Back to Journals » Research and Reports in Urology » Volume 8
Evaluation of biochemical recurrence in patients with high-risk prostate cancer treated with radical prostatectomy and radiotherapy plus androgen deprivation therapy
Authors Yamamoto Y , Kiba K, Yoshikawa M, Hirayama A, Kunikata S, Uemura H
Received 26 August 2016
Accepted for publication 4 November 2016
Published 2 December 2016 Volume 2016:8 Pages 225—231
DOI https://doi.org/10.2147/RRU.S120748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Yutaka Yamamoto,1 Keisuke Kiba,1 Motokiyo Yoshikawa,1 Akihide Hirayama,1 Seiji Kunikata,1 Hirotsugu Uemura2
1Department of Urology, Nara Hospital, Kindai University Faculty of Medicine, Ikoma, 2Department of Urology, Kindai University Faculty of Medicine, Osakasayama, Japan
Objective: The aim of this study was to evaluate the biochemical recurrence (BCR) in patients with high-risk prostate cancer (PCa) treated with radical prostatectomy (RP) or radiotherapy (RT) plus androgen deprivation therapy (ADT).
Methods: Subjects were patients with National Comprehensive Cancer Network-defined high-risk PCa treated with either RP or RT plus ADT. We calculated BCR-free survival in patients with those treatments and evaluated risk factor against BCR.
Results: A total of 114 patients, 71 RP and 43 RT plus ADT, were evaluated. A total of 59 and 20.9% of patients experienced BCR in the RP and RT treatment groups, respectively. The 5-year BCR-free survival probabilities improved significantly for patients who received RT compared to those who received RP (81.3 vs 37.3%, P<0.001). According to the number of risk factors, 59.2% of patients in the RP and 51.2% of patients in the RT treatment groups were classified with one risk factor (P<0.014). The 5-year BCR-free survival probabilities for patients treated with RP were 46.6 and 21.7% for one and multiple risk factors, respectively (P=0.008). On univariate analysis, only the number of risk factors had a significant impact on the risk of BCR. Meanwhile, there were no significant differences in the 5-year BCR-free survival probabilities between one and multiple risk factors in patients treated with RT.
Conclusion: Among patients treated with RP, a marked heterogeneity existed in the oncological outcomes. Based on these findings, the number of risk factors should be emphasized to decide the optimal treatments for patients with high-risk PCa.
Keywords: high-risk prostate cancer, radical prostatectomy, radiotherapy plus androgen deprivation therapy, biochemical recurrence, number of risk factors
Introduction
Prostate cancer (PCa) is the most common malignancy in men in Western industrialized countries.1 PCa is an extremely heterogeneous affliction that ranges from organ confined to a metastatic disease, and 15% of all PCa cases are diagnosed as high-risk disease.2 Radiotherapy (RT) plus androgen deprivation therapy (ADT) is recommended as the primary management for patients with high-risk PCa, and radical prostatectomy (RP) is a second option.3,4 However, due to the lack of prospective randomized controlled trials between these two modalities, the optimal treatment for high-risk PCa is still a matter of controversy. Several retrospective studies that compared outcomes after RP and RT for high-risk PCa have shown widely disparate results. Some reported better outcomes after RP,5–7 while others reported improved outcomes after RT,8,9 and a few reported equivalent efficacy.10,11 The reasons for the differences in treatment outcomes may be due to various factors, including selection biases, definition of high-risk disease and the use of adjuvant therapy after local treatment. It has been reported that patients who received RP are younger and healthier than patients who received RT.12,13 Also, according to the definition of high-risk category, D’Amico classification defines clinical T2c as a high-risk factor,14 whereas National Comprehensive Cancer Network (NCCN) classifies clinical T2c as an intermediate risk.3 Furthermore, adjuvant therapies, including RT and ADT, after surgery may significantly delay the time to biochemical recurrence (BCR). In this situation, it is important to precisely evaluate the clinical efficacy of RP monotherapy. However, few studies have compared the oncological outcomes after RP monotherapy and RT plus ADT, although guidelines recommend RP or RT plus ADT for high-risk PCa.
