Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Evaluation of α-synuclein and apolipoprotein E as potential biomarkers in cerebrospinal fluid to monitor pharmacotherapeutic efficacy in dopamine dictated disease states of Parkinson’s disease and schizophrenia
Authors Gupta AK, Pokhriyal R, Das U , Khan MI, Ratna Kumar D, Gupta R, Chadda RK, Ramachandran R, Goyal V, Tripathi M, Hariprasad G
Received 15 February 2019
Accepted for publication 5 June 2019
Published 19 July 2019 Volume 2019:15 Pages 2073—2085
DOI https://doi.org/10.2147/NDT.S205550
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Video abstract presented by Gururao Hariprasad.
Views: 304
Ashish Kumar Gupta,1 Ruchika Pokhriyal,1 Uddipan Das,1 Mohd Imran Khan,1 Domada Ratna Kumar,1 Rishab Gupta,2 Rakesh Kumar Chadda,2 Rashmi Ramachandran,3 Vinay Goyal,4 Manjari Tripathi,4 Gururao Hariprasad1
1Department of Biophysics; 2Department of Psychiatry; 3Department of Anaesthesia; 4Department of Neurology, All India Institute of Medical Sciences, New Delhi 110029, India
Background and objective: Dopamine plays an important role in the disease pathology of Parkinson’s disease and schizophrenia. These two neuropsychiatric disorders represent disease end points of the dopaminergic spectrum where Parkinson’s disease represents dopamine deficit and schizophrenia represents dopamine hyperactivity in the mid-brain. Therefore, current treatment strategies aim to restore normal dopamine levels. However, during treatment patients develop adverse effects due to overshooting of physiological levels of dopamine leading to psychosis in Parkinson’s disease, and extrapyramidal symptoms in schizophrenia. Absence of any laboratory tests hampers modulation of pharmacotherapy. Apolipoprotein E and α-synuclein have an important role in the neuropathology of these two diseases. The objective of this study was to evaluate cerebrospinal fluid (CSF) concentrations of apolipoprotein E and α-synuclein in patients with these two diseases so that they may serve as biomarkers to monitor therapy in Parkinson’s disease and schizophrenia.
Methods: Drug-naïve Parkinson’s disease patients and Parkinson’s disease patients treated with dopaminergic therapy, neurological controls, schizophrenic patients treated with antidopaminergic therapy, and drug-naïve schizophrenic patients were recruited for the study and CSF was collected. Enzyme-linked immunosorbent assays were carried out to estimate the concentrations of apolipoprotein E and α-synuclein. Pathway analysis was done to establish a possible role of these two proteins in various pathways in these two dopamine dictated diseases.
Results: Apolipoprotein E and α-synuclein CSF concentrations have an inverse correlation along the entire dopaminergic clinical spectrum. Pathway analysis convincingly establishes a plausible hypothesis for their co-regulation in the pathogenesis of Parkinson’s disease and schizophrenia. Each protein by itself or as a combination has encouraging sensitivity and specificity values of more than 55%.
Conclusion: The dynamic variation of these two proteins along the spectrum is ideal for them to be pursued as pharmacotherapeutic biomarkers in CSF to monitor pharmacological efficacy in Parkinson’s disease and schizophrenia.
Keywords: cerebrospinal fluid, Parkinson’s disease, schizophrenia, dopamine, apolipoprotein E, α-synuclein, biomarkers, treatment monitoring
Corrigendum for this paper has been published
Introduction
Parkinson’s disease is a progressive neurodegenerative disorder diagnosed based on the presence of motor symptoms like tremor, rigidity, bradykinesia, and postural instability.1 The prevalence increases with age and around 1–2% of the population over the age of 60 years is affected by Parkinson’s disease.2,3 Schizophrenia is a chronic mental disorder characterized by delusions, hallucinations, disorganized speech, or behavior and impaired cognitive ability.4 The worldwide prevalence of schizophrenia is 1%.1,5,6 Dopamine is an important neurotransmitter produced in the substantia nigra and ventral tegmental regions of the brain and its dysfunction plays a crucial role in both Parkinson’s disease and schizophrenia.7 In Parkinson’s disease, the decrease in dopamine in the substantia nigra of the mid-brain caused by selective loss of dopaminergic neurons has been implicated in disease pathology.8 On the contrary, dopamine hyperactivity is associated with schizophrenia.9 The treatment strategies for both the diseases exploit the difference in dopamine level from the baseline. In Parkinson’s disease, clinical intervention is aimed at increasing the concentration of dopamine in mid-brain.10 On the other hand, in schizophrenia, neuroleptics are prescribed which block dopamine receptors and decrease overall dopamine activity.11 However, there is a strong chance that during the treatment period patients develop symptoms related to the other extreme of dopamine spectrum, wherein Parkinson’s disease patients tend to develop psychosis, and schizophrenia patients tend to develop extrapyramidal side effects.12 This clinical scenario is depicted by the patients recruited in this study (Table 1).
