Back to Journals » Infection and Drug Resistance » Volume 13
Evaluating Cefiderocol in the Treatment of Multidrug-Resistant Gram-Negative Bacilli: A Review of the Emerging Data
Authors Giacobbe DR , Ciacco E, Girmenia C , Pea F, Rossolini GM , Sotgiu G, Tascini C, Tumbarello M, Viale P, Bassetti M
Received 19 September 2020
Accepted for publication 2 December 2020
Published 29 December 2020 Volume 2020:13 Pages 4697—4711
DOI https://doi.org/10.2147/IDR.S205309
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Daniele Roberto Giacobbe,1,2 Eugenio Ciacco,3 Corrado Girmenia,4 Federico Pea,5,6 Gian Maria Rossolini,7,8 Giovanni Sotgiu,9 Carlo Tascini,10 Mario Tumbarello,11,12 Pierluigi Viale,5,6 Matteo Bassetti1,2 On behalf of the ISGRI-SITA (Italian Study Group on Resistant Infections of the Italian Society of Anti-infective Therapy)
1Clinica Malattie Infettive, Ospedale Policlinico San Martino – IRCCS, Genoa, Italy; 2Department of Health Sciences, University of Genoa, Genoa, Italy; 3Pharmacy Unit, S. Salvatore Hospital, ASL1 Abruzzo, L’Aquila, Italy; 4Hematology, Dipartimento Medicina Traslazionale e di Precisione, AOU Policlinico Umberto I, Sapienza University of Rome, Rome, Italy; 5Department of Medical and Surgical Sciences, Alma Mater Studiorum, University of Bologna, Bologna, Italy; 6University Hospital IRCCS Policlinico Sant’Orsola Bologna, Bologna, Italy; 7Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; 8Clinical Microbiology and Virology Unit, Florence Careggi University Hospital, Florence, Italy; 9Department of Medical, Surgical and Experimental Sciences, University of Sassari, Sassari, Italy; 10SOC Malattie Infettive, Azienda Sanitaria Integrata, University of Udine, Udine, Italy; 11Dipartimento di Scienze di Laboratorio e Infettivologiche, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; 12Dipartimento di Sicurezza e Bioetica, Università Cattolica del Sacro Cuore, Rome, Italy
Correspondence: Daniele Roberto Giacobbe
Clinica Malattie Infettive, Ospedale Policlinico San Martino – IRCCS, L.go R. Benzi 10, Genoa 16132, Italy
Tel +390105554658
Email [email protected]
Abstract: Infections due to multidrug-resistant Gram-negative bacteria (MDR-GNB), especially when carbapenem resistant, have been very difficult to manage in the last fifteen years, owing to the paucity of dependable therapeutic options. Cefiderocol is a siderophore cephalosporin recently approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) that may have the potential to fill some of the remaining gaps in the treatment of MDR-GNB infections. Among others, cefiderocol demonstrated in vitro activity against carbapenem-resistant Acinetobacter baumannii and metallo-β-lactamases producers. Clinical data from both registrative studies and post-marketing experiences are essential to confirm whether these promises from in vitro studies could readily translate into clinical practice, as well as to delineate the precise place in therapy for cefiderocol for the treatment of MDR-GNB in the near future. Because of its unique potential, it is essential to provide both randomized controlled trials (RCT) and real-life data to improve the ability of clinicians to exploit its benefit in both empirical and targeted treatment of MDR-GNB infections. In this narrative review, we discuss the emerging data from pivotal RCT and initial real-life experiences on the use of cefiderocol for the treatment of MDR-GNB infections.
Keywords: cefiderocol, siderophore, Pseudomonas, Acinetobacter, Enterobacterales, antimicrobial resistance
Introduction
Infections due to multidrug-resistant Gram-negative bacteria (MDR-GNB), especially when carbapenem resistant, have been very difficult to manage in the past fifteen years, owing to the paucity of therapeutic options.1–5 Furthermore, available options such as polymyxins, aminoglycosides, and/or glycylcyclines, although certainly useful in presence or resistance to all other classes, have some disadvantages that clinicians would like to avoid, including nonnegligible toxicity and possible suboptimal pharmacokinetics in some sites of infection.6–8
Some precious additions to the antibiotic armamentarium such as ceftazidime/avibactam, ceftolozane/tazobactam, meropenem/vaborbactam, and imipenem/relebactam have recently allowed to renew the availability of β-lactam antibiotics (that usually display good safety profiles and pharmacokinetics) for treating some MDR-GNB.9–12 However, some gaps still need to be filled, for example, restoring the activity of β-lactams against metallo-β-lactamases (MBL)-producing GNB and carbapenem-resistant Acinetobacter baumannii.
In this narrative review, we discuss the available antimicrobial, pharmacological, and clinical data for cefiderocol, a siderophore cephalosporin recently approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) that may have the potential to fill some of the remaining gaps in the treatment of MDR-GNB infections.
Methods
The structure of the present narrative review was agreed by all authors and articulated in the following sections: (i) antimicrobial properties; (ii) pharmacological properties; (iii) results of randomized clinical trials: efficacy; (iv) safety of cefiderocol; (v) case reports and case series; (vi) place in therapy. Then, the authors were divided in small groups in order to draft the different sections, supported by inductive PubMed searches for relevant publications. Eventually, the different drafts were merged into a final manuscript that was approved by all authors.