Currently, the defined risk classification of PCa consists of risk features that include initial prostate-specific antigen (PSA), biopsy Gleason score (G-S) and clinical stage. Although this classification is simple and useful, the range is wide, especially in the high-risk category. Recently, several investigators have reported that a subclassification of high-risk PCa is beneficial for predicting PCa-specific mortality (PCSM) and BCR after RP and RT.15–21 They concluded that high-risk PCa is a heterogeneous cohort and does not have a uniform prognosis after definitive local treatment. Again, however, these reports also include patients who had received secondary interventions, such as RT and/or ADT after local treatment.
In this study, we compared BCR-free survival after RP monotherapy and RT plus ADT for patients with high-risk PCa. Furthermore, we determined whether the classification of high-risk PCa with the numbers of risk factors is associated with the incidence of BCR.
This study was approved by the Institutional Review Board (IRB) of Nara Hospital, Kindai University Faculty of Medicine. Patient consent was omitted by the IRB of our hospital by the following measures. To ensure a patient’s privacy, personal data were managed as unlinkable anonymous data. The personal data were deleted from the data of this research objective, and to hold anonymity of the research objective, a new number was given for each research objective. A correspondence list between new numbers and the research objective was not created.
Methods
We retrospectively investigated patients with high-risk PCa who had been treated with RP alone or RT plus ADT at Nara Hospital, Kindai University Faculty of Medicine, between January 2007 and December 2013. Patients with an Eastern Cooperative Oncology Group Performance Status of 0 or 1 at diagnosis were eligible for this study. Initial PSA >20 ng/mL, biopsy G-S 8–10, or clinical stage T3a is defined as a high-risk PCa according to the NCCN guidelines. Digital rectal examination (DRE), abdominal computed tomography (CT), pelvic magnetic resonance imaging (MRI), and bone scan were performed in all patients. Patients who had clinical evidence of regional lymph node disease or distant metastasis were excluded. In the RP group, patients who received concurrent ADT and/or adjuvant RT were also excluded. All needle biopsy specimens were reviewed by a single pathologist. The decision for patients to undergo RP or RT plus ADT was at the discretion of the attending physician and patient preference. RP was performed using an open retropubic approach, along with bilateral pelvic lymphadenectomy. BCR after RP was defined as PSA level >0.2 ng/mL with two consecutive increases or PSA not decreasing to <0.2 ng/mL after RP.22 If BCR was diagnosed, salvage ADT or RT was started immediately. As an external beam RT, three-dimensional conformal RT (3D-CRT) was carried out until December 2010 and, thereafter, intensity-modulated RT (IMRT) was delivered. 3D-CRT was delivered by a standard fractionation scheme, for a total dose of 70 Gy in 35 fractions. IMRT was delivered by a volumetric modulated arc therapy under computerized optimization to avoid critical organs with inverse planning for a total dose of 78 Gy in 39 fractions. All patients received concurrent ADT consisting of a luteinizing hormone–releasing hormone (LH–RH) agonist with an antiandrogen. Because of the retrospective nature of this study, the period of concurrent ADT was decided at the discretion of the attending physician. BCR after RT plus ADT was defined with the use of Phoenix definition (nadir plus 2 ng/mL).23 If BCR was diagnosed, salvage ADT was started immediately. Adverse events as a result of RT plus ADT were evaluated at each visit during and after treatment. Severity of adverse events was graded according to NCI-CTCAE v4.0. The primary objective was to compare the incidence of BCR between RP and RT plus ADT. Baseline patient characteristics were also evaluated for their impacts on the incidence of BCR. BCR-free survival was calculated by the Kaplan–Meier method. Univariate analyses were performed using the Cox proportional hazards regression model for evaluation of baseline patient characteristics to the incidence of BCR. P-value <0.05 was considered to be significant. Statistical Package for the Social Sciences Version 23.0 (SPSS, Chicago, IL, USA) was used for all statistical analyses.
