Back to Journals » Clinical Ophthalmology » Volume 14
Evaluation of Corneal Biomechanical Parameters in Psoriasis Patients: A Controlled Study
Authors Edris NA, Arfeen SA, Mosaad R, Nassar GA
Received 2 April 2020
Accepted for publication 16 June 2020
Published 30 June 2020 Volume 2020:14 Pages 1833—1837
DOI https://doi.org/10.2147/OPTH.S256629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Noha A Edris,1 Shaimaa A Arfeen,1 Rana Mosaad,2 Ghada A Nassar1
1Ophthalmology Department, Cairo University, Faculty of Medicine, Cairo, Egypt; 2Dermatology Department, Cairo University, Faculty of Medicine, Cairo, Egypt
Correspondence: Ghada A Nassar 10 Said Street, Heliopolis, Cairo 11757, Egypt
Tel +201222397300
Fax +20226677060
Email [email protected]
Purpose: To evaluate corneal biomechanical parameters with an ocular response analyzer (ORA) in patients with psoriasis and compare these parameters with age-matched control subjects.
Study Design: This was a cross-sectional observational case–control study.
Methods: Thirty eyes of 15 psoriasis patients were included in the study and compared with 30 eyes of 15 control subjects. Corneal biomechanical properties were calculated by ORA. Central corneal thickness (CCT) was measured by anterior-segment optical coherence tomography. The main outcome measures were corneal hysteresis (CH), corneal resistance factor (CRF), corneal-compensated intraocular pressure (IOPcc), and Goldmann-correlated IOP (IOPg). For dry-eye evaluation, Schirmer’s test was used.
Results: Mean CH in the psoriasis group was 10.20± 1.55 mmHg and in the control group 10.66± 1.36 mmHg (p=0.215). Mean CRF in the psoriasis group was 9.76± 1.60 mmHg and in the control group 10.97± 1.42 mmHg (p=0.003). Mean IOPcc in the psoriasis group was 14.84± 3.43 mmHg and in the control group 16.67± 3.17 mmHg (p=0.035). Mean IOPg in the psoriasis group was 13.92± 3.35 mmHg and in the control group 16.62± 3.10 mmHg (p=0.002). Mean CCT in the psoriasis group was 543.90± 37.27 μm and in the control group 551.23± 28.63 μm (p=0.392). Schirmer’s test results in the psoriasis group were 11.4± 1.57 mm/5 min and in the control group 17.5± 1.52 mm/5 min (p< 0.001).
Conclusion: Psoriasis affects corneal biomechanical properties with statistically significantly lower corneal biomechanics than normal. CH correlates negatively with disease activity. These corneal biomechanical changes should be considered when determining IOP values and during corneal evaluation for keratoconus-suspected patients.
Keywords: psoriasis, corneal hysteresis, corneal resistance factor, corneal-compensated intraocular pressure, ocular response analyzer
Introduction
Psoriasis is a chronic autoimmune disease of unknown etiology. One of the most accepted theories for its pathogenesis is abnormal infiltration by T lymphocytes and neutrophils as a result of inappropriate cellular immuno function. It may affect all ages, with high incidence in early adulthood.1 The disease is characterized by excessive growth of cutaneous epithelial cells with formation of inflamed, raised plaques that constantly shed scales. Other systemic manifestations include the nails, joints, and eyes.2–4 Ocular manifestations of psoriasis have been reported in the literature, with blepharitis, conjunctivitis, uveitis, and dry-eye syndrome being the most known.5
The effects of chronic autoimmune diseases on corneal biomechanics have been reported in different studies in which ocular response analyzers (ORAs) were used for evaluation of different biomechanical parameters, ie, corneal hysteresis (CH), corneal resistance factor (CRF), corneal-compensated intraocular pressure (IOPcc), and Goldmann-correlated IOP (IOPg).6,7 Limited studies have been done on psoriasis as a chronic inflammatory disease that may affect corneal biomechanical properties. In this study, we evaluated the corneal biomechanical properties of central corneal thickness and IOP with Goldmann applanation tonometry (GAT) in psoriasis patients and compared them with healthy normal controls.
