Back to Journals » Blood and Lymphatic Cancer: Targets and Therapy » Volume 10
Evaluating Blinatumomab for the Treatment of Relapsed/Refractory ALL: Design, Development, and Place in Therapy
Authors Sigmund AM, Sahasrabudhe KD, Bhatnagar B
Received 1 August 2020
Accepted for publication 29 September 2020
Published 3 November 2020 Volume 2020:10 Pages 7—20
DOI https://doi.org/10.2147/BLCTT.S223894
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Wilson Gonsalves
Audrey M Sigmund,* Kieran D Sahasrabudhe,* Bhavana Bhatnagar
Division of Hematology, Department of Internal Medicine, The Ohio State University and the Ohio State University Comprehensive Cancer Center, Columbus, OH 43210, USA
*These authors contributed equally to this work
Correspondence: Bhavana Bhatnagar
OSU Wexner Medical Center, 320 W 10th Avenue Tel +1-614-688-7939
Fax +1-614-293-6050
Email [email protected]
Abstract: Although adults with B-cell acute lymphoblastic leukemia (B-ALL) achieve high complete remission (CR) rates following treatment with intensive multi-agent chemotherapy regimens, up to two-thirds of these patients eventually relapse. Unfortunately, adults with relapsed or refractory (R/R) B-ALL have a poor prognosis, with variable responses to salvage chemotherapy regimens and allogeneic stem cell transplant. As such, the need to develop effective and well-tolerated treatments for this patient population has been of paramount importance over the past decade. In this regard, treatment options for R/R B-ALL patients have expanded considerably over a relatively short period of time, with the approvals of blinatumomab, inotuzumab ozogamicin and tisagenlecleucel occurring within only the past six years. Blinatumomab, a CD19 x CD3 bispecific T-cell engager (BiTE) was the first of these immune therapies to receive approval, and for many patients, is used as first-line salvage therapy. A number of large clinical trials have demonstrated improved progression-free survival and overall survival for R/R B-ALL patients receiving blinatumomab as compared to those receiving conventional salvage chemotherapy. In addition to being approved for both Philadelphia chromosome-negative and Philadelphia chromosome-positive R/R B-ALL, blinatumomab is also the only ALL therapy that carries approval for the treatment of measurable residual disease (MRD). Although blinatumomab has changed the therapeutic landscape for adults with R/R B-ALL, a number of important clinical considerations and questions remain, including the potential role of blinatumomab in the frontline setting, mechanisms of resistance, optimal goal MRD level, the role of transplant following MRD clearance, the optimal place for blinatumomab in the context of other recently approved immune-mediated therapies, and real world outcomes for patients treated outside the context of clinical trials. These issues are the focus of ongoing studies, which will hopefully inform future clinical practice regarding the utility of blinatumomab in the treatment of B-ALL patients.
Keywords: blinatumomab, BiTE antibody, B-cell acute lymphoblastic leukemia, relapsed and refractory disease, measurable residual disease, MRD
Introduction
Acute lymphoblastic leukemia (ALL) comprises the overwhelming majority of acute leukemia diagnoses in the pediatric population and approximately 20% of acute leukemia diagnoses in adults.1 Outcomes in children have dramatically improved since the 1950’s, with long-term survival rates of ~90%. Survival outcomes in adolescents and young adults (AYAs) as well as older adults, however, are not as comparable given the presence of high-risk genetic features and presence of co-morbidities that limit the use of several agents that are known to be especially active in pediatric ALL.2 Furthermore, approximately one-third of standard risk and two-thirds of high-risk adult patients will eventually relapse and long-term outcomes for such patients are exceedingly poor.3 Overall complete remission (CR) rates for relapsed or refractory (R/R) patients after first salvage therapy are typically 40% for those with Philadelphia chromosome-negative (Ph-) B-ALL with one and three year survival rates of only 26% and 11%, respectively.2 Factors that are known to be associated with poor overall prognosis include the presence of measurable residual disease (MRD) following induction therapy, older age and cytogenetics (such as Ph-like ALL, complex karyotype, hypodiploidy, and others).4
In light of the poor outcomes associated with R/R B-ALL, it became evident that novel, more effective treatment options were urgently needed for these patients. Blinatumomab (Blincyto, Amgen), a CD19 x CD3 bispecific T-cell engager (BiTE) antibody, was the first immune therapy to emerge as an effective treatment for adults with R/R B-ALL. Its unique mechanism of action, coupled with its tolerable toxicity profile and high response rates led to approval by the US Food and Drug Administration (FDA) in 2014 for the treatment of R/R B-ALL, based on the results of the MT103-211 trial.5 Additional studies showed efficacy for blinatumomab in patients with MRD positivity including the BLAST trial in which blinatumomab was shown to improve overall survival (OS) in B-ALL patients by effectively converting MRD+ patients to MRD-status.6,28–30 This led to FDA approval in 2018 for the treatment of B-ALL patients with MRD, defined as detectable disease of ≥0.1%. The aims of this review are to briefly describe the pharmacology of blinatumomab and to describe its current place in the therapeutic armamentarium for R/R and MRD+ B-ALL patients. We also discuss ongoing clinical investigations that will further inform the future of blinatumomab in the treatment of B-ALL patients.