Antimicrobial Properties
Cefiderocol is a novel siderophore cephalosporin active against GNB, including strains of Enterobacterales and nonfermenters that exhibit difficult-to-treat (DTR) resistance phenotypes (ie, resistant to fluoroquinolones and older β-lactams including carbapenems).13,14 This notable and thus far unique spectrum of activity is dependent on the following features: i) uptake across the bacterial outer membrane also via iron transporters, thus enhancing accumulation of the drug in the periplasmic space and overriding resistance mechanisms such as efflux pumps and porin alterations; and ii) remarkable stability, likely conferred by modifications in the C-7 and C-3 side chains, against all classes of beta-lactamases, including carbapenemases (both serine carbapenemases, such as KPC and OXA-types, and metalloenzymes such as NDM, VIM, IMP and the intrinsic L1 carbapenemase of Stenotrophomonas maltophilia).15–18
Similar to cefepime, cefiderocol carries a pyrrolidinium group on the C3 side chain, which enhances stability to β-lactamases and antimicrobial activity. Moreover, similar to ceftazidime, cefiderocol carries an aminothiazole ring and a carboxypropyl-oxyimino group on the C7 side chain, which also enhances stability to β-lactamases and activity against Gram-negative bacilli, including Pseudomonas aeruginosa. In addition, cefiderocol harbors a chlorocatechol group at the end of the C3 side chain, which is able to chelate ferric iron and confers siderophore activity: the complex cefiderocol-Fe3+ can thus be actively transported into the periplasmic space by specific iron-transporters, such as PiuA in P. aeruginosa, unlike other beta-lactams which only enter by passive diffusion across porin channels.14,18 Indeed, resistance acquisition studies revealed that mutations causing increased levels of pyoverdine production or higher level of FecA expression (both involved in the iron transport system) were associated with increased cefiderocol minimum inhibitory concentration (MIC) values in P. aeruginosa.19 Once in the periplasmic space, cefiderocol exerts its antimicrobial activity by inhibition of the penicillin-binding proteins (PBP)-mediated cell wall synthesis, leading to cell death.14,18 Cefiderocol was shown to have high affinity for PBP3 in clinically relevant Gram-negative rods (eg, Klebsiella pneumoniae, Escherichia coli, P. aeruginosa, and Acinetobacter baumannii), and also for PBP2 in K. pneumoniae and for PBP1a in P. aeruginosa.20,21
Similar to cefepime, cefiderocol is a weak AmpC inducer (possibly due to low PBP4 binding) with low affinity for chromosomal AmpC-type β-lactamases,22 which account for its overall good activity also against AmpC overproducing strains. Mutations causing alteration or loss of porin channels, such as OmpK35 and OmpK36 in K. pneumoniae, are associated with a marginal decrease of cefiderocol antimicrobial activity,18 while inactivation of the MexAB-OprM efflux pump only causes a slight cefiderocol MIC decrease in P. aeruginosa, suggesting that this mechanism is unable to efficiently expel the molecule outside the microbial cell.20
During infections, an iron depleted-milieu is expected to be encountered in the host tissues in response to which the bacterial iron transporters are up-regulated.23 This should be accounted for when testing in vitro susceptibility to cefiderocol, which must be carried out using iron-depleted media when using reference broth microdilution. The growth medium is prepared by treating conventional cation-adjusted Mueller-Hinton broth with a cation-binding resin in order to remove all the cations, and subsequently replenishing the cation-depleted broth with adequate concentrations of Mg2+, Zn2+ and Ca2+.24–26
For cefiderocol, the Clinical and Laboratory Standards Institute (CLSI) has set clinical breakpoints (CB) for Enterobacterales, P. aeruginosa, S. maltophilia, and A. baumannii, with MIC values of ≤4, 8, and ≥16 mg/L for susceptible, intermediate, and resistant categories, respectively.24 However, these breakpoints were not accepted by FDA for A. baumannii and S. maltophilia, since these two species were not included in the Apeks-UTI clinical trial that was designed for the approval of the drug in the USA.27 In Europe, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) has set cefiderocol CB with MIC values of ≤2 mg/L and >2 mg/L for susceptible and resistant categories, respectively, for both Enterobacterales and Pseudomonas spp., and also apharmacokinetic/pharmacodynamic (PK/PD) breakpoint MIC value of ≤2 mg/L for susceptibility, while CB was not set for A. baumannii and S. maltophilia due to insufficient clinical evidence.25 Epidemiological cut-off (ECOFF) values were also defined by the EUCAST as follows: 0.25 mg/L for E. coli, K. pneumoniae, and A. baumannii; 0.5 mg/L for P. aeruginosa, and 0.06 mg/L for S. maltophilia.25
Cefiderocol susceptibility has been investigated in large international surveillance studies carried out since 2014 (SIDERO-WT studies), covering over 28,000 Gram-negative isolates.13 Overall, the MIC90 for Enterobacterales (including E. coli, Klebsiella spp., Citrobacter spp., Enterobacter spp., Serratia spp., Morganella morganii, and Proteus spp.) ranged from 0.25 to 1 mg/L, with no significant geographical or temporal differences. Cumulative activity against Enterobacterales from surveillance studies revealed that >98% and >99% of isolates were inhibited at concentrations of 2 mg/L and 4 mg/L, respectively. Activity was retained against most isolates resistant to expanded-spectrum cephalosporins and carbapenems, including those producing different types of serine carbapenemases and metallo-β-lactamases. The MIC90 of Enterobacterales was 2–16 mg/L for strains producing different types of carbapenemases (Table 1). Against 1022 carbapenem-nonsusceptible Enterobacterales, of which 23% ceftazidime/avibactam resistant and 22% colistin resistant, cefiderocol MIC90 was 4 mg/L and 97% of the isolates were inhibited at a concentration of 4 mg/L.28
 |
Table 1 Susceptibility to Cefiderocol of Gram-Negative Isolates from Selected Surveillance Studies |
Data for Gram-negative nonfermenters from the international surveillance studies (see Table 1) reported MIC90 values of 0.5–2 mg/L for P. aeruginosa, 1–2 mg/L for Acinetobacter spp., 0.25–0.5 mg/L for S. maltophilia, and 0.12–0.5 mg/L for Burkholderia cepacia complex, underscoring the remarkable activity of cefiderocol against these difficult-to-treat pathogens. Cumulative activity data revealed that >99%, >95%, and >95% of isolates of P. aeruginosa, A. baumannii, and B. cepacia complex from surveillance studies, respectively, were inhibited at a concentration of 4 mg/L, while >99% of isolates of S. maltophilia were inhibited at a concentration of 2 mg/L. Activity was retained against most P. aeruginosa isolates resistant to carbapenems (>98% inhibited at 2 mg/L), including those resistant to ceftolozane/tazobactam and producing metalloenzymes. A notable activity was also retained against carbapenem-resistant A. baumannii, with an MIC90 of 1 mg/L for isolates producing OXA-type carbapenemases (Table 1).
Few isolates with elevated cefiderocol MIC values (≥8 mg/L) were detected from large surveillance studies. Some of these isolates were NDM-1 metallo-β-lactamase or PER-1 extended-spectrum β-lactamase producers; in such cases, the addition of enzyme inhibitors (eg, dipicolinic acid and/or avibactam) was capable of reducing cefiderocol MIC values, suggesting that production of these β-lactamases may contribute to increased cefiderocol MICs. However, cefiderocol exhibited good activity against several isolates producing these enzymes,29 suggesting that the presence of additional resistance mechanisms is likely necessary to increase MIC values above the susceptibility breakpoint.
Concerning other pathogens, cefiderocol was shown to be active in vitro against Vibrio spp. Haemophilus influenzae, Moraxella catarrhalis, and Bordetella parapertussis,20 and also against less common Gram-negative pathogens including Pantoea spp., Sphingomonas paucimobilis, and Elizabethkingia meningoseptica.30 On the other hand, activity is variable against anaerobes, likely due to the variable importance of the siderophore-iron transport systems for growth under anaerobic conditions.20
In conclusion, cefiderocol is a new antibiotic with a unique mechanism of cell entry in Gram-negative pathogens, while being stable to most beta-lactamases. It is a potentially useful drug for treating infections caused by carbapenemase-producing Enterobacterales and non-fermenters. As such, cefiderocol appears to be one of the most innovative antibiotics among those recently approved.