 |
Table 1 Clinical profile of patients receiving pharmacological therapy and showing side effects |
Currently, there is no definite parameter to monitor the treatment and assist the clinicians to modulate therapy to avoid adverse effects. In this regard, biomarkers provide a convenient tool that can be objectively evaluated and used as an indicator of biological processes and pharmacologic response in the human body.13–15 Biomarker discovery for various diseases including neurological conditions has provided an efficient medium to monitor various disease conditions.16–18 Despite the discovery of many protein biomarkers for diagnosis or prognosis of Parkinson’s disease and schizophrenia, there is no significant clinical proteomic study to monitor drug therapy in these two diseases.19,20 The unavailability of reliable biomarkers to monitor drug therapy in Parkinson’s disease and schizophrenia provides opportunities for clinical proteomic-based biomarker discovery in this field. In the recent past, our group has been dedicatedly involved in protein biomarker discovery to assess treatment in both Parkinson’s disease and schizophrenia.21,22
Apolipoprotein E is a ligand for low-density lipoprotein receptors and is the most important lipid transport protein present in the brain.23 The gene is located on the chromosome19q13.2 with three alleles e2, e3, and e4.24 It is involved in many complex biological processes such as regulation of intracellular signaling, lipid metabolism, modulation of nitric oxide synthase-mediated cell proliferation, immune system regulation, and extracellular signaling.25–27 Apolipoprotein E is mainly synthesized by astrocytes in the brain and is known to be associated with various neurodegenerative disorders including Alzheimer’s disease and Parkinson's disease.28,29 It is a predominant genetic risk factor for Parkinson’s disease as it imparts vulnerability to early semantic memory impairment.30 In schizophrenia, aberrant apolipoprotein E signaling and the evidence of common receptors with schizophrenia susceptibility gene, reelin, supports its role in the disease pathology.31
α-synuclein is encoded by the SNCA gene located on the chromosome 4q22.1.32 It is abundantly expressed in the brain and is known to interact with lipids, presynaptic vesicles, and plasma membrane by lipid rafts.33–35 It is a core component of Lewy bodies which is a clinical hallmark for Parkinson’s disease.36 In addition, point mutations in the α-synuclein gene are known to be a risk factor for Parkinson’s disease.37 The association between α-synuclein expression and schizophrenia has been shown by a previous study.38 α-synuclein expression at the mRNA level is down regulated in lymphocytes of schizophrenic patients.39
The intricate association of apolipoprotein E and α-synuclein, with neuropsychiatric disorders, prompted us to study the expression of these proteins in cerebrospinal fluid (CSF) along the clinical dopaminergic spectrum, with a view to developing them as therapeutic efficacy monitoring biomarkers in Parkinson’s disease and schizophrenia.
Methods
Ethics, patient selection criteria, and consent
The study was approved by the ethics committee of All India Institute of Medical Sciences, New Delhi (Reference no.: IESC/T-418/26.08.2015), and the methods followed were as per the ethical standard formulated in the Helsinki declaration. The Parkinson’s disease and schizophrenia patients were screened and recruited for the study at the Department of Neurology and Department of Psychiatry, All India Institute of Medical Sciences, New Delhi, respectively. The neurological control group comprised of patients with bladder, prostate, and uterine pathologies, who were screened at urology and gynecology clinics at the institute. These patients were recruited for surgeries under spinal anesthesia. Before enrolling the patients in the study written informed consent was obtained. Briefly, 1.5 mL of CSF was collected under sterile conditions in microfuge tubes and was centrifuged at 4°C for 5 min at 3,000 rpm. The supernatant was taken in a separate microfuge tube and stored in −80°C until further experiments. Proper care was taken while collecting the CSF samples to avoid blood contamination, and samples with even minute contamination with blood were excluded from the study.
Patient inclusion and exclusion criteria
Inclusion criteria: The Unified Parkinson's Disease Rating Scale was used for screening patients with Parkinson’s disease according to which a score of zero represents no disability and a score of 199 represents complete disability.40 For describing the progress of symptoms in Parkinson’s disease patients, the Hoehn and Yahr scale was used and was graded from stage 1 to stage 5.41 ICD 10 was used to diagnose schizophrenia.42 Exclusion criteria: The patients with other disease or coexisting pathology or those under any therapeutic interventions were excluded from the study.
Enzyme-linked immunosorbent assay (ELISA)
The estimation of apolipoprotein E and α-synuclein in the recruited patients was done using ELISA kits (Elabscience, China). The methodology used was as per the manufacturer’s instruction protocol. The concentrations of apolipoprotein E and α-synuclein were extrapolated from the standard curves.
Statistical analysis
The mean concentrations for all the five groups were plotted and a linear curve with line equations and R2 value was obtained. Correlation coefficient and p-value (<0.05) for CSF apolipoprotein E and α-synuclein were obtained using Student’s t-test. Receiver operating characteristic (ROC) curve was obtained using GraphPad Prism (GraphPad Prism software, San Diego, CA, USA) to derive cut-off levels and area-under-the-curve for apolipoprotein E and α-synuclein in Parkinson’s disease and schizophrenia.
