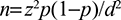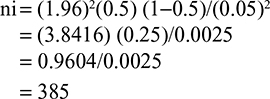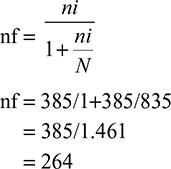Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 8
Evaluation of cotrimoxazole use as a preventive therapy among patients living with HIV/AIDS in Gondar University Referral Hospital, northwestern Ethiopia: a retrospective cross-sectional study
Authors Gebresillassie BM, Biruk M, Abegaz TM, Erku DA , Mekuria AB , Tadesse YD
Received 24 December 2015
Accepted for publication 21 April 2016
Published 7 July 2016 Volume 2016:8 Pages 125—133
DOI https://doi.org/10.2147/HIV.S103081
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Begashaw Melaku Gebresillassie,1 Minaleshewa Biruk Gebeyehum,1 Tadesse Melaku Abegaz,1 Daniel Asfaw Erku,2 Abebe Basazn Mekuria,3 Yokabd Dechassa Tadesse2
1Department of Clinical Pharmacy, 2Department of Pharmaceutical Chemistry, 3Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Purpose: Cotrimoxazole preventive therapy (CPT) is a feasible, inexpensive, and well-tolerated way of using cotrimoxazole intervention for patients living with HIV/AIDS to reduce HIV/AIDS-related morbidities and mortalities caused by various bacteria, fungi, and protozoa. The aim of this study was to evaluate the use of cotrimoxazole as a prophylaxis therapy among patients living with HIV/AIDS at Gondar University Referral Hospital (GURH), northwestern Ethiopia.
Materials and methods: A retrospective cross-sectional study was used to evaluate the use of cotrimoxazole as a prophylaxis therapy among people living with HIV/AIDS at GURH, northwestern Ethiopia from September 2013 to October 2015. Medical records of 264 patients were selected by using systematic random sampling technique from the sampling frame list of all patients’ medical records. Data were collected from patients’ medical records using the structured checklist and evaluated against World Health Organization (WHO) guidelines on the use of cotrimoxazole prophylaxis. The quantitative data were analyzed using the statistical packages for social sciences Version 20. Descriptive and binary logistic regression analyses were used to describe and assess the association between different variables.
Results: Approximately 95 (36.0%) patients were at WHO clinical stage III at the start of CPT. The use of CPT was consistent with the guidelines in the rationale for indication 200 (75.75%) and dose 263 (99.62%), despite the presence of contraindications in 24 (9.90%) patients. The occurrence of cotrimoxazole-associated side effects was higher in the first month of therapy. Problems regarding drug–drug interactions were identified in 63 (23.86%) patients, and 92 (34.84%) patients discontinued CPT due to different reasons.
Conclusion: Although the practice of discontinuation of CPT and follow-up for adverse drug effects were not consistent with WHO guidelines on the rational use of cotrimoxazole prophylaxis, the use of CPT among people living with HIV/AIDS at GURH was appropriate. Health professionals who were working in the antiretroviral therapy units should update themselves and adhere to the available updated guidelines to reduce the occurrence of adverse effects and prophylaxis failure.