Pharmacological Properties
Pharmacokinetic Properties
The pharmacokinetic profile of cefiderocol was studied in healthy subjects both after single-ascending dose (100 to 1000 mg) and multiple-ascending dose (1000 mg q8h and 2000 mg q8h).31 Overall, cefiderocol showed a linear pharmacokinetic behavior with ascending doses and a mean elimination half-life of 2.0–2.7h. The mean total clearance was of 4.6–6.0 L/h and the fraction excreted unchanged into urine was of 60–70%. The pharmacokinetic characteristics of cefiderocol at the dose of 2000 mg q8h over 1 h in healthy subjects are summarized in Table 2.31
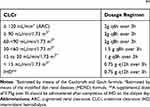 |
Table 2 Dosage Regimens of Cefiderocol Focused at Achieving 90% of PTAs of 75% T>MIC Against Pathogens with an MIC Up to 4 mg/L in Patients with Different Classes of Renal Function44 |
The pharmacokinetics of cefiderocol was compared in healthy subjects with those in subjects with mild [estimated glomerular filtration rate (eGFR) 60-<90 mL/min/1.73 m2], moderate (eGFR 30-<60 mL/min/1.73 m2) and severe impairment of renal function (eGFR <30 mL/min/1.73 m2) after a single dose of 1000 mg.32 Overall, total drug clearance and elimination half-life were inversely and linearly related with renal dysfunction. The mean ratio of drug exposure, in terms of area under the concentration–time curve from zero-to-infinity (AUC0-∞), in subjects with mild, moderate, and severe renal impairment compared with those with normal renal function was 1.0, 1.5, and 2.5. The volume of distribution (Vd) and the fraction unbound (fu) to the plasma proteins were very similar between groups (mean Vd ranged from 13.5 to 16.4 L; mean fu ranged from 0.42 to 0.35).32
The potential for drug–drug interaction of cefiderocol with different human drug transporters [organic anion transporter (OAT) 1 and 3, organic cation transporter (OCT) 1 and 2, multidrug and toxin extrusion (MATE) 2-K and organic anion transporting polypeptide (OATP) 1B3] was assessed in three cohorts of healthy subjects.33 Substrates of these transporters were administered concomitantly to cefiderocol for assessing whether cefiderocol might or not inhibit drug transport. Overall, the study did not show any clinically significant drug–drug interaction of cefiderocol via drug transporters.33
The intrapulmonary pharmacokinetics of cefiderocol was assessed in healthy adult subjects after administration of a single 2000 mg dose infused over 1 h.34 The mean epithelial lining fluid (ELF)-to-plasma ratio was of 0.101 and 0.239 based on total drug in plasma and on free drug in plasma, respectively, similar to other cephalosporins.34
A recent study assessed the concentration-time profile of total radioactivity equivalent and unchanged cefiderocol after administration of 1000 mg [14C] cefiderocol over 1 h in healthy subjects.35 The findings showed that cefiderocol accounted for 92.3% of total radioactivity in plasma and for 90.6% of the administered dose into urine, thus confirming that metabolism is a minor route of elimination of cefiderocol.35
Pharmacodynamic Properties
Cefiderocol is a beta-lactam antibiotic for which the pharmacodynamic determinant of efficacy is the time that the plasma concentration exceeds the MIC of the pathogen (t>MIC) during the dosing interval.36 Experimental animal models of infections showed that a t>MIC of around 75% is associated with an effective microbiological response to cefiderocol in terms of 1–2 log of bacterial killing.36,37 In a P. aeruginosa neutropenic murine model, the t>MIC targets needed for stasis, 1 log and 2 log decrease in bacterial burden against strains with MICs of 0.064–0.5 mg/L ranged 44.4–94.7%, 50.2–97.5%, and 62.1–100%, respectively.36 In murine thigh and lung infection models, the mean t>MIC needed for 1-log reduction in bacterial burden against various Gram-negative bacteria (E. coli, K. pneumoniae, P. aeruginosa, A. baumannii, and S. maltophilia) differed according to the site of infection and to the pathogen.37 In the thigh infection model, the mean t>MIC was of 73.3% and 77.2% against Enterobacterales and P. aeruginosa, respectively; in the lung infection model, it was of 64.4%, 70.3%, 88.1%, and 53.9% against Enterobacterales, P. aeruginosa, A. baumannii, and S. maltophilia, respectively.
Matsumoto et al assessed in an immunocompetent rat respiratory tract infection model the influence that infusion time of administration (3 h vs 1 h) may have on the efficacy of humanized cefiderocol exposure (2g q8h) against carbapenem-resistant Gram-negative bacilli (P. aeruginosa, A. baumannii and K. pneumoniae). Administration by extended infusion (EI) over 3 h resulted in more sustained reduction in lung bacterial burden (3.04–4.41 log10) compared with intermittent infusion (II) over 1 h (0.7–3.7 log10).38 This provided the rationale for considering the use of EI for ameliorating t>MIC with cefiderocol.
Consistently, the efficacy of humanized exposures of 2g q8h EI over 3 h cefiderocol was assessed against various species of Gram-negative bacteria with variable patterns of susceptibility to other antibiotics in several murine neutropenic thigh infection models.39–43 Monogue and colleagues assessed the efficacy of cefiderocol against a collection of 15 P. aeruginosa, A. baumannii, and Enterobacterales isolates. They found that the humanized exposures of cefiderocol were able to cause a ≥1-log drop in bacterial burden against all pathogens with an MIC up to 4 mg/L.42 Similar findings were observed also by Ghazi and colleagues who assessed the efficacy of humanized exposures of cefiderocol against eight different strains of P. aeruginosa with an MIC ranging from 0.063 to 0.5 mg/L for cefiderocol, from 2 to 64 mg/L for cefepime, and from 1 to 32 mg/L for levofloxacin.40 Stainton and colleagues assessed the sustainability of humanized exposure of cefiderocol over 72 h against a collection of 12 P. aeruginosa, A. baumannii, and Enterobacterales isolates. Sustained kill was observed at 72 h against 9 out of 11 strains with an MIC ranging from 0.5 and 8 mg/L, and no adaptive resistance was observed during therapy.43 In another study, the effect of cefiderocol human-simulated exposures was compared with that of ceftazidime human-simulated exposures (2g q8h over 2 h) against 24 S. maltophilia strains that were fully susceptible to cefiderocol (MICs 0.015–0.5 mg/L) and either ceftazidime-susceptible (10/24) or ceftazidime-nonsusceptible (14/24). For cefiderocol bacterial killing was potent against all strains (mean ± SD bacterial burden log10 reduction at 24 h - 2.76 ± 0.68; ≥2 log in 87.5% and ≥1 log in the remaining 12.5% of isolates) whereas for ceftazidime it was present but less potent against the 10 ceftazidime-susceptible strains (- 1.38 ± 1.49) and absent against the 14 ceftazidime-nonsusceptible strains (mean ± SD bacterial growth of 0.64 ± 0.79).39 Recently, a neutropenic thigh infection model confirmed that the efficacy of humanized cefiderocol exposure at 72 h against Enterobacterales, P. aeruginosa, and A. baumannii isolates is unaltered even by host iron overload (mean ± SD log10 bacterial decrease – 2.5 ± 1.5 vs – 2.5 ± 1.4 in standard and iron-overloaded models, respectively).41 Consistently with these findings, the dosage regimens proposed for cefiderocol were focused at predicting by means of Monte Carlo simulations a high probability of success (≥90% of PTAs of 75% t>MIC) against pathogens with an MIC up to 4 mg/L in patients belonging to all of the different classes of renal function (Table 2).44
Noteworthy is that cefiderocol is one of the few antibiotics in the therapeutic armamentarium with a well-defined dosing strategy specified in the manufacturer’s fact sheet also for patients with augmented renal clearance (ARC). ARC is defined as a CLCr >120–130 mL/min/1.73 m2 and is a pathophysiological condition that may accelerate the elimination of beta-lactams like cefiderocol, thus theoretically causing underexposure if standard dosages are administered.45 The strategy of a more intensified dosage is extremely relevant in preventing the risk of therapeutic failure associated with drug underexposure when using cefiderocol in populations of critically ill patients at high prevalence of ARC, like those with febrile neutropenia (16.4%), sepsis (39.5–56%), burns (65%), trauma (85%), and subarachnoid hemorrhage (100%).45
The strategy of administering cefiderocol by EI among all patients irrespective of the degree of renal function may be helpful also at minimizing the development of multidrug antimicrobial resistance. Administering beta-lactams by EI rather than by II may represent a step forward in suppressing resistance amplification, as it may ensure better exposures in terms of t>MIC compared with II.46
Results of Randomized Clinical Trials: Efficacy
The efficacy of cefiderocol in complicated urinary tract infections (cUTI) was evaluated in a randomized (2:1), Phase II, double-blind, parallel-group, non-inferiority trial (APEKS-cUTI), started in 2014. In this trial, cefiderocol was compared with imipenem-cilastatin. Patients infected by carbapenem-resistant organisms were not enrolled. The primary endpoint was clinical cure/microbiological eradication (as a composite endpoint) at the test of cure (TOC), which was set at 7±2 days after the end of treatment (EOT). The study was planned with a non-inferiority margin of 20%. However, following discussion with the FDA on the possible decrease of the non-inferiority margin to 15% and the increase of the sample size, the study protocol was amended accordingly.47
The APEKS-cUTI study was conducted in 67 hospitals in 15 countries, from February 2015 to August 2016. Only individuals aged ≥18 years and with a diagnosis of cUTI (with or without pyelonephritis) or acute uncomplicated pyelonephritis (30% of the total sample size) were recruited. A one/two-week intravenous therapy was planned in the study protocol: cefiderocol (2 g) q8h vs imipenem-cilastatin (1g) q8h. Dosages were adjusted depending on renal function and body weight. The high dose of imipenem was chosen to allow inclusion of patients with P. aeruginosa infection.