Pathway analysis
The entire information of genes corresponding to the identified proteins, their related functions were obtained from UniProt and from published literature in PubMed. Using this information, the proteins were analyzed for their biological interactions in Parkinson’s disease and schizophrenia pathways using KEGG and Schizo-Pi database.43 For visualizing the interaction and pathways of identified proteins and its interactors Cytoscape v2.8.0 software was used.44,45 Michigan Molecular Interactions plugin was used to collect the human gene regulatory interactome obtained from the public databases including STRING, MINT, MENTHA, and HPRD and merge the information.46–50 From this complete network, sub-networks for Parkinson’s disease and schizophrenia were obtained up to the first neighboring nodes using the plugin BiNoM v2.5. The resulting networks were merged using Cytoscape. Venn/Euler diagram was used to analyze the intersection between Parkinson’s disease and schizophrenia. The corresponding interactions of the identified proteins were noted and analyzed.
Results
Clinical profile
A total of 61 CSF samples of patients with Parkinson’s disease and schizophrenia were obtained from the neurology and psychiatry out-patient departments. The sample group included drug- naïve patients and those treated for Parkinson’s disease and schizophrenia and neurological controls. The demographic profile of the patients recruited for the study is mentioned in Table 2. The sex distribution of the patients has fewer females as compared to males. Also, the mean age of Parkinson’s disease patients is almost twice that of schizophrenia patients, and the mean age of the neurological control group is 61.4 years.
 |
Table 2 Demographic profile of patients recruited in the study |
Apolipoprotein E and α-synuclein expression in CSF of Parkinson’s disease and schizophrenia
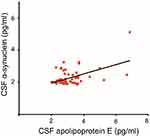
ELISA was done to determine the CSF concentrations of apolipoprotein E and α-synuclein across five groups; (1) drug-naïve Parkinson’s disease, (2) treated Parkinson’s disease, (3) neurological controls, (4) drug-naïve schizophrenia and, (5) treated schizophrenia. The relationship between apolipoprotein E and α-synuclein concentrations and dopamine level in CSF is represented in Figure 1. It should also be noted that the concentrations of both apolipoprotein E and α-synuclein correlate with each other as indicated by a positive correlation coefficient value of 0.5 in Figure 2. ROC curve was plotted for apolipoprotein E and α-synuclein levels in CSF in Parkinson’s disease, neurological control, and schizophrenia as shown in Figure 3. Individual values corresponding to the cut-off values, sensitivity, and specificity are given in Table 3. It can be observed that when either of the two proteins, apolipoprotein E and α-synuclein, were considered for evaluation with the individual estimated cut-off values, the sensitivity and specificity values ranged from 53.3% to 79.3%.
 |
Figure 1 ELISA for expression of (A) apolipoprotein E and (B) α-synuclein in the cerebrospinal fluid (CSF) of Parkinson’s disease, neurological controls, and schizophrenia patients. Clinical phenotypes comprise of Parkinson’s disease naïve (P), Parkinson’s treated (PRx), neurological controls of patients with urological and gynecological diseases needing surgical intervention (NC), schizophrenia treated (SRx), and schizophrenia naïve patients (S). Mean ± Standard error of mean of the values is shown by horizontal lines. The bars represent the concentrations as the average of duplicate readings of each patient sample. Trend lines of apolipoprotein E (y=−0.25x+3.78; R2=0.91) and α-synuclein (y=−0.14x+2.63; R2=0.94) across the five clinical phenotypes is shown as a blue dotted line in (A) and (B), respectively. Diagrammatic representation of the dopamine concentration in cerebrospinal fluid (CSF) is shown along the x-axis. Concentrations of dopamine in the CSF across the clinical phenotypes has been estimated in Gao et al and Jensen et al.47,48 * indicates statistical significance with p<0.05. |
 |
Table 3 Pharmacotherapeutic monitoring value of Apolipoprotein E and α-synuclein in Parkinson’s disease and schizophrenia |
Pathway analysis
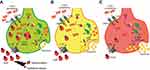
Pathway analysis was carried out to study the interactions of these proteins in these dopamine dictated diseases. A total of 25 proteins were found to be directly interacting with apolipoprotein E and α-synuclein in Parkinson’s disease, and 18 proteins were found to be directly interacting with apolipoprotein E and α-synuclein in schizophrenia, with 13 proteins being common amongst the two groups (Figure 4). The functions of these proteins and their relevance in this study have been delineated in Table 4. A hypothesis has been proposed based on the ELISA results, highlights of the pathway analysis, information from previous studies, and the same has been diagrammatically represented in Figure 5.
 |
Table 4 Interactions of apolipoprotein E and α-synuclein in the pathogenesis of Parkinson’s disease and schizophrenia |
Discussion
Clinical profile
The incidence of Parkinson’s disease and schizophrenia majorly affects the male population; therefore, the sex distribution of the patients has fewer females as compared to males.69 Secondly, the mean age of Parkinson’s disease patients is almost double that of schizophrenia patients because the incidence of Parkinson’s disease increases above the age of 60 years, with only 4% of the affected being under the age of 50 years.70 On the other hand, the incidence of schizophrenia occurs between 16 and 25 years.71 The mean age of the neurological control group is 61.4 years since the patients selected as neurological controls were those requiring surgical intervention for urological disorders which presents around this age.72 The drug-naïve patients of Parkinson’s disease and schizophrenia represent the extreme end points of dopamine spectrum, patients who have been treated represent time frames within this spectrum, and neurological controls represent the mid-point of the spectrum that defines the physiological range of dopamine.