Keywords: drug use evaluation, cotrimoxazole, HIV/AIDS, Gondar
Introduction
Antibiotics have major role in reducing the burden of communicable diseases all over the world. However, the ability of the drugs to cure an infection is not infinite. Antimicrobial resistance threatens the efficacy of successful treatment of infections, and it is a public health-related issue with national and global dimensions.1 As a group, antibiotics contribute significantly to the cost of medications and claimed globally to account for 15%–30% of total budget for health. They account for >50% of the value of medication sold in India. The increasing overuse of antimicrobials associated with the development of resistance as well as adverse drug reactions. In several occasions, the rational use of antimicrobials has been reported to reduce the emergence of resistant strains.2 Rational drug use is concerned with promoting the quality of care, preventing unnecessary drug exposure that leads to unwanted side effects, and cost-effective treatment, while maximizing therapeutic benefits and improving patient compliance.3
People living with HIV/AIDS (PLWHA) or coinfected by tuberculosis (TB), although whether or not they have access to ART, will develop HIV-related opportunistic infections due to their compromised immune system.4 So that, World Health Organization (WHO) has developed guidelines on cotrimoxazole preventive therapy (CPT) for HIV-related infections for children, adolescents, and adults in resource limited areas.5
Cotrimoxazole is a combination of two antimicrobial drugs (sulfa-methoxazole and trimethoprim) that is a broad spectrum antimicrobial agent targeting a variety of aerobic Gram-positive and Gram-negative organisms and protozoa. Therefore, it is of particular interest for prophylactic treatment, since it has a broad coverage against common bacteria, parasites, including toxoplasmosis and those causing chronic diarrhea, and fungi/yeasts, such as pneumocystics carinii pneumonia (PCP).6 The recommended dose of cotrimoxazole for adults living with HIV is 960 mg daily (800 mg sulfa-methoxazole +160 mg trimethoprim, either as a 960 mg double strength tablet or as a single 480 mg). The dosing of cotrimoxazole prophylaxis for children is optimized based on their age or body weight bands.7
In spite of this, wide scale use of cotrimoxazole prophylactic may increase the occurrence of antimicrobial resistance in communities to different pathogens.8 The emergency of bacterial resistance to antimicrobials has become a major problem of the world. Studies have reported that 22%–65% of antibiotic prescriptions were inappropriate.9,10
Lack of competent diagnostic methods and inadequate/poor knowledge of treatment regimens have contributed to incorrect drug choices, incorrect dosing, adverse drug reactions, and interactions, and use of more expensive drugs when less expensive drugs would be equally or more effective. Considering this problem, drug use evaluation has been recommended as a method for exploring and identifying inappropriate or unnecessary drug use that monitor, evaluate, and promote the rational drug use.11,12
Majority of the limited studies in the area of pharmaceutical sciences and services in Ethiopia have been focused on the assessment of prescription pattern and drug use evaluation in health institution-based setups. So far, in Gondar University Referral Hospital (GURH), there are no published reports aimed at evaluating the actual use of cotrimoxazole for prophylaxis in PLWHA. So the objective of this study was to dig out the drawbacks in the use of CPT and will generate information regarding cotrimoxazole drug use that will be used as a source of information to give emphasis on the gap between the current practice and the current guidelines for CPT use among PLWHA.
Materials and methods
Study area and design
An institution-based retrospective cross-sectional study was used on the rational use of cotrimoxazole as a preventive therapy among patients living with HIV/AIDS at GURH, northwestern Ethiopia. The hospital has 400 beds and a range of specialty departments, including internal medicine, pediatric, gynecology and obstetrics, surgery, psychiatry, HIV/AIDS care unit, and outpatient clinic (Gondar University Referral Hospital Statics and Information Center, unpublished data, 2015).
The source population consists of all medical records of PLWHA on CPT in the ART care unit of GURH. The study population includes all medical records of PLWHA on CPT in the ART clinic of GURH during the data collection period.
Sample size calculation and sampling technique
Using a single mean formula, the sample size was determined as follows:13,14
|
|
where n is the required sample size; d is the marginal error that is 5% (d=0.05); p is the proportion of CPT use among patients living with HIV/AIDS who came to Gondar University Referral Hospital (0.5 [50%]); and z is the required degree of accuracy at 95% confidence level =1.96.
Using the above formula
|
|
Since the sample was taken from the total population of 835 PLWHA who have been taking CPT, ie, <10,000, the final sample size was determined after using the correction factor. So that,
|
|
From the sampling frame of list of all medical records of patients living with HIV/AIDS who were on CPT at the study area, study subjects’ medical records were selected using systematic random sampling technique, and the sampling interval was calculated by using the following formula:
|
|
The starting number from the first three medical records was selected by simple random sampling technique, and it was 2. Starting from the second medical record, every Kth (third) medical record was included in the sample. Those medical records which did not fulfill the inclusion criteria or incomplete was substituted by the next patient’s medical record.