A total of 371 patients were enrolled in the primary study population (modified intention-to-treat [mITT]). More than half of patients were aged ≥65 years (in both arms) and complicated patients were more frequent than in contemporary studies. The most frequent uropathogens were E. coli and K. pneumoniae, whereas P. aeruginosa was isolated from 7% and 4% of patients treated with cefiderocol and imipenem-cilastatin, respectively. Several bacterial isolates were resistant to cefepime and levofloxacin. The primary endpoint was achieved by 73% (183/252) and 55% (65/119) of the patients enrolled in the cefiderocol and imipenem-cilastatin arms, respectively (adjusted difference: 18.6%; 95% confidence interval [CI] 8.2 to 28.9, thereby demonstrating not only non-inferiority but also superiority of cefiderocol as a post hoc result). When analyzing the single components of the composite endpoint, microbiological response was higher in patients treated with cefiderocol (73% [184/252]) as opposed to those treated with imipenem-cilastatin (56% [67/119]), with an adjusted difference of 17.3% (95% CI 6.9 to 27.6). Conversely, clinical response was similar in the two arms (90% [226/252] in cefiderocol-treated patients vs 87% [104/119] in the imipenem-cilastatin treated-patients; adjusted difference 2.4%, 95% CI −4.7 to 9.4).
The efficacy of cefiderocol in patients with hospital-acquired bacterial pneumoniae (HABP), ventilator-associated bacterial pneumonia (VABP), or healthcare-associated bacterial pneumonia (HCABP) caused by GNB was evaluated in the study APEKS-NP, a Phase III, double-blind, randomized, non-inferiority trial. The results of the APEKS-NP trial have been recently published.48,49 The patients were randomized to cefiderocol 2 g every 8 h or to meropenem 2 g every 8 h, both as a 3-h infusion. Linezolid was administered in both arms for a duration of at least 5 days while cefiderocol or meropenem was administered for 7–14 days. The primary endpoint was all-cause mortality at day 14 for the mITT population, with a non-inferiority margin of 12.5%. Cefiderocol was non-inferior to meropenem with respect to all-cause mortality at day 14 (12.4% [18/145] in cefiderocol arm vs 11.6% [17/146] in meropenem arm; difference 0.8%; 95% CI −6.6 to 8.2).48
The CREDIBLE-CR study was an open-label, international, multicenter, Phase 3 RCT that was pathogen-oriented rather than indication oriented:50,51 this was a descriptive study, not powered for inferential testing. Indeed, cefiderocol was compared with best available therapy (BAT) for the treatment of severe infections (HCABP, HABP, VABP, cUTI, or bloodstream infections [BSI]/sepsis) due to carbapenem-resistant (CR) GNB. The results of the CREDIBLE-CR study have also been recently published.52 Cefiderocol 2 g every 8 h was given as a 3-h infusion and BAT was chosen by the investigator and consisted of up to three antibiotics. Patients were randomized 2:1 to receive cefiderocol or BAT. Duration of therapy (either with cefiderocol or with BAT) was 7 to 14 days, possibly extended up to 21 days based on reasonable explanation. In patients with cUTI, a minimum length of therapy of 5 days was allowed. The primary efficacy endpoint for patients with HABP/VABP/HCABP and for those with BSI/sepsis was clinical cure at TOC visit. For patients with cUTI, the primary efficacy endpoint was microbiological cure (eradication) at TOC. In the CR-mITT population (primary study population) clinical cure rates at TOC were comparable between groups, overall (52.5% [42/80] in cefiderocol-treated vs 50% [19/38] in BAT-treated patients) and in subgroups of patients with HABP/VABP/HCABP (50% [20/40] in cefiderocol-treated vs 52.6% [10/19] in BAT-treated patients), and patients with BSI/sepsis (43.5% [10/23] in cefiderocol-treated vs 42.9% [6/14] in BAT-treated patients). Microbiological cure in patients with cUTI was 52.9% (9/17) and 20% (1/5) in cefiderocol-treated and BAT-treated patients, respectively. However, all-cause mortality at day 14, day 28, and day 49 was numerically higher in the cefiderocol group (19%, 25%, 34%, respectively) compared to BAT (12%, 18%, 18%, respectively). This mortality imbalance was greatest at days 14, 28, and 49 for patients with HABP/VABP/HCABP (cefiderocol 24%, 31%, and 42% vs BAT 14%, 18%, and 18%). It is worth noting that, in the safety population, a greater number of deaths occurring up to day 3 were reported in the cefiderocol arm (cefiderocol 4% vs 0% BAT), which may be considered unrelated to study drug efficacy. Moreover, a greater number of deaths were reported in the cefiderocol arm (9% cefiderocol vs 0% BAT) after day 28 through the end of study as opposed to BAT, whereas proportions were similar from day 4 to day 28 (21% and 18% in cefiderocol and BAT arms, respectively).