Correlation of apolipoprotein E and α-synuclein expression in CSF of Parkinson’s disease and schizophrenia
The concentrations of both apolipoprotein E and α-synuclein inversely correlate with the dopamine concentrations. It is higher in drug-naïve Parkinson’s disease patients and linearly decreases through treated Parkinson’s disease, neurological controls, treated schizophrenia patients and drug-naïve schizophrenia patients. Such a relationship of apolipoprotein E and α-synuclein concentrations with the dopamine levels provides a window of opportunity to modulate treatment in a way that patients do not develop side effects. According to the ROC curve each protein, apolipoprotein E and α-synuclein, individually or as a combination has sensitivity and specificity values of around 54%. This would, therefore, mean that using these protein biomarkers for monitoring therapeutic efficacy would help to reduce the number of patients affected by drug-induced side effects in these two diseases by more than half. These results and data are very encouraging from a translational point of view in the field of neuropsychiatry. It may be noted that though the patients were phenotypes and grouped based on certain clinical criteria, there exists a vast heterogeneity among the patients with respect to the age of onset of the disease, stage of the disease, quality of drug intervention, duration of therapy, personal habits, and habitat. This explains the subtle variations in the concentrations of these two proteins.
Interaction-based pathway analysis involving apolipoprotein E and α-synuclein in Parkinson’s disease and schizophrenia
In order to understand the role of apolipoprotein E and α-synuclein in the pathogenesis of Parkinson’s disease and schizophrenia, it becomes important to study the interaction of these proteins in the dopaminergic pathway and subsequent cellular damage. Based on these interactions, pathway analysis was carried out to place the observed experimental outcomes in the right perspective. The protein interactions and cellular mechanisms explaining the observed results are shown in Figure 5 and is discussed below.
Conclusion
Apolipoprotein E and α-synuclein CSF concentrations have an inverse correlation along the entire dopaminergic clinical spectrum comprising of Parkinson’s disease and schizophrenia. Each protein by itself or as a combination has the ability to differentiate either of the pathological states from the physiological state. Pathway analysis supports the mechanism of coregulation in the pathogenesis of the two diseases. The dynamic variation of these two proteins along the spectrum is ideal for them to be pursued as pharmacotherapeutic biomarkers in CSF to monitor pharmacological efficacy in Parkinson’s disease and schizophrenia with a reasonable accuracy. Outcome of this study will be helpful for the clinicians and patients to monitor pharmacotherapy and make informed treatment decisions in Parkinson’s disease and schizophrenia.
Acknowledgments
An abstract of this paper titled ‘Evaluation of apolipoprotein E and α-synuclein as potential biomarkers in CSF to monitor pharmaco-therapeutic efficacy in dopamine dictated disease states of Parkinson’s disease and schizophrenia’ was published in the 2019 Science Program, by the American Academy of Neurology as a part of the Annual Meeting held in Philadelphia, PA, USA, in May 2019 ((http://indexsmart.mirasmart.com/AAN2019/PDFfiles/AAN2019-000045.pdf).
GH acknowledges Department of Science and Technology, Government of India for the grant SO/BB-0122/2013 (D-348). The work was partly carried out at the Proteomics Division at Central Core Research Facility at AIIMS, New Delhi, India.
Disclosure
The authors report no conflicts of interest in regard to this work.
References
1. Mollenhauer B, Weintraub D. The depressed brain in Parkinson’s disease: implications for an inflammatory biomarker. Proc Natl Acad Sci U S A. 2017;114(12):3004–3005. doi:10.1073/pnas.1700737114
2. de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi:10.1016/S1474-4422(06)70471-9
3. Mehanna R, Moore S, Hou JG, Sarwar AI, Lai EC. Comparing clinical features of young onset, middle onset and late-onset Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(5):530–534. doi:10.1016/j.parkreldis.2014.02.013
4. Patel KR, Cherian J, Gohil K, Atkinson D. Schizophrenia: overview and treatment options. PT. 2014;39(9):638–645.
5. Gore FM, Bloem PJ, Patton GC, et al. Global burden of disease in young people aged 10–24 years: a systematic analysis. Lancet. 2011;377(9783):2093–2102. doi:10.1016/S0140-6736(11)60512-6
6. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485–515. doi:10.1038/nrd.2016.28
7. Bogerts B, Häntsch J, Herzer M. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. BiolPsychiatry. 1983;18(9):951–969.
