infants. J Korean Med Sci. 2010;25(3):418–424. | ||
Letshwiti JB, Semberova J, Pichova K, Dempsey EM, Franklin OM, Miletin J. A conservative treatment of patent ductus arteriosus in very low birth weight infants. Early Hum Dev. 2017;104:45–49. | ||
Linder N, Haskin O, Levit O, et al. Risk factors for intraventricular hemorrhage in very low birth weight premature infants: a retrospective case-control study. Pediatrics. 2003;111(5 Pt 1):e590–e595. | ||
Lian C, Xie Z, Wang Z, et al. Pediatric preoperative risk factors to predict postoperative ICU admission and death from a multicenter retrospective study. Paediatr Anaesth. 2016;26(6):637–643. | ||
Kaplan HC, Lannon C, Walsh MC, Donovan EF; Ohio Perinatal Quality Collaborative. Ohio statewide quality-improvement collaborative to reduce late-onset sepsis in preterm infants. Pediatrics. 2011;127(3):427–435. | ||
Wirtschafter DD, Powers RJ, Pettit JS, et al. Nosocomial infection reduction in VLBW infants with a statewide quality-improvement model. Pediatrics. 2011;127(3):419–426. | ||
Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. J Infect. 2014;68(Suppl 1):S24–S32. | ||
Schulman J, Stricof R, Stevens TP, et al; New York State Regional Perinatal Care Centers. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127(3):436–444. | ||
Jensen US, Knudsen JD, Wehberg S, Gregson DB, Laupland KB. Risk factors for recurrence and death after bacteraemia: a population-based study. Clin Microbiol Infect. 2011;17(8):1148–1154. | ||
Jensen US, Knudsen JD, Ostergaard C, Gradel KO, Frimodt-Møller N, Schønheyder HC. Recurrent bacteraemia: A 10-year regional population-based study of clinical and microbiological risk factors. J Infect. 2010;60(3):191–199. | ||
Perlman SE, Saiman L, Larson EL. Risk factors for late-onset health care-associated bloodstream infections in patients in neonatal intensive care unit. Am J Infect Control. 2007;35(3):17–82. | ||
Samanta S, Farrer K, Breathnach A, Heath PT. Risk factors for late onset gram-negative infections: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2011;96(1):F15–F18. | ||
Benjamin DK, DeLong E, Cotten CM, Garges HP, Steinbach WJ, Clark RH. Mortality following blood culture in premature infants: increased with Gram-negative bacteremia and candidemia, but not Gram-positive bacteremia. J Perinatol. 2004;24(3):175–180. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