Of note, the difference in 49-day mortality stratified for pathogen was the highest for Acinetobacter spp. (50% [21/42] vs 18% [3/17] in cefiderocol and BAT-treated patients, respectively), although it is of note that some variables indicating severity of presentation or of baseline diseases (ICU at randomization, severe renal dysfunction, ongoing shock, and shock within 31 days before randomization) were more frequent in the cefiderocol than BAT arms in patients with Acinetobacter spp. infections.52
Safety of Cefiderocol
Two Phase 1 studies showed mild, clinically not significant adverse events mainly represented by diarrhea and skin reactions (maculopapular rash, urticarial) in less than 20% of patients, with only one treatment discontinuation due to urticaria.31,32 In another phase 1 study in healthy adult subjects, cefiderocol in normal doses (2 g) and supratherapeutic doses (3–4 g) had no apparent clinically significant effect on QT and corrected QT (QTcF) interval.53
Phase 2 and phase 3 studies confirmed that cefiderocol is comparable to other cephalosporins in terms of tolerability and safety profile. In the APEKS-cUTI RCT, safety was assessed in all randomly assigned individuals who received at least one dose of study drug.47 Adverse events occurred in 41% (122/300) and 51% (76/148) of patients in the cefiderocol and in the imipenem-cilastatin groups, respectively, with the majority being mild or moderate. Overall, diarrhea and constipation were observed in 7.7% of patients in the cefiderocol group and in 10.1% of those in the imipenem-cilastatin group. Serious adverse events were reported in 5% and 8% of patients in the cefiderocol and imipenem-cilastatin groups, respectively. Among serious adverse events, Clostridioides difficile infection (CDI) occurred in one patient in the cefiderocol group and in two patients in the imipenem-cilastatin group. One death due to cardiac arrest, considered unrelated to study drug by the investigator, was reported in the cefiderocol group.
In the APEKS-NP study, adverse events were observed in 88% (130/148) and 86% (129/150) in cefiderocol and meropenem groups, respectively.48 In both arms, urinary tract infections (15.5% and 10.7% in cefiderocol and meropenem arms, respectively) and hypokalemia (10.8% and 15.3% in cefiderocol and meropenem arms, respectively) were the most frequently observed adverse events. Serious adverse events were observed in 36% (54/148, of which 3 drug-related) and 30% (45/150, of which 5 drug-related) in cefiderocol and meropenem groups, respectively. Among patients treated with cefiderocol and meropenem, 4/148 (3%) and 4/150 (3%) developed C. difficile infection.48
According to the results of the CREDIBLE-CR study, the rate of adverse events (evaluated in 101 patients who received cefiderocol and 49 patients who received BAT) was similar in the two arms, with over 90% of patients experiencing at least one adverse event.52 Diarrhea, pyrexia, septic shock, vomiting, and hypokalemia were the most frequently observed adverse events in both groups and diarrhea (19% vs 12%), ALT increased (7% vs 0%), AST increased (8% vs 2%), pleural effusion (8% vs 2%), and chest pain (6% vs 0%) were observed more frequently in the cefiderocol than in the BAT groups. The majority of chest pain episodes reported in the cefiderocol group were considered to be of non-cardiovascular origin and not related to cefiderocol. Most adverse events occurred at a low frequency and were considered manifestations of the patients’ underlying disease. Indeed, the frequency of adverse events considered to be treatment-related by the investigator was 15% (15/101) in the cefiderocol arm and 22% (11/49) in the BAT arm. Diarrhea (2%), abnormal liver function tests (2%), ALT increased (3%), and AST increased (3%) were the most frequently reported treatment-related, treatment-emergent adverse events in the cefiderocol group; while acute kidney injury (8%) was the most frequently reported treatment-related, treatment-emergent adverse event in the BAT group. Serious adverse events were reported for 50% and 47% of patients in cefiderocol group and BAT group, respectively. Septic shock was the most frequently reported serious adverse event in both cefiderocol (12%) and BAT (12%) groups. Overall, only 1/101 patient in the cefiderocol group (1%) experienced a treatment-related serious adverse event, that is, an increase in transaminases levels which led to study drug discontinuation and resolved in 30 days. Conversely, treatment-related serious adverse events were observed in 5/49 patients in the BAT group (10%). Discontinuation due to treatment-related adverse events occurred in 3% and 4% of patients in the cefiderocol group and BAT group, respectively.
Case Reports and Case Series
Case reports and case series of patients with severe GNB infections treated with cefiderocol in compassionate use are detailed in Table 3.54–66 All these cases highlight unique challenges in managing patients infected by MDR-GNB including MBL-producing GNB.
 |
 |
 |
Table 3 Published Case Reports and Case Series of Compassionate Use of Cefiderocol |
Place in Therapy
Cefiderocol is a first-in-class antibiotic, a siderophore intravenous cephalosporin that binds ferric iron and is actively transported into the periplasm of GNB.14,67,68 Beyond its novel mechanism of action, from a practical standpoint what makes it attractive for clinicians is the displayed in vitro activity against carbapenem-resistant A. baumannii and MBL-producing GNB, ie, those MDR-GNB for which there are currently no marketed active β-lactams (without forgetting its in vitro activity against Stenotrophomonas spp. and Burkholderia spp.).69 Therefore, clinical data from both registrative studies and post-marketing experiences are essential to confirm whether these promises from in vitro studies could readily translate into clinical practice, as well as to delineate a precise place in therapy for cefiderocol for the treatment of MDR-GNB in the near future. Real-life data would also be important for further delineating the safety of cefiderocol through Phase 4 surveillance studies.
While the results of the APEKS-cUTI and APEKS-NP studies have eventually led to the FDA approval of cefiderocol for cUTI and HABP/VABP,47,48 the recent EMA approval of cefiderocol for the treatment of infections due to Gram-negative bacteria in adults with limited treatment options70,71 opens doors to its use also for other pressing priorities, such as BSI caused by carbapenem-resistant A. baumannii or MBL producers, or for its empirical use in endemic settings or in colonized patients with severe infection. In this regard, it would be critical to clarify the remaining issue of the increased mortality in cefiderocol-treated patients in the CREDIBLE-CR study, especially in the case of infections due to non-fermenting GNB. In our opinion, this could be achieved in two different, complementary ways: (i) through conduction of further RCT (the open-label GAMECHANGER RCT, which is comparing cefiderocol vs BAT for the treatment of BSI due to GNB, is currently recruiting patients [NCT03869437]); (ii) through post-marketing observational experiences, which, although unable to provide high-quality evidence for guiding treatment due to the inherent limitations of observational studies (even when properly adjusting for confounding variables) may provide useful hypothesis-generating data and clinical success/mortality rates for fine-tuning the design of future RCT (perhaps by identifying those categories of patients that may benefit the most from cefiderocol administration) should also the GAMECHANGER study provide inconclusive evidence.