8. Kinoshita K, Tada Y, Muroi Y. Selective loss of dopaminergic neurons in the substantia nigra pars compacta after systemic administration of MPTP facilitates extinction learning. Life Sci. 2015;137:28–36. doi:10.1016/j.lfs.2015.07.017
9. Grace A. Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 2012;62(3):1342–1348. doi:10.1016/j.neuropharm.2011.05.011
10. Jankovic J, Aguilar LG. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2008;4(4):743–757.
11. Li P, Snyder GL, Vanover KE. Dopamine targeting drugs for the treatment of schizophrenia: past, present, and future. Curr Top Med Chem. 2016;16(29):3385–3403.
12. Caroff SN, Hurford I, Lybrand J, Campbell EC. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29(1):127–128. doi:10.1016/j.ncl.2010.10.002
13. Hariprasad G, Hariprasad R, Kumar L, Srinivasan A, Kola S, Kaushik A. Apolipoprotein A1 as a potential biomarker in the ascitic fluid for the differentiation of advanced ovarian cancers. Biomarkers. 2013;18(6):532–541. doi:10.3109/1354750X.2013.822561
14. Rukmangadachar LA, Makharia GK, Mishra A, et al. Proteome analysis of the macroscopically affected colonic mucosa of Crohn’s disease and intestinal tuberculosis. Sci Rep. 2016;6:23162. doi:10.1038/srep23162
15. Sehrawat U, Pokhriyal R, Gupta AK, et al. Proteomic analysis of advanced ovarian cancer tissue to identify potential biomarkers of responders and nonresponders to first-line chemotherapy of carboplatin and paclitaxel. Biomark Cancer. 2016;16(8):43–56.
16. Kataria J, Rukmangadachar LA, Hariprasad G, et al. Two-dimensional difference gel electrophoresis analysis of cerebrospinal fluid in tuberculous meningitis patients. J Proteomics. 2011;74(10):2194–2203. doi:10.1016/j.jprot.2011.06.020
17. Rukmangadachar LA, Kataria J, Hariprasad G, Samantaray JC, Srinivasan A. Two-dimensional difference gel electrophoresis (DIGE) analysis of sera from visceral leishmaniasis patients. Clin Proteomics. 2011;8(1):4. doi:10.1186/1559-0275-8-2
18. Manral P, Sharma P, Hariprasad G, Chandralekha TM, Srinivasan A. Can apolipoproteins and complement factors be biomarkers of Alzheimer’s disease? CurrAlzheimer Res. 2012;9(8):935–943.
19. Chahine LM, Stern MB, Chen-Plotkin A. Blood-based biomarkers for Parkinson’s disease. ParkinsonismRelatDisord. 2014;20:S99–S103.
20. Sabherwal S, English JA, Föcking M, Cagney G, Cotter DR. Blood biomarker discovery in drug-free schizophrenia: the contribution of proteomics and multiplex immunoassays. Expert Rev Proteomics. 2016;13(12):1141–1155. doi:10.1080/14789450.2016.1252262
21. Gupta AK, Rani K, Swarnkar S, et al. Evaluation of serum apolipoprotein E as a potential biomarker for pharmacological therapeutic efficacy monitoring in dopamine dictated disease spectrum of schizophrenia and Parkinson’s disease. J Cent NervSyst Dis. 2018;10:1179573518803585.
22. Gupta AK, Kumar GK, Rani K, et al. 2D-DIGE as a strategy to identify serum protein biomarkers to monitor pharmacological efficacy in dopamine dictated states of Parkinson’s disease and schizophrenia. Neuropsychiatr Dis Treat. 2019;15:1031–1044. doi:10.2147/NDT.S198559
23. Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240(4852):622–630.
24. Yu CE, Cudaback E, Foraker J, et al. Epigenetic signature and enhancer activity of the human APOE gene. Hum Mol Genet. 2013;22(24):5036–5047. doi:10.1093/hmg/ddt354
25. Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40(1):1–6.
26. Mahley RW, Rall SC
27. Zerba KE, Ferrell RE, Sing CF. Complex adaptive systems and human health: the influence of common genotypes of the apolipoprotein E (ApoE) gene polymorphism and age on the relational order within a field of lipid metabolism traits. Hum Genet. 2000;107(5):466–475.
28. Harhangi BS, de Rijk MC, Van Duijn CM, et al. APOE and the risk of PD with or without dementia in a population-based study. Neurology. 2000;54(6):1272–1276. doi:10.1212/wnl.54.6.1272
29. Souza DR, de Godoy MR, Hotta J, et al. Association of apolipoprotein E polymorphism in late-onset Alzheimer’s disease and vascular dementia in Brazilians. Braz J Med Biol Res. 2003;36(7):919–923. doi:10.1590/s0100-879x2003000700013
30. Mata IF, Leverenz JB, Weintraub D, et al. APOE, MAPT, SNCA, and cognitive performance in Parkinson disease. JAMA Neurol. 2014;71(11):1405–1412. doi:10.1001/jamaneurol.2014.1455
31. Gibbons AS, Udawela M, Jeon WJ, Seo MS, Brooks L, Dean B. The neurobiology of APOE in schizophrenia and mood disorders. Front Biosci. 2011;16:962–979. doi:10.2741/3729
32. Chen X, de Silva HA, Pettenati MJ, et al. The human NACP/α-synuclein gene: chromosome assignment to 4q21.3-q22 and TaqI RFLP analysis. Genomics. 1995;26(2):425–427.