Inclusion and exclusion criteria
All medical records of patients living with HIV/AIDS who were on CPT at GURH were included in the study; however, patient medical records with incomplete data and unclear documentation were excluded.
Study variables
The independent variables include sociodemographic characteristics of the patient (age, sex, pregnancy status, educational, and marital status), patient’s clinical conditions (including WHO HIV clinical stages and comorbidities), and laboratory profiles (including CD4 counts and other laboratory tests). The dependent variables were indication/use, daily prescribed dose, frequency, duration of treatment, occurrence of side effects, monitoring of CPT, drug interaction, contraindications, and discontinuation of CPT treatment.
Data collection and quality assurance
Data were collected by six principal investigators using a data collection checklist, which was structured and adopted from different guidelines on medication use evaluation.3,7 The study subjects’ medical records were reviewed retrospectively for the rational use of cotrimoxazole as a preventive therapy in PLWHA. A pretest was also carried out on 20 randomly selected patients’ medical records that are not included in the study. After that, necessary modifications (on recording of laboratory results) were applied to the data collection checklist.
Operational definitions
CPT
CPT is a feasible, inexpensive, and well-tolerated way of using cotrimoxazole intervention in PLWHA to reduce HIV/AIDS-related morbidities and mortalities caused by various bacteria, fungi, and protozoa.
Drug use evaluation
It is a systematic, continuous, and criteria-based drug use evaluation that ensures the appropriate use of medications. The criteria to be reviewed in different studies include indications, prescribed doses, dosage form, route of administration, duration of therapy, contraindications, and drug interactions.
Rational/appropriate use
CPT is used according to the current WHO guideline on the rational use of cotrimoxazole prophylaxis and supplementary section to the 2013 WHO consolidated guideline.
Incomplete data
It is defined as insufficient data or loss of data regarding the disease and the medication (such as indication, dose, frequency, and duration) and/or patient profiles.
Unclear data
It is defined as data that do not clearly indicate about the case of the patient such as clinical characteristics and different doses given for a single patient.
Contraindications
The followings were considered as contraindications for CPT in this study: severe allergy to sulfa drugs, first trimester of pregnancy, severe renal insufficiency (Cr >1.5 mg dL), severe hepatic disease (SGPT >115 IU/L for male and 90 IU/L for female), and bone marrow suppression (severe pancytopenia).
Data processing and analysis
The data collected using the quantitative method was entered and analyzed using statistical packages for social sciences Version 20 statistical software computer program. Statistical analyses were performed using SPSS v.20 (SPSS Inc., Chicago, IL, USA). Frequencies, percentages, and regression analysis were used to analyze different variables. CI 95% and P-value <0.05 were used as cut points for determining the significance of association.
Ethical consideration
This study was conducted after ethical clearance was requested and gained from the research and ethics review committees of School of Pharmacy and the Clinical Directorate of GURH. Then the permission to collect data was obtained after official letters were written to the heads of ART clinic and information and statistics department of the hospital, since data collection was conducted by reviewing the medical records of patients on cotrimoxazole use as a preventive therapy among PLWHA. Moreover, all other concerned bodies were informed about aim and purpose of the study. Since the study did not directly involve the patients, there was no direct risk to study subjects for participating in the study. Therefore, informed consent was not sought from the study participants, but we made sure that confidentiality of the information was assured in such a way that no disclosure of any patient’s or health care provider’s name and drug product in relation to the finding was made.
Results
Sociodemographic characteristics
During a 4-week data collection period, a total of 264 study subjects’ medical records were included in the study for analysis. The mean age of respondents was 28 years, with SD of 10 years. Majority of the participants were females (61%) and 15 of them were pregnant. About two-thirds (61.74%) of the patients were married, and most of them (43.39%) had attended primary school (1–8) (Table 1).