Until then, some uncertainties in delineating the precise place in therapy of cefiderocol for the treatment of MDR-GNB infections will remain. Indeed, on the one hand, we now have a β-lactam that, at least in vitro, fills the gaps against some high-priority MDR-GNB, taking also into account the consideration that the increased mortality observed in the CREDIBLE-CR study may be merely due to chance alone in view of the low power related to the small sample size (especially in subgroups) of the CREDIBLE-CR study and the current lack of a clear explanation for the observed result. On the other hand, further studies remain necessary to verify this hypothesis, and cefiderocol should not be used indiscriminately. In our opinion, the potential advantages of having restored β-lactam activity against highly resistant A. baumannii and MBL producers should not be wasted while waiting for further evidence. What remains largely unclear is whether cefiderocol should be used alone or in combination with BAT (eg, polymyxins) until more solid evidence is provided. There is still no clear answer to this question, which, notably, does not involve the classical (and still unresolved) dilemma of the general comparison of monotherapy vs combinations for MDR-GNB infections in terms of efficacy, but the novel one of not using cefiderocol alone considering the possible imbalance in mortality registered in the CREDIBLE-CR study. In our opinion, it could be ultimately reasonable to consider using cefiderocol-including combinations in the case of severe clinical presentations, in which a de-escalation rather than escalation strategy could be more indicated (authors opinion only, not supported by published evidence at the present time). The same may apply to the inclusion of cefiderocol in empirical regimens in patients with severe infections and hospital-level or patient-level risk factors for infections due to carbapenem-resistant A. baumannii and/or MBL producers. Of note, in this scenario reliable and rapid microbiological tests for the detection of causative agents and involved resistance mechanisms will increasingly play a crucial role in the optimization of the empirical use of cefiderocol (initiation/discontinuation) according to antimicrobial and diagnostic stewardship principles.72
In conclusion, cefiderocol expands the spectrum of MDR-GNB that can be treated again with β-lactams and will likely offer a precious addition to the clinician armamentarium. Because of this unique potential, it remains essential to provide both RCT (eg, GAMECHANGER) and real-life data to improve the clinicians’ ability to exploit its benefit in both empirical and targeted treatment of MDR-GNB infections.
Acknowledgments
This research was conducted on behalf of ISGRI-SITA (Italian Study Group on Resistant Infections of the Italian Society of Anti-infective Therapy).
Funding
This work was funded by an unrestricted grant by Shionogi Srl. The sponsor had no role in selecting the participants, reviewing the literature, defining recommendations, drafting the paper, or in the decision to submit the manuscript for publication. All views expressed are solely those of the authors.
Disclosure
Outside the submitted work, DRG reports honoraria from Stepstone Pharma GmbH and unconditional grants from MSD Italia and Correvio Italia.
FP reports grants from Shionogi, during the conduct of the study; personal fees from Angelini, Basilea Pharmaceutica, Gilead, Hikma, Merck Sharp & Dohme, Nordic Pharma, Novartis, Shionogi, Thermo Fisher, and Sandoz, outside the submitted work. Outside the submitted work, he has participated in advisory boards and/or received speaker honoraria from Angelini, Basilea Pharmaceutica, Correvio, Gilead, Hikma, Merck Sharp & Dohme, Novartis, Sanofi Aventis, and Thermo-Fisher.
GMR reports grants, personal fees from Shionogi, during the conduct of the study; grants, personal fees, non-financial support from Accelerate and Menarini; grants from Angelini, bioMérieux, Cepheid, Elitech, Merck, Nordic Pharma, Seegene, Zambon, Symcel, DID, Hain Lifescience GmbH, GenePoc, Setlance, Biomedical Service, Qvella, and Qlinea, outside the submitted work; personal fees from Becton Dickinson, Angelini, bioMérieux, Cepheid, Merck, Nordic Pharma, Pfizer, Venatorx, Zambon, Roche, Thermo Fisher, Beckman Coulter, Qpex, and Qiagen, outside the submitted work.
CT reports personal fees from Correvio, Basilea, Hikma, MSD, Pfizer, Thermo Fisher, Zambon, Biomerieux, and Shionogi, and personal fees from Angelini, outside the submitted work. Outside the submitted work, he has received research grants, and/or been a consultant and/or received a fee for speaking from bioMérieux, Zambon, Basilea, Merck, Nordic Pharma, Angelini, Thermo Fisher, Biotest, Pfizer, Astra Zeneca, Shionogi, Hikma, Avir Pharma, Biotest.
MT reports personal honoraria for participating in advisory boards and/or for meeting presentations from Angelini, Astellas, Menarini, MSD, Nordic Pharma, Pfizer, Roche, Shionogi, outside the submitted work.
MB reports grants and personal fees from Shionogi, outside the submitted work. Outside the submitted work, he has participated in advisory boards and/or received speaker honoraria from Achaogen, Angelini, Astellas, Bayer, Basilea, BioMérieux, Cidara, Gilead, Menarini, MSD, Nabriva, Paratek, Pfizer, Roche, Melinta, Shionogi, Tetraphase, VenatoRx and Vifor and has received study grants from Angelini, Basilea, Astellas, Shionogi, Cidara, Melinta, Gilead, Pfizer and MSD.
References
1. Giani T, Arena F, Pollini S, et al. Italian nationwide survey on Pseudomonas aeruginosa from invasive infections: activity of ceftolozane/tazobactam and comparators, and molecular epidemiology of carbapenemase producers. J Antimicrob Chemother. 2018;73:664–671. doi:10.1093/jac/dkx453
2. Kadri SS, Adjemian J, Lai YL, et al. Difficult-to-treat resistance in Gram-negative Bacteremia at 173 US Hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67:1803–1814.
3. Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13:785–796. doi:10.1016/S1473-3099(13)70190-7
4. Theuretzbacher U. Global antimicrobial resistance in Gram-negative pathogens and clinical need. Curr Opin Microbiol. 2017;39:106–112. doi:10.1016/j.mib.2017.10.028
5. Bassetti M, Giacobbe DR, Giamarellou H, et al. Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect. 2018;24:133–144. doi:10.1016/j.cmi.2017.08.030
6. Bassetti M, Peghin M, Vena A, Giacobbe DR. Treatment of infections due to MDR Gram-negative bacteria. Front Med. 2019;6:74. doi:10.3389/fmed.2019.00074
7. Panidis D, Markantonis SL, Boutzouka E, et al. Penetration of gentamicin into the alveolar lining fluid of critically ill patients with ventilator-associated pneumonia. Chest. 2005;128:545–552. doi:10.1378/chest.128.2.545
8. Tsuji BT, Pogue JM, Zavascki AP, et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39:10–39.
9. Giacobbe DR, Bassetti M, De Rosa FG, et al. Ceftolozane/tazobactam: place in therapy. Expert Rev Anti Infect Ther. 2018;16:307–320. doi:10.1080/14787210.2018.1447381
10. Pogue JM, Bonomo RA, Kaye KS. Ceftazidime/avibactam, meropenem/ vaborbactam, or both? Clinical and formulary considerations. Clin Infect Dis. 2019;68:519–524. doi:10.1093/cid/ciy576
11. Tumbarello M, Trecarichi EM, Corona A, et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis. 2019;68:355–364. doi:10.1093/cid/ciy492
12. Zhanel GG, Lawrence CK, Adam H, et al. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-beta-lactamase inhibitor combinations. Drugs. 2018;78:65–98. doi:10.1007/s40265-017-0851-9
13. Yamano Y. In vitro activity of cefiderocol against a broad range of clinically important gram-negative bacteria. Clin Infect Dis. 2019;69:S544–S551. doi:10.1093/cid/ciz827
14. Zhanel GG, Golden AR, Zelenitsky S, et al. Cefiderocol: a siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant gram-negative bacilli. Drugs. 2019;79:271–289. doi:10.1007/s40265-019-1055-2
15. Ito A, Nishikawa T, Matsumoto S, et al. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2016;60:7396–7401.
16. Ito-Horiyama T, Ishii Y, Ito A, et al. Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother. 2016;60:4384–4386. doi:10.1128/AAC.03098-15
17. Poirel L, Kieffer N, Nordmann P. Stability of cefiderocol against clinically significant broad-spectrum oxacillinases. Int J Antimicrob Agents. 2018;52:866–867. doi:10.1016/j.ijantimicag.2018.11.005
18. Sato T, Yamawaki K. Cefiderocol: discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin Infect Dis. 2019;69:S538–S543. doi:10.1093/cid/ciz826
19. Ito ANT, Kuriowa M, Ishioka Y, et al. Mechanism of cefiderocol high MIC mutants obtained in non-clinical FoR studies.