Results
Patient characteristics
A total of 114 patients, 71 RP alone and 43 RT plus ADT group, met the eligibility criteria and were evaluable for this analysis. The median follow-up periods were 59.1 (range, 9.0–106.9) and 54.5 (range, 29.2–107) months in the RP and RT plus ADT groups, respectively (P=0.345). Table 1 shows the baseline characteristics of the patients in each group. Patients who received RT plus ADT were significantly older than those who received RP, with a median age of 73 and 70 years, respectively (P=0.027). Also, patients who received RT plus ADT had higher PSA (P=0.007) and higher biopsy G-S (P<0.001) than those who received RP. According to the number of risk factors, 59.2, 36.6 and 4.2% of patients in the RP alone and 51.2, 23.2 and 25.6% of patients in the RT plus ADT were classified with one, two and three high-risk factors, respectively (P=0.014). In the RT plus ADT groups, 15 patients received 3D-CRT and 28 patients received IMRT. All patients received concurrent maximal androgen blockade. LH–RH agonists had been administered in all patients. Thirty-one patients received leuprolide, and 13 patients received goserelin acetate. A total of 93.2% of the patients received bicalutamide, and the remaining patients received flutamide. Sixteen patients received neoadjuvant ADT and, the remaining 27 patients received both neoadjuvant ADT and adjuvant ADT. The median duration of concurrent ADT was 21.4 (range, 9.2–28.9) months.
Treatment outcomes
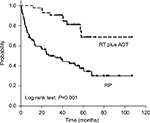
Overall BCR-free survival is shown in Figure 1. Patients who experienced BCR were 59.2% (n=42) in the RP and 20.9% (n=9) in the RT plus ADT. No patients died in the RP group, whereas two patients died, including one patient who died from PCa, in the RT plus ADT group. The 5-year BCR-free survival probabilities improved significantly for patients who received RT plus ADT (81.3%) compared with patients who received RP (37.3%) (P<0.001). In the RP group, surgical pathology showed T3a in 52% (n=37) and T3b in 12.7% (n=9). Two patients with pT3b had pathological lymph node metastasis (Table 1). Patients with pT3b received immediate RT because their PSA level did not decrease to <0.2 ng/mL after surgery. Patients with pT3a were observed without any adjuvant therapy until BCR was confirmed. Salvage treatments including RT and ADT were administered immediately following BCR. Eventually, of the 42 patients with BCR, 30 patients received salvage RT and the remaining 12 patients received salvage ADT. No significant difference was observed between 3D-CRT and IMRT for BCR-free survival probabilities (data not shown).
In the RP group, 42 patients had one risk factor and 29 patients had multiple risk factors. Patients with multiple risk factors were observed to have significantly worse BCR-free survival than those with one risk factor. The 5-year BCR-free survival probabilities of patients with one and multiple risk factors were 46.6 and 21.7%, respectively (P=0.008, Figure 2). In the RT plus ADT group, 22 patients had one risk factor and 21 patients had multiple risk factors. In contrast to the RP, there was no significant difference in the 5-year BCR-free survival probabilities between one and multiple risk factors. The 5-year BCR-free survival probabilities of patients with one and multiple risk factors were 86.3 and 80.1%, respectively (P=0.589, Figure 3).
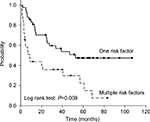  | Figure 2 Kaplan–Meier analysis of biochemical recurrence-free curves according to the number of high-risk factors for patients treated with radical prostatectomy. |
  | Figure 3 Kaplan–Meier analysis of biochemical recurrence-free curves according to the number of high-risk factors for patients treated with radiotherapy plus androgen deprivation therapy. |
As shown in Table 2, the univariate analysis shows that neither the age at diagnosis nor the risk factors including primary PSA, baseline G-S and clinical T stage were associated with the incidence of BCR in the RP group. Only the number of risk factors had a significant impact on the incidence of BCR (hazard ratio [HR], 1.826; 95% confidence interval [CI], 1.043–3.534; P=0.018). On the other hand, in the RT plus ADT group, no baseline variables were associated with risk of BCR based on the univariate analysis. Additionally, differences in the risk of BCR were not observed relative to the number of risk factors (HR, 1.487; 95% CI, 0.436–7.320; P=0.589).