Description about CPT use among PLWHA
Indication to start
Ninety-five (35.98%) of the PLWHA presented with WHO clinical stage III, and 79 (29.92%) of the PLWHA presented with WHO clinical stage II of HIV infection at the start of CPT. Thirty-seven (14.01%) of the PLWHA developed TB, 26 (9.84%) of the PLWHA developed fungal infection, and two (0.75%) of the PLWHA developed PCP as a comorbid illness. Majority of the PLWHA, 190 (72.00%), had a CD4 count of <350 cell/mm3 (Table 2).
Dose and timing of CPT initiation
Approximately 259 (98.10%) of the PLWHA received correct dose of CPT, and majority of the patients, 139 (52.65%), initiated CPT before ART initiation; from these, 72 (27.30%) of the patients started before 2 weeks of ART initiation, and 65 (24.60%) of the patients started at 2 weeks prior to ART. Both CPT and ART were initiated concurrently in 119 (45.10%) of the patients, and ART was started before CPT in a few number of patients (Table 3).
Cotrimoxazole use when there is contraindication
Cotrimoxazole was used by 24 (9.09%) patients in spite of its contraindication in these patients. Majority of these patients were first trimester pregnant (eight [33.33%]), followed by patients who had severe allergy to sulfa drugs (seven [29.16%]) (Table 4).
Discontinuation of CPT
In 63 (23.86%) patients, cotrimoxazole interaction with zidovudine (the prior may enhance the myelosuppressive effect of zidovudine and lead to anemia) was documented, and no other interactions involving cotrimoxazole were encountered. From these, 92 (34.84%) patients discontinued CPT for different reasons, including peptic ulcer (16 [17.39%]), CD4 count >350 cells/mm3 (12 [13.03%]), and skin rash (13 [14.13%]), were found, but in 39 patients, the reason for cessation was not documented (Table 5).
Duration of CPT use and monitoring
The duration of cotrimoxazole prophylaxis therapy was assessed among the study population, and majority of the patients (73.9%) stayed on the prophylaxis therapy for >6 months followed by 1–6 months duration (20.1%) as illustrated in Figure 1. During the follow-up visits, only in eleven (4.2%) patients, regular monitoring for every 3 months (clinical examination and laboratory tests, such as CD4, complete blood count [CBC], serum creatinine, liver function tests, and blood urea nitrogen) was done and recorded for any adverse reactions and efficacy, whereas monitoring results were not recorded for 1.5% patients (Figure 2).
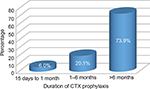  | Figure 1 Length of stay of PLWHA on CPT. Abbreviations: CPT, cotrimoxazole preventive therapy; CTX, cotrimoxazole; PLWHA, people living with HIV/AIDS. |
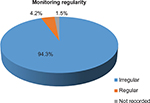  | Figure 2 Monitoring schedule for PLWHA on CPT. Abbreviations: CPT, cotrimoxazole preventive therapy; PLWHA, people living with HIV/AIDS. |
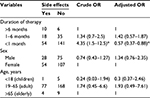
In the binary logistic regression test, sex and age were not found to have statistically significant effects on the occurrence of side effects. However, among the duration of therapy, patients who took <1 month (AOR =0.57, 95% CI =0.37–12.5) were more likely to encounter side effects than those who took the medication beyond a month. This statistically significant association was observed in the multivariate analysis when controlled for confounders (Table 6).
Appropriateness of CPT use
In summary, only 170 (64.40%) of the patients were found to be fully appropriate. The remaining patients were inappropriate for different reasons. One is incorrect discontinuation for nondocumented reasons. The others include continuation of CPT when CD4 count was >350 cells/mm3 after 6 months of ART and inappropriate initiation (Figure 3).