Methods
The study was performed following the tenets of the Declaration of Helsinki and was approved by the ethics committee of the Ophthalmology Department. Informed consent was obtained from all subjects before participating in the study. This controlled study took place between January 2019 and September 2019 at an institutional hospital. Psoriasis patients were consecutively recruited from the Dermatology Department. Control subjects included patients coming to the ophthalmology outpatient clinic for routine eye checkups. We included 30 eyes of 15 patients with psoriasis vulgaris and 30 eyes of 15 age- and sex-matched controls. Exclusion criteria were keratoconus, corneal opacities, severe dry-eye syndrome, glaucoma, ocular hypertension, use of systemic steroids or contact lenses, and previous surgery or trauma. The diagnosis of psoriasis was established by dermatological examination of the patients. All patients were treated with vitamin D analogues, salicylic acid, phenolic compounds, and topical steroids.
The scoring system used for psoriasis was the Psoriasis Area and Severity Index (PASI).10 This is the gold standard for the assessment of extensive psoriasis, but has the limitation of interobserver variation, so two independent observers were involved in evaluating patients. Four sites affected — the head (h), upper limbs (u), trunk (t), and lower limbs (l), are scored separately using three parameters: erythema, induration, and desquamation. Each of these parameters is graded on a severity scale of 0, 4, where 0 = nil, 1 = mild, 2 = moderate, 3 = severe, and 4 = very severe. The area-wise percentage involvement of the involved sites is calculated as 1 = <10% area, 2 = 10%–29%, 3 = 30%–49%, 4 = 50%–69%, 5 = 70%–89%, and 6 = >90%. The final formula for a PASI score is: 0.1 (Eh +lh +Dh) Ah + 0.2 (Eu + lu +Du) Au + 0.3 (Et + lt + Dt) At + 0.4 (El + ll + Dl) Al. The maximum PASI score is 72. A score of 75 represents a 75% reduction of baseline PASI score, and is commonly considered a denominator for satisfactory results of psoriasis treatment. The PASI score can measure the disease activity and the control of this activity by treatment.8
Ophthalmologic Examination
All subjects underwent a detailed ophthalmologic examination, including medical history and best-corrected visual acuity using a Snellen chart measured in decimally. The anterior segment was examined by slit-lamp biomicroscopy. Schirmer’s test under topical anesthesia and corneal fluorescein staining were done for all subjects. Two minutes after one drop of proparacaine 0.5% had been instilled, a Schirmer strip was placed at the outer third of the lower-lid margin. After 5 minutes, the strip was removed and the wet part measured in millimeters.9 IOP was measured with GAT. The fundus examination was done using a 90 D lens, and any pathological retinal findings were recorded.
Corneal Biomechanical Measurement by Ocular Response Analyzer
Corneal biomechanical measurements were evaluated with an ORA(Reichert Ophthalmic Instruments, Buffalo, NY, USA) for all patients and control groups. All measurements were taken between 10 to 12 am to avoid the effect of diurnal variation in corneal parameters.10 Parameters were evaluated before any contact procedures to avoid their effect on corneal biomechanical properties. CH, CRF, IOPcc, and IOPg were recorded. For analysis of measurements, the formula of best waveform score (WS) was used to obtain the best-quality readings. This is a numerical parameter of 0–10 that provides an estimation of the reliability of each ORA measurement. ORA measurements with WS <3.7 should be discarded in normal healthy subjects. For satisfactory corresponding quality of the WS ORA scan, a single measurement with WS>7.5 can be considered sufficient.11 Central corneal thickness (CCT) was measured by anterior-segment optical coherence tomography using an RTVue RT100 (Optovue, Fremont, CA, USA). Primary outcome measures were CH, CRF, IOPcc, and IOPg in all patients and controls. Secondary outcome measures were correlations between PASI scores and corneal biomechanical parameters in the psoriasis group.
Statistical Analysis
Data were coded and entered using SPSS version 25 (IBM, Armonk, NY, USA). The Shapiro–Wilk test was done before data analysis to check data distribution for numerical values. Data were summarized using means ± SD and ranges for quantitative data and frequency (count) and relative frequency (percentage) for categorical data. Comparisons between quantitative variables were done using unpaired t-tests. For comparing categorical data, χ2 tests were performed. Fisher’s exact test was used instead when the expected frequency was <5. Correlations between quantitative variables were calculated using Spearman’s correlation coefficient. Linear regression analysis was done with ocular parameters as dependent variables and groups as independent predictor to adjust for sex. p<0.05 was considered statistically significant.