These results suggest that neither baseline variables nor the number of risk factors may influence the incidence of BCR for patients who received RT plus ADT.
Adverse events
According to the RT plus ADT group, adverse events of any grade were experienced by 14 patients (32.6%). The most common adverse events were diarrhea (16.3%), anal pain (9.3%) and urinary frequency (9.3%). Only two patients experienced grade 3 adverse events (Table 3). No patients experienced therapy interruption due to adverse events.
  | Table 3 Adverse events following RT plus ADT Abbreviations: ADT, androgen deprivation therapy; RT, radiotherapy. |
Discussion
In this study, we evaluated patients with NCCN-defined high-risk PCa treated with RP alone or RT plus ADT. The 5-year BCR-free survival probabilities were significantly lower in the RP group compared to those in the RT plus ADT group, even though the RT plus ADT group had higher PSA and higher biopsy G-S than those in the RP group. Although no large-scale prospective randomized trials have been established, several retrospective studies have compared RP with RT. Boorjian et al11 reported the outcomes after RP and external beam RT (EBRT) for 1,847 patients who were classified with high-risk PCa according to the NCCN criteria. Nearly two-thirds of the patients received RP, and the remaining patients received EBRT with or without ADT. The oncological outcome was evaluated with the use of systemic progression defined as metastases on bone scan or on biopsies outside of the prostatic bed. The estimated 10-year probabilities of systemic progression-free survival rates were 85, 88 and 81% for patients treated with RP, EBRT plus ADT, and EBRT alone, respectively. They concluded that treatment modality did not affect the systemic progression. Arcangeli et al9 reported outcomes for 284 patients with high-risk PCa treated with RP or RT in combination with 9 months of ADT. They showed an improvement in 3-year BCR-free survival favoring RT in combination with ADT (86.8 vs 69.8%; P=0.001). With regard to RT plus ADT, our result on the BCR rate is similar to that of this previous report. However, the BCR rate after RP may be rather inferior to previous reports and the reason for that may be that no patients received adjuvant RT and/or ADT in our study, whereas in the previous study, 68% of the patients received adjuvant therapies. Indeed, the EORTC trial 22911 reported that adjuvant RT after RP demonstrates an improvement in BCR-free survival compared with no postsurgical treatment (74.0 vs 52.6%, P<0.001).24
In this study, we compared the incidence of BCR as an early oncological outcome. However, a recent study described that BCR after RP and RT is associated with different risks of PCSM because of the different definitions of BCR for each modality.25 Another study has also shown that the time to BCR after RT may be longer when compared with RP.26 On the contrary, some evidence suggests that the interval from BCR to metastatic progression is shorter after RT when compared with RP.27,28 Their findings suggest that BCR may not be an adequate surrogate marker of metastatic progression or PCSM when the different definitions of BCR are used. Therefore, our results should be interpreted with caution and further follow-up is needed. Furthermore, with respect to RT plus ADT, the possible influence of the concurrent androgen suppression should be taken into account. These patients experienced transient testosterone suppression. Arcangeli et al9 reported that the median interval to reach normal testosterone level (≥3 nmol/L) after ADT was 25 months for patients with high-risk PCa. In this study, the median follow-up period was relatively short and may reduce the incidence of BCR. Based on these factors, our results should be interpreted.