20. Ito A, Sato T, Ota M, et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against gram-negative bacteria. Antimicrob Agents Chemother. 2018;62.
21. Moya B, Zamorano L, Juan C, et al. Affinity of the new cephalosporin CXA-101 to penicillin-binding proteins of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54:3933–3937. doi:10.1128/AAC.00296-10
22. Ito A, Nishikawa T, Ota M, et al. Stability and low induction propensity of cefiderocol against chromosomal AmpC beta-lactamases of Pseudomonas aeruginosa and Enterobacter cloacae. J Antimicrob Chemother. 2018;73:3049–3052. doi:10.1093/jac/dky317
23. Kidd JM, Abdelraouf K, Nicolau DP. Development of neutropenic murine models of iron overload and depletion to study the efficacy of siderophore-antibiotic conjugates. antimicrob agents chemother. 2019;64. doi:10.1128/AAC.01961-19
24. Clinical and Laboratory Standards Institute. Performance standards for anti-microbial susceptibility testing, 30th informational supplement. CLSI supplement M100–Ed. 30. Wayne, PA: CLSI; 2020.
25. EUCAST clinical breakpoints cefiderocol addendum. Available from: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Addenda/Cefiderocol_addendum_20200501.pdf.
26. Hackel MA, Tsuji M, Yamano Y, et al. Reproducibility of broth microdilution MICs for the novel siderophore cephalosporin, cefiderocol, determined using iron-depleted cation-adjusted Mueller-Hinton broth. Diagn Microbiol Infect Dis. 2019;94:321–325. doi:10.1016/j.diagmicrobio.2019.03.003
27. FDA website. Available from: https://www.fda.gov/drugs/development-resources/cefiderocol-injection.
28. Hackel MA, Tsuji M, Yamano Y, et al. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother. 2018;62.
29. Kohira N, Hackel MA, Ishioka Y, et al. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from global surveillance program (SIDERO-WT-2014). J Glob Antimicrob Resist. 2020;22:738–741. doi:10.1016/j.jgar.2020.07.009
30. Rolston KVI, Gerges B, Shelburne S, et al. Activity of cefiderocol and comparators against isolates from cancer patients. Antimicrob Agents Chemother. 2020;64. doi:10.1128/AAC.01955-19
31. Saisho Y, Katsube T, White S, et al. Pharmacokinetics, safety, and tolerability of cefiderocol, a novel siderophore cephalosporin for gram-negative bacteria, in healthy subjects. Antimicrob Agents Chemother. 2018;62. doi:10.1128/AAC.02163-17
32. Katsube T, Echols R, Arjona Ferreira JC, et al. Cefiderocol, a siderophore cephalosporin for gram-negative bacterial infections: pharmacokinetics and safety in subjects with renal impairment. J Clin Pharmacol. 2017;57:584–591. doi:10.1002/jcph.841
33. Katsube T, Miyazaki S, Narukawa Y, et al. Drug-drug interaction of cefiderocol, a siderophore cephalosporin, via human drug transporters. Eur J Clin Pharmacol. 2018;74:931–938. doi:10.1007/s00228-018-2458-9
34. Katsube T, Saisho Y, Shimada J, Furuie H. Intrapulmonary pharmacokinetics of cefiderocol, a novel siderophore cephalosporin, in healthy adult subjects. J Antimicrob Chemother. 2019;74:1971–1974. doi:10.1093/jac/dkz123
35. Miyazaki S, Katsube T, Shen H, et al. Metabolism, excretion, and pharmacokinetics of [(14) C]-cefiderocol (S-649266), a siderophore cephalosporin, in healthy subjects following intravenous administration. J Clin Pharmacol. 2019;59:958–967. doi:10.1002/jcph.1386
36. Ghazi IM, Monogue ML, Tsuji M, Nicolau DP. Pharmacodynamics of cefiderocol, a novel siderophore cephalosporin, in a Pseudomonas aeruginosa neutropenic murine thigh model. Int J Antimicrob Agents. 2018;51:206–212. doi:10.1016/j.ijantimicag.2017.10.008
37. Nakamura R, Ito-Horiyama T, Takemura M, et al. In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models. Antimicrob Agents Chemother. 2019;63. doi:10.1128/AAC.02031-18
38. Matsumoto S, Singley CM, Hoover J, et al. Efficacy of cefiderocol against carbapenem-resistant gram-negative bacilli in immunocompetent-rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrob Agents Chemother. 2017;61. doi:10.1128/AAC.00700-17
39. Chen IH, Kidd JM, Abdelraouf K, Nicolau DP. Comparative in vivo antibacterial activity of human-simulated exposures of cefiderocol and ceftazidime against stenotrophomonas maltophilia in the murine thigh model. Antimicrob Agents Chemother. 2019. doi:10.1128/AAC.01558-19
40. Ghazi IM, Monogue ML, Tsuji M, Nicolau DP. Humanized exposures of cefiderocol, a siderophore cephalosporin, display sustained in vivo activity against siderophore-resistant Pseudomonas aeruginosa. Pharmacology. 2018;101:278–284.
41. Kidd JM, Abdelraouf K, Nicolau DP. Efficacy of humanized cefiderocol exposure is unaltered by host iron overload in the thigh infection model. Antimicrob Agents Chemother. 2019;64. doi:10.1128/AAC.01767-19
42. Monogue ML, Tsuji M, Yamano Y, et al. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of gram-negative bacteria in a murine thigh infection model. Antimicrob Agents Chemother. 2017;61. doi:10.1128/AAC.01022-17
43. Stainton SM, Monogue ML, Tsuji M, et al. Efficacy of humanized cefiderocol exposures over 72 hours against a diverse group of gram-negative isolates in the neutropenic murine thigh infection model. Antimicrob Agents Chemother. 2019;63.
44. Katsube T, Wajima T, Ishibashi T, et al. Pharmacokinetic/pharmacodynamic modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, for dose adjustment based on renal function. Antimicrob Agents Chemother. 2017;61. doi:10.1128/AAC.01381-16
45. Cook AM, Hatton-Kolpek J. Augmented renal clearance. Pharmacotherapy. 2019;39:346–354. doi:10.1002/phar.2231
46. Ambrose PG, Lomovskaya O, Griffith DC, et al. beta-Lactamase inhibitors: what you really need to know. Curr Opin Pharmacol. 2017;36:86–93. doi:10.1016/j.coph.2017.09.001
47. Portsmouth S, van Veenhuyzen D, Echols R, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18:1319–1328. doi:10.1016/S1473-3099(18)30554-1
48. Wunderink RG, Matsunaga Y, Ariyasu M, et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2020. doi:10.1016/S1473-3099(20)30731-3
49. Shionogi Inc. FDA accepts Shionogi’s supplemental new drug application with priority review for FETROJA® (cefiderocol) for the treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia; [cited August 12, 2020]. Available from: https://www.shionogi.com/us/en/news/2020/6/FDA-Accepts-Shionogis-Supplemental-New-Drug-Application-with-Priority-Review-for-FETROJA.html.