Results
Epidemiology and Clinical Data
Sixty eyes were included in the study: 30 eyes of 15 patients that fulfilled the inclusion criteria in the psoriasis group and 30 eyes of 15 subjects in the control group. Mean duration of psoriasis in the study group was 5.8±4.6 years. Mean age in the psoriasis group was 44.73±11.44 years, while in the control group it was 41.94±9.94 years. The difference in age was not statistically significant (p=0.312). In the psoriasis group, 18 (60%) were male and 12 (40%) female. In the control group, 19 (63.33%) were male and eleven (36.67%) were female. The difference in sex distribution between the two groups was not statistically significant (p=0.7). In the psoriasis group, mean best-corrected visual acuity(expressed decimally) in the right eye was 0.7±0.2 and in the left eye 0.8±0.2, while in the control group it was 0.82±0.1 in the right eye and 0.87±0.1 in the left eye (p=0.174). In the psoriasis group, IOP measured by GAT was 13.89±1.05 mmHg, while in the control group it was 15.33±1.76 mmHg (p=0.001). No significant difference was found between IOPg and IOP measured by GAT (p=0.85). The psoriasis patients showed significant dry eye on Schirmer’s test under topical anesthesia. In the psoriasis group, this was 11.4±1.57 mm/5 min, while in the control group it was 17.5±1.52 mm/5 min (p<0.001).
Corneal Biomechanical Properties
Clinical measurements with the ORA revealed that mean CH was lower in the psoriasis group than in the control group, but the difference was not statistically significant.Mean CRF was significantly lower in the psoriasis group. Mean IOPcc and IOPg were significantly lower in the psoriasis group. Mean CCT was lower in the psoriasis group, but the difference was not statistically significant. Measurements of corneal biomechanical properties for both groups are shown in Table 1. There was a significant positive correlation between CCT, IOPcc, and IOPg and the PASI scoring system. Both CH and CRF showed negative correlations with the activity score. Values of correlations between corneal parameters in the psoriasis group and the PASI are summarized in Table 2.
 |
Table 1 Corneal Biomechanical Measurements in the Psoriasis And Control Groups |
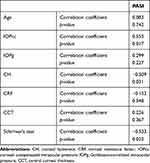 |
Table 2 Correlations Between Different Parameters of the Psoriasis Group And the Scoring System (PASI) |
Discussion
The relationship between the eye and psoriasis has been known for a long period. Ocular manifestations associated with psoriatic arthritis were first reported in 1976 by Lambert and Wright, with conjunctivitis being the most common complication, followed by iritis. Many studies have correlated the severity of ocular inflammation with the psoriatic skin disease rather than with the extent of joint impact.12,13
The effect of psoriasis on the cornea has not been extensively studied. The main pathology that has been reported to affect the cornea secondary to psoriasis is keratoconjunctivitis sicca; however, the effect of psoriasis as a chronic autoimmune disease on the corneal structure has not been well studied.14 T lymphocytes, other types of inflammatory leukocytes, and inflammatory cytokines play an important role in the pathogenesis of psoriasis.1,15 Hogan et al proved the presence of T lymphocytes in the epithelium and stroma of the normal human cornea, even in the absence of any pathological process. They suggested their migration into the cornea from limbal vessels, but their exact function is still not well understood.16
In this study, the authors hypothesized that infiltration of the cornea by lymphocytes and other inflammatory cytokines as a result of inappropriate cellular immuno function might occur with subsequent alteration in corneal biomechanics. On studying the corneal biomechanics of psoriatic patients, it was found that both CRF and CH were lower in the psoriatic group than the normal control group, and the difference was statistically significant for CRF.
CH represents the viscous properties of the cornea and indicates collagen lamella-organization change, while CRF reflects the elastic characteristics of the cornea. There has been no reported evidence for an association between CH and CRF; however, CRF is thought to be a better indicator of the total elastic property of the cornea.17,18 Corneal biomechanical properties may be determined by two corneal components: corneal collagen fibers and the viscous ground substance in which the fibers are embedded, consisting of proteoglycans and glucosaminoglycans, both providing viscoelasticity.19
As our results showed a significant negative correlation between disease activity and CH, we suggest an effect of altered immun- response and T lymphocytes on the viscous background of corneal lamellar fibers with subsequent weakening of proteoglycans and glucosaminoglycans. However, this theory needs further investigation with electron microscopy. Previous studies have reported the opposite strengthening effect in the corneas of diabetic patients with an increase in corneal biomechanical properties.20
We also found a significant negative correlation between disease activity and CH. As such, CH may play a role as an indicator of disease activity; however, this finding needs further studies. IOPcc in psoriatic patients was higher than both IOPg and IOP GAT. Although that was statistically in significant (p=0.29), it may be a flag when measuring IOP in psoriatic patients, as it may lead to underestimation of IOP in patients susceptible to IOP elevation as a complication of steroid intake throughout their treatment course.