This study has also shown that the number of risk factors had a significant impact on risk of BCR for patients treated with RP. Meanwhile, in the RT plus ADT, differences in the incidence of BCR were not observed according to the number of risk factors. Several studies have reported the association between preoperative variables and oncological outcomes for patients with high-risk PCa. Yossepowitch et al20 stratified high-risk PCa patients who received RP into eight subgroups based on preoperative variables. According to this classification system, the 5-year relapse-free probability after surgery alone ranged from 49 to 80%. They concluded that high-risk PCa does not have a uniform prognosis after RP. Joniau et al18 proposed more simplified classification of high-risk PCa treated with RP. They stratified patients into three groups, and the 10-year OS rates were 88.3, 88.8 and 79.7% for the good, intermediate and poor prognosis subgroups, respectively. Considering these reports, the oncological outcomes of RP in high-risk PCa may vary with the use of novel classification system. Some investigators have also showed the association between the number of risk factors and oncological outcome for patients who received RP. Walz et al15 reported that in the 887 RP series, patients with only one risk factor had the most favorable 5-year BCR-free survival probabilities (50.3%), relative to patients with more than two risk factors (27.5%). The authors concluded that the BCR-free survival may vary substantially, depending on the number of high-risk factors. Reese et al29 also investigated the 5-year BCR-free survival rates of 798 patients with high-risk PCa treated with RP. They reported that the 5-year BCR-free survival probabilities with one and multiple risk factors were 51.2 and 40.0%, respectively (P<0.01). Our result on the 5-year BCR-free survival is similar with these reports. In our study, the 5-year BCR-free survival probabilities of RP were 46.6 and 21.7% for one and multiple risk factors, respectively (P=0.008). These results suggest that nearly half of the patients with one high-risk factor may be cured with RP monotherapy. On the other hand, it may be difficult to cure with RP alone for patients with multiple risk factors. Yamamoto et al17 reported that curing patients with multiple high-risk factors using RP monotherapy is difficult; therefore, RP for them should be considered as a first step to be followed with further treatments that include ADT and RT. Meanwhile, in the RT plus ADT group, differences in the risk of BCR were not observed relative to the number of risk factors. Most studies focused on surgically managed high-risk PCa. To the best of our knowledge, only one retrospective study has evaluated a classification system of high-risk PCa treated with RT. Muralidhar et al examined 1,185 patients with high-risk PCa treated with RT.21 They showed that favorable high-risk patients defined as T1c with either G-S 4+4=8 and PSA <10 ng/mL or G-S 6 and PSA >20 ng/mL had significantly better PCSM than other patients with high-risk disease (adjusted HR 0.42, P=0.049). Our findings differed from these results, and the differences could be attributable as follows. First, they excluded patients with any Gleason grade 5 disease because of the significantly worse outcomes in prior work. However, our study included patients with Gleason grade 5 disease (70.5%). In men with high-risk PCa treated with definitive therapy, Gleason grade 5 disease had worsened the time to PSA failure compared to those without grade 5 component.30 Second, they included patients who were not given concurrent ADT. One-third of the patients did not receive ADT, and the remaining patients received only 4 months of ADT. Moreover, patients with favorable high-risk disease were less likely to receive ADT than those with other high-risk diseases. Our study excluded patients who had not received concurrent ADT. NCCN recommends 2–3 years ADT for high-risk PCa when treated with RT. These factors may have contributed to the different outcomes.
There are some limitations in this study, including its retrospective nature, the relatively small number of patients analyzed and the relatively short duration of concurrent ADT for RT patients. A significant limitation of our analyses is the use of BCR as a primary endpoint. Different definitions of BCR for RP and RT may cause difficulty in comparing oncological outcomes. Furthermore, with not a long follow-up period resulting in a very few events, especially in RT plus ADT, further studies with longer follow-up will be needed.
Conclusion
We reported the oncological outcomes of RP monotherapy and RT plus ADT for patients with high-risk PCa. The 5-year BCR-free survival probabilities were significantly lower in the RP group than in the RT plus ADT group. However, among patients who received RP, a marked heterogeneity existed in the oncological outcomes. Our findings suggest that the number of risk factors should be taken into account when deciding the optimal treatment for patients with high-risk PCa.
Acknowledgment
We would like to thank Mr Marco A De Velasco for his assistance in proofreading the article.
Disclosure
The authors report no conflicts of interest in this work.