33. Withers GS, George JM, Banker GA, Clayton DF. Delayed localization of synelfin (synuclein, NACP) to presynaptic terminals in cultured rat hippocampal neurons. Brain Res Dev Brain Res. 1997;99:87–94.
34. Jo E, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE. α-synuclein-membrane interactions and lipid specificity. J Biol Chem. 2000;275(44):34328–34334. doi:10.1074/jbc.M004345200
35. Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of α-synuclein. J Neurosci. 2004;24(30):6715–6723. doi:10.1523/JNEUROSCI.1594-04.2004
36. Xu L, Pu J. α-synuclein in Parkinson’s disease: from pathogenetic dysfunction to potential clinical application. Parkinsons Dis. 2016;2016:1720621.
37. Olanow CW, Brundin P. Parkinson’s disease and alpha-synuclein: is Parkinson’s disease a prion-like disorder? Mov Disord. 2013;28(1):31–40. doi:10.1002/mds.25373
38. Demirel ÖF, Cetin İ, Turan Ş, Sağlam T, Yıldız N, Duran A. Decreased expression of α-Synuclein, Nogo-A, and UCH-L1 in patients with schizophrenia: a preliminary serum study. Psychiatry Investig. 2017;14(3):344–349. doi:10.4306/pi.2017.14.3.344
39. Noori-Daloii MR, Kheirollahi M, Mahbod P, et al. Alpha- and beta-synucleins mRNA expression in lymphocytes of schizophrenia patients. Genet Test Mol Biomarkers. 2010;14(5):725–729. doi:10.1089/gtmb.2010.0050
40. Chou KL, Taylor JL, Patil PG. The MDS–UPDRS tracks motor and non– a motor improvement due to subthalamic nucleus deep brain stimulation in Parkinson disease. Parkinsonism Relat Disord. 2013;19(11):966–969. doi:10.1016/j.parkreldis.2013.06.010
41. Goetz CG, Poewe W, Rascol O, et al. Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendation. Mov Disord. 2004;19:1020–1028. doi:10.1002/mds.20213
42. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders. Clinical descriptions and diagnostic guidelines. Available from: https://apps.who.int/iris/handle/10665/37958. Accessed June 28, 2019.
43. Ganapathiraju MK, Thahir M, Handen A, et al. Schizophrenia interactome with 504 novel protein-protein interactions. NPJ Schizophr. 2016;2:16012. doi:10.1038/npjschz.2016.12
44. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi:10.1101/gr.1239303
45. Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27(3):431–432. doi:10.1093/bioinformatics/btq675
46. Chatr-Aryamontri A, Ceol A, Palazzi LM, et al. MINT: the Molecular INTeraction database. Nucleic Acids Res. 2007;35:D572–D574. doi:10.1093/nar/gkl950
47. Gao J, Ade AS, Tarcea VG, et al. Integrating and annotating the interactome using the MiMI plugin for Cytoscape. Bioinformatics. 2009;5(1):137–138. doi:10.1093/bioinformatics/btn501
48. Jensen LJ, Kuhn M, Stark M, et al. STRING 8-a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37:D412–D416. doi:10.1093/nar/gkn760
49. Keshava Prasad TS, Goel R, Kandasamy K, et al. Human protein reference database-2009 update. Nucleic Acids Res. 2009;37(Database issue):D767–D772. doi:10.1093/nar/gkn892
50. Calderone A, Castagnoli L, Cesareni G. Mentha: a resource for browsing integrated protein-interaction networks. Nat Methods. 2013;10(8):690–691. doi:10.1038/nmeth.2561
51. Rostovtseva TK, Gurnev PA, Protchenko O, et al. α-synuclein shows high-affinity interaction with voltage-dependent anion channel, suggesting mechanisms of mitochondrial regulation and toxicity in Parkinson disease. J Biol Chem. 2015;290(30):18467–18477. doi:10.1074/jbc.M115.641746
52. Lu L, Zhang C, Cai Q, et al. Voltage-dependent anion channel involved in the α-synuclein-induced dopaminergic neuron toxicity in rats. Acta Biochim Biophys Sin. 2013;45(3):170–178. doi:10.1093/abbs/gms114
53. Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46(6):821–831. doi:10.1016/j.yjmcc.2009.02.021
54. Beutner G, Rück A, Riede B, Brdiczka D. Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. The implication for regulation of permeability transition by the kinases. Biochim Biophys Acta. 1998;1368(1):7–18.
55. Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12(5):835–840. doi:10.1007/s10495-006-0525-7
56. Schinzel AC, Takeuchi O, Huang Z, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA. 2005;102(34):12005–12010. doi:10.1073/pnas.0505294102
57. Gincel D, Shoshan-Barmatz V. Glutamate interacts with VDAC and modulates the opening of the mitochondrial permeability transition pore. J Bioenerg Biomembr. 2004;36(2):179–186.
58. McFarland MA, Ellis CE, Markey SP, Nussbaum RL. Proteomics analysis identifies phosphorylation-dependent α-synuclein protein interactions. Mol Cell Proteomics. 2008;7(11):2123–2137. doi:10.1074/mcp.M800116-MCP200
59. Liani E, Eyal A, Avraham E, et al. Ubiquitylation of synphilin-1 and α-synuclein by SIAH and its presence in cellular inclusions and Lewy bodies imply a role in Parkinson’s disease. Proc Natl Acad Sci. 2004;101(15):5500–5555. doi:10.1073/pnas.0401081101
60. Dashtipour K, Tafreshi A, Adler C, et al. Hypermethylation of synphilin-1, Α-synuclein-interacting protein (SNCAIP) gene in the cerebral cortex of patients with sporadic Parkinson’s disease. Brain Sci. 2017;7:7. doi:10.3390/brainsci7070074
61. Stafa K, Trancikova A, Webber PJ, Glauser L, West AB, Moore DJ. GTPase activity and neuronal toxicity of Parkinson’s disease-associated LRRK2 is regulated by ArfGAP1. GTPase activity and neuronal toxicity of Parkinson’s disease-associated LRRK2 is regulated by ArfGAP1. PLoS Genet. 2012;8(2):e1002526. doi:10.1371/journal.pgen.1002526
62. Wu J, Lou H, Alerte TN, et al. Lewy-like aggregation of α-synuclein reduces protein phosphatase 2A activity in vitro and in vivo. Neuroscience. 2012;07:288–297. doi:10.1016/j.neuroscience.2012.01.028
63. Hua G, Xiaolei L, Weiwei Y, et al. Protein phosphatase 2A is involved in the tyrosine hydroxylase phosphorylation regulated by α-synuclein. Neurochem Res. 2015;40(3):428–437. doi:10.1007/s11064-014-1477-x
64. Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG. α-Synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci. 2005;118(15):3523–3530. doi:10.1242/jcs.02481
65. Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of α-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. Faseb J. 2001;15(6):916–926. doi:10.1096/fj.00-0334com
66. Wersinger C, Sidhu A. Attenuation of dopamine transporter activity by α-synuclein. Neurosci Lett. 2003;340(3):189–192.
67. Kawakami F, Yabata T, Ohta E, et al. LRRK2 phosphorylates tubulin-associated tau but not the free molecule: LRRK2-mediated regulation of the tau-tubulin association and neurite outgrowth. PLoS One. 2012;7(1):e30834. doi:10.1371/journal.pone.0030834
68. Ohi K, Hashimoto R, Yasuda Y, et al. The AKT1 gene is associated with attention and brain morphology in schizophrenia. World J Biol Psychiatry. 2013;14(2):100–113. doi:10.3109/15622975.2011.591826
69. Loke H, Harley V, Lee J. Biological factors underlying sex differences in neurological disorders. Int J Biochem Cell Biol. 2015;65:139–150. doi:10.1016/j.biocel.2015.05.024
70. Van Den Eeden SK, Tanner CM, Bernstein AL, et al. The incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157(11):1015–1022. doi:10.1093/aje/kwg068
71. Sham PC, MacLean CJ, Kendler KS. A typological model of schizophrenia based on age at onset, sex, and familial morbidity. Acta Psychiatr Scand. 1994;89(2):135–141.
72. Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL. Harrisons Principles of Internal Medicine.
73. Wilhelmus MM, Bol JG, Van Haastert ES, et al. Apolipoprotein E and LRP1 increase early in Parkinson’s disease pathogenesis. Am J Pathol. 2011;179(5):2152–2156. doi:10.1016/j.ajpath.2011.07.021
74. Vance JE, Hayashi H. Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim Biophys Acta. 2010;1801(8):806–818. doi:10.1016/j.bbalip.2010.02.007
75. Vitali C, Wellington CL, Calabresi L. HDL and cholesterol handling in the brain. Cardiovasc Res. 2014;103(3):405–413. doi:10.1093/cvr/cvu148
76. de Chaves EP, Narayanaswami V. Apolipoprotein E and cholesterol in aging and disease in the brain. Future Lipidol. 2008;3(5):505–530.
77. Iwai A. Properties of NACP/α-synuclein and its role in Alzheimer’s disease. Biochim Biophys Acta. 2000;1502(1):95–109.