Our study is limited by its small sample and cross-sectional design. We recommend conducting further prospective studies with larger samples and follow-up for better understanding of the correlation between disease activity and changes in corneal biomechanical findings.
In conclusion, this study revealed that psoriasis affects corneal biomechanical properties measured with an ORA. Statistically significant negative correlations between disease activity and CH and CRF were recorded. These corneal biomechanical changes should be considered when determining IOP values. These measurements are important in detection of glaucoma suspects or early glaucoma. It may identify the risk of glaucoma progression and differentiate early keratoconus from healthy corneas in psoriasis patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lebwohl M. Psoriasis. Lancet. 2003;361(9364):1197–1204. doi:10.1016/S0140-6736(03)12954-6
2. Bolognia JL, Jorizzo JL, Rapini RP, et al. Psoriasis. Dermatology. Vol. 1. London: Mosby; 2003:125–149.
3. Wolf K, Goldsmith LA, Katz SI, et al. Psoriasis. Fitzpatrick Dermatology in General Medicine.
4. Sugai J, Ozawa A, Kawakubo Y, Izuka M, Miyahara M, Okhido M. New method for determining prognosis of patients with psoriasis (E-PAP). J Dermatol Sci. 1998;16:165–169. doi:10.1016/S0923-1811(97)00047-9
5. Kirby B, Fortune DG, Bhushan M, Chalmers RJ, Griffiths CE. The salford psoriasis index: an holistic measure of psoriasis. Br J Dermatol. 2000;142:728–732. doi:10.1046/j.1365-2133.2000.03418.x
6. Tas M, Oner V, Ozkaya E, et al. Evaluation of corneal biomechanical properties in patients with rheumatoid arthritis: a study by ocular response analyzer. Ocul Immunol Inflamm. 2014;22:224–227. doi:10.3109/09273948.2013.841957
7. Emre S, Kayikçioglu O, Ates H, et al. Corneal hysteresis, corneal resistance factor, and intraocular pressure measurement in patients with scleroderma using the Reichert ocular response analyzer. Cornea. 2010;29:628–631. doi:10.1097/ICO.0b013e3181c3306a
8. Bhor U, Pande S. Scoring systems in dermatology. Indian J Dermato lVenereolLeprol. 2006;72:315–321. doi:10.4103/0378-6323.26722
9. Her Y, Lim JW, Han SH. Dry eye and tear film functions in patients with psoriasis. Jpn J Ophthalmol. 2013;57:341–346. doi:10.1007/s10384-012-0226-4
10. Laiquzzaman M, Bhojwani R, Cunliffe I, Shah S. Diurnal variation of ocular hysteresis in normal subjects: relevance in clinical context. Clin Experiment Ophthalmol. 2006;34(2):114–118. doi:10.1111/j.1442-9071.2006.01185.x
11. Vantomme M, Pourjavan S, Detry-Morel M. The range of the waveform score of the ocular response analyzer (ora) in healthy subjects. Bull SocBelgOphthalmol. 2013;322:91–97.
12. Lambert JR, Wright V. Eye inflammation in psoriatic arthritis. Ann Rheum Dis. 1976;35(4):354–356. doi:10.1136/ard.35.4.354
13. Okamoto F, Umebayasi Y, Ohtsuka F, Hommura S. Factors associated with increased aqueous flare in psoriasis. Jpn J Ophthalmol. 2001;45(2):172–176. doi:10.1016/S0021-5155(00)00359-2
14. Kilic B, Dogan U, Parlak AH, et al. Ocular findings in patients with psoriasis. Int J Dermatol. 2013;52(5):554–559. doi:10.1111/j.1365-4632.2011.05424.x
15. Gottlieb AB. TNF-alpha and apoptosis: implications for the pathogenesis and treatment of psoriasis. J Drugs Dermatol. 2002;1:264–275.
16. Hogan MJ, Zimmerman LE. Ophthialinic Pathology.
17. Dupps WJ
18. Lau W, Pye D. A clinical description of ocular response analyzer measurements. Invest Ophthalmol Vis Sci. 2011;52:2911–2916. doi:10.1167/iovs.10-6763
19. Terai N, Raiskup F, Haustein M, Pillunat LE, Spoerl E. Identification of biomechanical properties of the cornea: the ocular response analyzer. CurrEye Res. 2012;37(7):553–562.
20. Scheler A, Spoerl E, Boehm A. Effect of diabetes mellitus on corneal biomechanics and measurement of intraocular pressure. Acta Ophthalmol (Copenh). 2012;90(6):447–451. doi:10.1111/j.1755-3768.2012.02437.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