  | Figure 3 Appropriateness of CPT use among PLWHA in GURH. Abbreviations: CPT, cotrimoxazole preventive therapy; GURH, Gondar University Referral Hospital; PLWHA, people living with HIV/AIDS. |
Discussion
This study has tried to assess the pattern of rational use of CPT among PLWHA in GURH. Drug use evaluation (DUE) is one among the ways to ensure that drugs are used appropriately. If the use was deemed inappropriate, intervention with patients or providers was necessary to optimize the drug therapy.15 The goal of antibiotic therapy is to achieve the best possible clinical outcomes with a reduced risk for developing resistance while consuming the least amount of hospital resources. Therefore, studying the use of cotrimoxazole as a preventive therapy among PLWHA helps to understand the way that cotrimoxazole is being utilized in this hospital. In this study, majority of the patients (61%) on CPT were females, which was in agreement with the observations reported from Hawassa Referral Hospital (64.80%) and Jimma Teaching Specialized Hospital (70.99%).15,16
For the initiation of cotrimoxazole prophylaxis, about three-fourths of the patients initiated CPT with CD4 counts <350 cell/mm3 or symptomatic for the prevention of opportunistic infections, such as PCP, TB, and toxoplasmosis, which was in agreement with the findings reported from study conducted by WHO HIV/AIDS program officers in 69 selected countries and Jimma University Specialized Hospital.17,18 However, in some of the patients (who were WHO stage 1 and CD4 level >350 cells/mL), CPT was initiated without any symptomatic disease, which was not in-line with WHO guideline on the rational use of cotrimoxazole prophylaxis and supplementary section to the 2013 WHO consolidated guideline; this finding was also higher than the value obtained from Boru Meda Hospital, which was 2%.19 This might result in an increase in the risk of cotrimoxazole adverse effects and increases the risk of antibiotic resistance since at this time there is no need to initiate the prophylaxis because the patients’ immunity is strong enough to protect against opportunistic infections.20
Almost all (98.10%) of the patients received appropriate dose of CPT, which was higher than the finding obtained from a retrospective evaluation study conducted in Hawassa (87%) and Jimma (85.93%). Even though the rational use CPT regarding the dose was encouraging, the prescribers should strictly adhere to the available national guidelines beyond the dose of the prophylaxis.
Despite its contraindication, ∼9.09% patients used cotrimoxazole against its contraindications. This finding was in-line with the finding reported from Boru Meda, which was 8.06%. Compared with the study conducted in Jimma, which was 6.41%, this finding was higher. This might have resulted because of the lack of health professionals paying attention to contraindications and nonadherence to the available updated guidelines, such as WHO guideline on the rational use of cotrimoxazole prophylaxis and supplementary section to the 2013 WHO consolidated guideline.
WHO and Ethiopian Federal Ministry of Health recommended the reasons for discontinuation of CPT in PLWHA for better treatment outcome and for preventing potential drug-related problems.21–23 Regarding this, out of 92 patients who discontinued CPT, approximately two-thirds of them were discontinued according to the WHO guideline on the rational use of cotrimoxazole prophylaxis and supplementary section to the 2013 WHO consolidated guideline, whereas the rest (approximately one-third) of them discontinued without any documented data for their discontinuation. These findings were lower than that reported from Boru Meda and Jimma, which were 75% and 76.60%, respectively.15,19 The reason attributed for this was the lack of organized documentation system during every visit to the hospital.
Regarding the occurrence of cotrimoxazole-associated adverse events, 6.06% patients developed gastrointestinal discomfort, such as peptic ulcer disease, while 4.92% patients developed skin rash during the course of prophylaxis. Similarly, a study conducted in Zambia on CPT use among children (1–14 years) showed that 0.3% of the respondents developed skin rash.24 Compared to this study, the occurrence of adverse events in GURH was higher. This might have resulted because of health professionals’ ignorance in assessing their patients thoroughly and recording the results appropriately during each visit or it could be related to maldispensing practice/common dispensing short-comings in the ART pharmacy, such as poor counseling, medication history taking, and lack of organized recording system of the patient profile.