50. Bassetti M, Ariyasu M, Binkowitz B, et al. Designing A pathogen-focused study to address the high unmet medical need represented by carbapenem-resistant gram-negative pathogens - the international, multicenter, randomized, open-label, Phase 3 CREDIBLE-CR Study. Infect Drug Resist. 2019;12:3607–3623.
51. Echols R, Ariyasu M, Nagata TD. Pathogen-focused clinical development to address unmet medical need: cefiderocol targeting carbapenem resistance. Clin Infect Dis. 2019;69:S559–S564. doi:10.1093/cid/ciz829
52. Bassetti M, Echols R, Matsunaga Y, et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis. 2020. doi:10.1016/S1473-3099(20)30796-9
53. Sanabria C, Migoya E, Mason JW, et al. Effect of cefiderocol, a siderophore cephalosporin, on QT/QTc interval in healthy adult subjects. Clin Ther. 2019;41:1724–1736 e1724.
54. Alamarat ZI, Babic J, Tran TT, et al. Long-term compassionate use of cefiderocol to treat chronic osteomyelitis caused by extensively drug-resistant Pseudomonas aeruginosa and extended-spectrum-beta-lactamase-producing klebsiella pneumoniae in a pediatric patient. Antimicrob Agents Chemother. 2020;64.
55. Contreras DA, Fitzwater SP, Nanayakkara DD, et al. Coinfections of two strains of NDM-1- and OXA-232-Coproducing Klebsiella pneumoniae in a kidney transplant patient. Antimicrob Agents Chemother. 2020;64.
56. Edgeworth JD, Merante D, Patel S, et al. Compassionate use of cefiderocol as adjunctive treatment of native aortic valve endocarditis due to extremely drug-resistant Pseudomonas aeruginosa. Clin Infect Dis. 2019;68:1932–1934. doi:10.1093/cid/ciy963
57. Stevens RW, Clancy M. Compassionate use of cefiderocol in the treatment of an intraabdominal infection due to multidrug-resistant Pseudomonas aeruginosa: a case report. Pharmacotherapy. 2019;39:1113–1118. doi:10.1002/phar.2334
58. Trecarichi EM, Quirino A, Scaglione V, et al. Successful treatment with cefiderocol for compassionate use in a critically ill patient with XDR Acinetobacter baumannii and KPC-producing Klebsiella pneumoniae: a case report. J Antimicrob Chemother. 2019;74:3399–3401. doi:10.1093/jac/dkz318
59. Lampejo T, Cherian BP, Tan MGM, et al. Cefiderocol in the treatment of systemic carbapenemase-producing multi-drug resistant Klebsiella pneumoniae infection. J Glob Antimicrob Resist. 2020. doi:10.1016/j.jgar.2020.10.008
60. Grande Perez C, Maillart E, Miendje Deyi VY, et al. Compassionate use of cefiderocol in a pancreatic abscess and emergence of resistance. Med Mal Infect. 2020. doi:10.1016/j.medmal.2020.10.022
61. Oliva A, Ceccarelli G, De Angelis M, et al. Cefiderocol for compassionate use in the treatment of complicated infections caused by extensively and pan-resistant Acinetobacter baumannii. J Glob Antimicrob Resist. 2020;23:292–296. doi:10.1016/j.jgar.2020.09.019
62. Falcone M, Tiseo G, Nicastro M, et al. Cefiderocol as rescue therapy for Acinetobacter baumannii and other carbapenem-resistant Gram-Negative infections in ICU patients. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa1410
63. Siméon S, Dortet L, Bouchand F, et al. Compassionate use of cefiderocol to treat a case of prosthetic joint infection due to extensively drug-resistant enterobacter hormaechei. Microorganisms. 2020;8:1236. doi:10.3390/microorganisms8081236
64. Kufel WD, Steele JM, Riddell SW, et al. Cefiderocol for treatment of an empyema due to extensively drug-resistant Pseudomonas aeruginosa: clinical observations and susceptibility testing considerations. IDCases. 2020;21:e00863. doi:10.1016/j.idcr.2020.e00863
65. Zingg S, Nicoletti GJ, Kuster S, et al. Cefiderocol for extensively drug-resistant gram-negative bacterial infections: real-world experience from a case series and review of the literature. Open Forum Infect Dis. 2020;7:ofaa185. doi:10.1093/ofid/ofaa185
66. Dagher M, Ruffin F, Marshall S, et al. Case report: successful rescue therapy of extensively drug-resistant acinetobacter baumannii osteomyelitis with cefiderocol. Open Forum Infect Dis. 2020;7:ofaa150. doi:10.1093/ofid/ofaa150
67. Mollmann U, Heinisch L, Bauernfeind A, et al. Siderophores as drug delivery agents: application of the “Trojan Horse” strategy. Biometals. 2009;22:615–624. doi:10.1007/s10534-009-9219-2
68. Page MGP. The role of iron and siderophores in infection, and the development of siderophore antibiotics. Clin Infect Dis. 2019;69:S529–S537. doi:10.1093/cid/ciz825
69. Wu JY, Srinivas P, Pogue JM. Cefiderocol: a novel agent for the management of multidrug-resistant gram-negative organisms. Infect Dis Ther. 2020;9:17–40. doi:10.1007/s40121-020-00286-6
70. Fectroja. Summary of product characteristics; [cited August 15, 2020]. Available from: https://www.ema.europa.eu/en/documents/product-information/fetcroja-epar-product-information_en.pdf.
71. Fetcroja EMA assessment report 27 February 2020. EMA/136096/2.
72. Giacobbe DR, Giani T, Bassetti M, et al. Rapid microbiological tests for bloodstream infections due to multidrug resistant Gram-negative bacteria: therapeutic implications. Clin Microbiol Infect. 2020;26:713–722. doi:10.1016/j.cmi.2019.09.023
73. Hackel MA, Tsuji M, Yamano Y, et al. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant gram-negative bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 Study). Antimicrob Agents Chemother. 2017;61. doi:10.1128/AAC.00093-17
74. Karlowsky JA, Hackel MA, Tsuji M, et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against gram-negative bacilli isolated by clinical laboratories in North America and Europe in 2015–2016: SIDERO-WT-2015. Int J Antimicrob Agents. 2019;53:456–466. doi:10.1016/j.ijantimicag.2018.11.007
75. Kazmierczak KM, Tsuji M, Wise MG, et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-beta-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents. 2019;53:177–184.
76. Jacobs MR, Abdelhamed AM, Good CE, et al. ARGONAUT-I: activity of cefiderocol (S-649266), a siderophore cephalosporin, against gram-negative bacteria, including carbapenem-resistant nonfermenters and enterobacteriaceae with defined extended-spectrum beta-lactamases and carbapenemases. Antimicrob Agents Chemother. 2019;63.
77. Dobias J, Denervaud-Tendon V, Poirel L, Nordmann P. Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant Gram-negative pathogens. Eur J Clin Microbiol Infect Dis. 2017;36:2319–2327. doi:10.1007/s10096-017-3063-z
78. Kohira N, West J, Ito A, et al. In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother. 2016;60:729–734. doi:10.1128/AAC.01695-15
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