78. Marzolo MP, von Bernhardi R, Bu G, Inestrosa NC. Expression of alpha(2)-macroglobulin receptor/low-density lipoprotein receptor-related protein (LRP) in rat microglial cells. J Neurosci Res. 2000;60(3):401–411. doi:10.1002/(SICI)1097-4547(20000501)60:3<401::AID-JNR15>3.0.CO;2-L
79. Lee HJ, Bae EJ, Lee SJ. Extracellular α–synuclein-a novel and the crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10(2):92–98. doi:10.1038/nrneurol.2013.275
80. Danzer KM, Kranich LR, Ruf WP, et al. Exosomal cell-to-cell transmission of alpha-synuclein oligomers. Mol Neurodegener. 2012;24:7–42.
81. Dzamko N, Gysbers A, Perera G, et al. Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to α-synuclein pathology. Acta Neuropathological. 2017;133(2):303–319. doi:10.1007/s00401-016-1648-8
82. Holmes BB, DeVos SL, Kfoury N, et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci U S A. 2013;110(33):E3138. doi:10.1073/pnas.1301440110
83. Fantini J, Carlus D, Yahi N. The fusogenic tilted peptide (67–78) of α-synuclein is a cholesterol binding domain. Biochim Biophys Acta. 2011;1808(10):2343–2351. doi:10.1016/j.bbamem.2011.06.017
84. Bar-On P, Crews L, Koob AO, et al. Statins reduce neuronal α-synuclein aggregation in vitro models of Parkinson’s disease. J Neurochem. 2008;105(5):1656–1667. doi:10.1111/j.1471-4159.2008.05254.x
85. Emamzadeh FN, Allsop D. α-Synuclein interacts with lipoproteins in plasma. J Mol Neurosci. 2017b;63(2):165–172. doi:10.1007/s12031-017-0967-0
86. Emamzadeh FN. Role of apolipoproteins and α-synuclein in Parkinson’s disease. J Mol Neurosci. 2017a;62(3–4):344–355. doi:10.1007/s12031-017-0942-9
87. Gao N, Li YH, Li X, et al. Effect of α-synuclein on the promoter activity of the tyrosine hydroxylase gene. Neurosci Bull. 2007;23(1):53–57. doi:10.1007/s12264-007-0008-z
88. Kastner A, Hirsch EC, Herrero MT, Javoy-Agid F, Agid Y. Immunocytochemical quantification of tyrosine hydroxylase at a cellular level in the mesencephalon of control subjects and patients with Parkinson’s and Alzheimer’s disease. J Neurochem. 1993;61(3):1024–1034.
89. Peterson L, Ismond KP, Chapman E, Flood P. Potential benefits of the therapeutic use of β2-adrenergic receptor agonists in neuroprotection and Parkinson’s disease. J Immunol Res. 2014;2014:103780. doi:10.1155/2014/394127
90. Mittal S, Bjørnevik K, Im DS, et al. β2-Adrenoreceptor is a regulator of the α-synuclein gene driving the risk of Parkinson’s disease. Science. 2017;357(6354):891–898. doi:10.1126/science.aaf3934
91. Hsiao JT, Halliday GM, Kim WS. α-synuclein regulates neuronal cholesterol efflux. Molecules. 2017;19(22):10.
92. Hirsch-Reinshagen V, Zhou S, Burgess BL, et al. Deficiency of ABCA1 impairs apolipoprotein E metabolism in the brain. J Biol Chem. 2004;279(39):41197–41207. doi:10.1074/jbc.M407962200
93. Kamisuki S, Mao Q, Abu-Elheiga L, et al. A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. Chem Biol. 2009;16(8):882–892. doi:10.1016/j.chembiol.2009.07.007
94. Cheng D, Kim WS, Garner B. Regulation of α-synuclein expression by liver X receptor ligands in vitro. Neuroreport. 2008;19(17):1685–1689. doi:10.1097/WNR.0b013e32831578b2
95. Wheatley VR, Brind JL. Sebaceous gland differentiation: III. The uses and limitations of freshly isolated mouse preputial gland cells for the in vitro study of hormone and drug action. J Invest Dermatol. 1981;76(4):293–296.
96. Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann N YAcad Sci. 2003;991(1):120–131. doi:10.1111/j.1749-6632.2003.tb07470.x
97. Bosco DA, Fowler DM, Zhang Q, et al. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate α-synuclein fibrilization. Nat Chem Biol. 2006;2(5):249–253. doi:10.1038/nchembio782
98. Gallardo G, Schlüter OM, Südhof TC. A molecular pathway of neurodegeneration linking α-synuclein to ApoE and Aβ peptides. Nature Neurosci. 2008;11(3):301. doi:10.1038/nn2058
99. Ruipérez V, Darios F, Davletov B. α-synuclein, lipids and Parkinson’s disease. Prog Lipid Res. 2010;49(4):420–428. doi:10.1016/j.plipres.2010.05.004
100. Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci. 2006;103(15):5644–5651. doi:10.1073/pnas.0600549103
101. Chang S, Ran Ma T, Miranda RD, et al. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci. 2005;102(51):18694–18699. doi:10.1073/pnas.0508254102
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.