References
Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localized disease. Eur Urol. 2011;59(1):61–71. | ||
Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–1123. | ||
National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology, Prostate Cancer. Fort Washington, PA: NCCN; 2015. | ||
Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65(1):124–137. | ||
Nguyen PL, Chen MH, Catalona WJ, Moul JW, Sun L, D’amico AV. Predicting prostate cancer mortality among men with intermediate to high-risk disease and multiple unfavorable risk factors. Int J Radiat Oncol Biol Phys. 2009;73(3):659–664. | ||
Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28(9):1508–1513. | ||
Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer. 2010;116(22):5226–5234. | ||
Fletcher SG, Mills SE, Smolkin ME, Theodorescu D. Casematched comparison of contempory radiation therapy to surgery in patients with locally advanced prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66(4):1092–1099. | ||
Arcangeli G, Strigari L, Arcangeli S, et al. Retrospective comparison of external beam radiotherapy and radical prostatectomy in high-risk, clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75(4):975–982. | ||
Akakura K, Suzuki H, Ichikawa T, et al. A randomized trial comparing radical prostatectomy plus endocrine therapy versus external beam radiotherapy plus endocrine therapy for locally advanced prostate cancer: results at median follow-up of 102 months. Jpn J Clin Oncol. 2006;36(12):789–793. | ||
Boorjian SA, Karnes RJ, Viterbo R, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117(13):2883–2891. | ||
Meng MV, Elkin EP, Latini DM, Duchane J, Carroll PR. Treatment of patients with high risk localized prostate cancer: results from Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE). J Urol. 2005;173(5):1557–1561. | ||
Klein EA, Ciezki J, Kupelian PA, Mahadevan A. Outcomes for intermediate risk prostate cancer: are there advantages for surgery, external radiation, or brachytherapy? Urol Oncol. 2009;27(1):67–71. | ||
D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. | ||
Walz J, Joniau S, Chun FK, et al. Pathological results and rates of treatment failure in high-risk prostate cancer patients after radical prostatectomy. BJU Int. 2011;107(5):765–770. | ||
Lodde M, Harel F, Lacombe L, Fradet Y. Substratification of high-risk localized prostate cancer treated by radical prostatectomy. World J Urol. 2008;26(3):225–229. | ||
Yamamoto S, Kawakami S, Yonese J, et al. Long-term oncological outcome and risk stratification in men with high-risk prostate cancer treated with radical prostatectomy. Jpn J Clin Oncol. 2012;42(6):541–547. | ||
Joniau S, Briganti A, Gontero P, et al. Stratification of high-risk prostate cancer into prognostic categories: a European multi-institutional study. Eur Urol. 2015;67(1):157–164. | ||
Spahn M, Joniau S, Gontero P, et al. Outcome predictors of radical prostatectomy in patients with prostate-specific antigen greater than 20 ng/ml: a European multi-institutional study of 712 patients. Eur Urol. 2010;58(1):1–7. | ||
Yossepowitch O, Eggener SE, Bianco FJ Jr, et al. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. J Urol. 2007;178(2):493–499. | ||
Muralidhar V, Chen MH, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828–835. | ||
Heidenreich A, Aus G, Bolla M, et al. European Association of Urology. EAU guidelines on prostate cancer. Eur Urol. 2008;53(1):68–80. | ||
Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–974. | ||
Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380(9858):2018–2027. | ||
Lee BH, Kibel AS, Ciezki JP, et al. Are biochemical recurrence outcomes similar after radical prostatectomy and radiation therapy? Analysis of prostate cancer-specific mortality by nomogram-predicted risks of biochemical recurrence. Eur Urol. 2015;67(2):204–209. | ||
Hernandez DJ, Nielsen ME, Han M, et al. Natural history of pathologically organ-confined (pT2), Gleason score 6 or less, prostate cancer after radical prostatectomy. Urology. 2008;72(1):172–176. | ||
Lee WR, Hanks GE, Hanlon A. Increasing prostate-specific antigen profile following definitive radiation therapy for localized prostate cancer: clinical observations. J Clin Oncol. 1997;15:230–238. | ||
Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597. | ||
Reese AC, Pierorazio PM, Han M, Partin AW. Contemporary evaluation of the National Comprehensive Cancer Network prostate cancer risk classification system. Urology. 2012;80(5):1075–1079. | ||
Nanda A, Chen MH, Renshaw AA, D’Amico AV. Gleason pattern 5 prostate cancer: further stratification of patients with high-risk disease and implications for future randomized trials. Int J Radiat Oncol Biol Phys. 2009;74(5):1419–1423. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.