In addition, the binary logistic regression analysis test revealed that the duration of prophylaxis has statistically significant predictor in the occurrence of side effects. Among the duration of therapies, patients who took <1 month (AOR =0.57, 95% CI =0.37–12.5) were more likely to develop side effects (peptic ulcer and skin reactions) than those who took the medication beyond a month. This statistically significant association was observed in the multivariate analysis while controlling for confounders. This report was in agreement with evidence from a multicenter study conducted by Dutch AIDS treatment group, which reported that most of the adverse reactions that required discontinuation frequently were observed during the first month of cotrimoxazole use.25
In approximately quarter of the patients, cotrimoxazole had interaction with zidovudine, which is lower compared with the finding from Boru Meda that was 49.59%. Cotrimoxazole may enhance the myelosuppressive effect of zidovudine, and this interaction might cause bone marrow suppression, and thus, hematologic abnormalities, such as anemia, thrombocytopenia, and neutropenia, might occur.4,19 This in turn leads to the lack of adherence and poor patient outcomes.
According to the WHO guideline on the rational use of cotrimoxazole prophylaxis and supplementary section to the 2013 WHO consolidated guideline, it is recommended for patients to have laboratory tests before initiating cotrimoxazole and regular follow-up initially monthly and then every 3 months. If the drug is well tolerated, children should be evaluated monthly.7,20 Regarding this, in ∼94.31% of patients, follow-up monitoring was made irregularly, whereas in 1.51%, it was not recorded. Monitoring parameters in adults should include CD4 count and CBC measurements every 3 months,7,20 but in this study, during follow-up, CD4 count and CBC were determined for only 4.20% and 2.30% patients, respectively. Although other tests, such as renal and liver function tests, were available in this hospital, due to lack of giving attention/ignorance, majority of the patients took CPT without being assessed for their kidney function level, which may lead to crystalluria for those with renal failure. On the other hand, the report from Hawassa Referral Hospital did not address laboratory monitoring of CBC (hemoglobin) due to the lack of data on these profile.16 As compared with this hospital, laboratory measurement of hematological profile was good in GURH, even though it was made for less number of patients.
Strengths and limitations
Even though this research was conducted at right time where resistance, treatment failure, and adverse reactions occur, the major concern was the retrospective nature of the study that might be taken as a limitation. However, considering the time and cost needed for conducting prospective research, retrospective research was feasible and better option in a resource-limited setup, such as GURH. Moreover, use of the updated CPT treatment guideline was also taken as a strength.
Conclusion
In this study, the overall use of cotrimoxazole as a prophylaxis therapy among patients living with HIV/AIDS was consistent with WHO guidelines on the rational use of cotrimoxazole prophylaxis. The highest consistency was found regarding to rationale for dose, indication, and discontinuation. However, its use despite the presence of contraindications and inappropriate discontinuation of CPT was not in-line with the WHO guideline. In certain patients, the lowest consistency was observed in monitoring the adverse reactions during follow-up visits; along with this, factors that affect the adherence to the monitoring schedule and problems regarding drug–drug interaction were identified and justified. So, to improve the outcome, the practice of timing of initiation, discontinuation, follow-up for adverse drug effects, and documentation during each visit should be improved through continuously updating the health professionals and implementing and adhering strictly to the updated national guidelines.
Acknowledgments
The authors acknowledge the support of GURH Statistics and Information Center and ART pharmacy staffs for making the required materials accessible. Financial support was provided only by the authors.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Longo DL, Kasper DL, Fauci AS, Jameson JL, Hauser SL, Loscalzo J. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012. | ||
Stanley SW. Concepts in Drug Utilization Reviews. Washington, DC: Washington State University; 1999. | ||
Drug Administration and Control Authority of Ethiopia (DACA). Training Modules on Operation and Management of Special Pharmacies. 2nd ed. Addis Ababa: DACA; 2002. | ||
Keith LP, Laurence LB, John SL. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill; 2005:1029–1057. | ||
Graham SM. Cotrimoxazole prophylaxis for infants exposed to HIV infection. Bull World Health Organ. 2004;82:297–298. | ||
Kubacka RT. A primer on drug utilization review. J Am Pharm Assoc (Wash). 1996;36(4):257–261. | ||
World Health Organization. The Use of Co-Trimoxazole Prophylaxis for HIV-Related Infections Among Adults, Adolescents and Children. Geneva: WHO Press; 2013. | ||
Gunten V, Troillet N, Beney J, et al. Impact of an interdisciplinary strategy on antibiotic use: a prospective controlled study in three hospitals. J Antimicrob Chemother. 2005;55(3):362–366. | ||
Rehana HS, Nagarani MA, Rehan M. A study on the drug prescribing pattern and use of antimicrobial agents at a tertiary care teaching hospital in Eastern Nepal. Indian J Pharmacol. 2013;30(3):175–180. | ||
World Health Organization. WHO Expert Consultation on Cotrimoxazole Prophylaxis in HIV Infection. Geneva: WHO; 2006. | ||
Palaian S, Mishra P, Shankar PR, Dubey AK, Bista D, Almeida R. Safety monitoring of drugs. Kathmandu Univ Med J. 2006;4(1):119–127. | ||
WHO Collaborating Center for International Drug Monitoring; Uppsala. International Monitoring of Adverse Reactions to Drugs: Adverse Reaction Terminology. Geneva: WHO; 2010. | ||
Lwanga SK, Lemeshow S. Sample Size Determination for Health Studies: A Practical Manual. Geneva, Switzerland: World Health Organization; 1991:1–5. | ||
Getu D, Tegbar Y. Lecture Notes for Health Sciences: Research Methodology. Gondar: University of Gondar; 2006:47–50. | ||
Diriba L, Worku F, Girma T. Evaluation of prophylactic use of cotrimoxazole for people living with HIV/AIDs in Jimma University Specialized Hospital, Ethiopia. Ethiop J Health Sci. 2008;18(3):59–64. | ||
Deresse D, Alemayehu T. Evaluation of the use of co-trimoxazole prophylaxis in people living with HIV/AIDS in Hawassa Referral Hospital, a retrospective evaluation. Asian J Med Sci. 2009;1(3):88–90. | ||
Anand AD, Marco V, Reuben G, Mazuwa B, Mayada Youssef F, Charlie G. Implementation of cotrimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull World Health Organ. 2010;88(4):253–259. | ||
Degu AD, Kefale BG, Temesgen GT. Drug use evaluation of cotrimoxazole prophylaxis in people living with human immunodeficiency virus/acquired immune deficiency syndrome at Jimma University Specialized Hospital, Jimma, Ethiopia. Int J Basic Clin Pharmacol. 2014;3(2):343–349. | ||
Geresu B, Misganaw D, Beyene Y. Retrospective evaluation of cotrimoxazole use as preventive therapy in people living with HIV/AIDS in Boru Meda Hospital. BMC Pharmacol Toxicol. 2014;15:4. | ||
WHO. Guidelines on Cotrimoxazole Prophylaxis for HIV-Related Infections Among Children, Adolescents and Adults: Recommendation for Public Health Approach. Geneva, Switzerland: WHO; 2006. | ||
World Health Organization/UNAIDS Provisional (WHO/UNAIDS). Recommendations on the use of cotrimoxazole prophylaxis in adults and children living with HIV/AIDS in Africa. Afr Health Sci. 2001;1(1):30–31. | ||
Chambers F, Jawetz E. Sulfonamides-trimethoprim and quinoloes. In: Katzung BG, editor. Basic and Clinical Pharmacology. 11th ed. New York, NY: McGraw Hill; 2009:778. | ||
Federal Ministry of Health. Guidelines for Cotrimoxazole Prophylaxis in HIV/AIDS Care and Treatment. Addis Ababa: Federal Ministry of Health; 2006. | ||
Sarah W, Veronica M, Deborah F, et al. The impact of daily co-trimoxazole prophylaxis and antiretroviral therapy on mortality and hospital admissions in HIV-infected Zambian children. Clin Infect Dis. 2007;44(10):1361–1367. | ||
Schneider MM, Nielson TL, Nelsing S, et al. Efficacy and toxicity of two doses of trimethoprim-sulfamethoxazole as primary prophylaxis against Pneumocystis carinii pneumonia in patients with human immunodeficiency virus. J Infect Dis. 1995;171(6):1632–1636. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.