Blinatumomab: Mechanism of Action and Management of Toxicities
Mechanism of Action
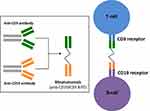
Blinatumomab is a BiTE antibody that transiently links CD19-positive B-cells with CD3-positive T-cells, subsequently inducing both T-cell mediated lysis of B-cells as well as T-cell proliferation. It targets the pan-B-cell antigen, CD19, which is present on B-lymphocytes throughout early stages of differentiation, as early as pre-B cells.7 CD19 is expressed on more than 90% of B-cell lineage cancers and thus is an ideal target for drugs designed to treat B-cell leukemias and lymphomas. Importantly, stem cells lack CD19 expression and thus are protected from the effects of CD19 targeted therapies.8
Blinatumomab is composed of two single chain variable antibody fragments that are connected by a flexible linker (Figure 1).9 One of the single-chain variable antibody fragments binds to the epsilon chain of the T-cell receptor/CD3 complex and the other single-chain variable antibody fragment binds to CD19. By binding to CD19 expressed on the surface of cells of B-cell lineage and CD3 expressed on the surface of T-cells, blinatumomab acts as a bridge to bring B-cells and T-cells into closer proximity and results in transient engagement of tumor cells by T-cells. A signaling cascade is subsequently triggered which leads to activation of T-cells and release of cytokines which then prompts the release of cytolytic proteins, such as granzymes and perforins, into the synapse. This ultimately leads to the destruction of tumor cells by either directly inducing apoptosis or leading to the activation of caspases which then precipitate cell death. These physiologic effects caused by blinatumomab are similar to T-cell attack against other tumor cell types.10,11
Preclinical and Early Clinical Trials
Preclinical trials of blinatumomab were conducted in animal models including mice and chimpanzees. In one study to assess efficacy of blinatumomab, mice were engineered with human B-cell lymphoma xenografts and subsequently treated with blinatumomab. The authors found that blinatumomab could prevent growth of the lymphoma and essentially result in cure when administered early in tumor development.12
Safety assessment was performed in chimpanzees as blinatumomab was found only to be cross-reactive with human and chimpanzee C19 and CD3. In vivo analysis in chimpanzees showed that blinatumomab caused a significant but temporary increase in several serum cytokine levels including IL-2, IL-6, and IFN-γ. Cytokine levels were highest after the first infusion compared to subsequent infusions. The authors also evaluated peripheral B-cell counts and found that there was no significant decrease during the treatment period. However, in the post-treatment period 3–5 weeks after last infusion, rates of B-cell depletion were as high as 40–70%.13
The first Phase I trials in humans were initially opened in 2001 and included patients with R/R indolent Non-Hodgkin’s Lymphoma (NHL) and chronic lymphocytic leukemia (CLL). Blinatumomab was administered in short infusions and significant toxicities were noted including neurotoxicity, which led to early termination of these trials.14 A subsequent phase I trial (MT103-104) went on to evaluate blinatumomab administered in continuous intravenous infusion (cIV). This trial included 76 patients with indolent NHL and dose escalation was utilized with doses of 0.5–90 μg/m2/d given over 4–8 weeks. Of the patients evaluated, 73% experienced a neurologic event with 21% experiencing a grade 3 neurologic event. The maximum tolerated dose (MTD) was 60 μg/m2/d. Of those patients that were treated with the MTD, the overall response rate was 69%.15 The authors also found that stepwise dosing with corticosteroid prophylaxis led to few treatment discontinuations due neurotoxicity.15 These early clinical trials led to Phase II/III studies to further evaluate dosage pattern and utility in CD19+ malignancies including B-ALL.
Clinical Pharmacology and Administration
Blinatumomab has similar pharmacokinetic properties to small molecules, including linear pharmacokinetics over a dose range of 5 to 90 µg/m2/day as well as a short half-life.16 When administered as a cIV over 4 weeks, blinatumomab typically achieves steady state serum concentrations within one day. The mean volume of distribution is 4.52 L, similar to plasma volume, and the mean clearance is 2.72 L/hr.16 The pharmacokinetics of blinatumomab demonstrates high variability, with fluctuations in average steady-state concentrations of up to 100% between individuals.16
Pharmacokinetics in adult patients have not been shown to be significantly impacted by body weight, body surface area, age, or sex. The impact of hepatic and renal dysfunction on blinatumomab clearance has not been evaluated with a formal study, however, hepatic impairment is not thought to significantly impact catabolism of blinatumomab given that it is a therapeutic protein; thus, dose adjustments are not recommended for those with hepatic impairment. Although renal impairment has been shown to result in an approximately 21% decrease in clearance of blinatumomab, no dosage adjustments are required for patients who have baseline mild [Creatinine clearance (CrCl) ranging from 60 to 89 mL/min] or moderate (CrCl ranging from 30 to 59 mL/min) renal impairment. At present, there is no data available for patients with severe renal impairment (CrCl <30 mL/min) or for those on hemodialysis.16
For patients with R/R B-ALL, blinatumomab is administered as a cIV over 4 weeks with dosage schedule of 4 weeks on, 2 weeks off. Hospital admission is recommended for the initial 9 days of the first cycle and the initial 2 days of cycle 2 for close monitoring for signs of cytokine release syndrome (CRS) and neurotoxicity. To reduce the magnitude of cytokine elevation and resultant toxicities, stepwise dosing is recommended with initiation of cycle 1, starting at 9 μg/d cIV for the first week followed by 28 μg/d cIV for the following 3 weeks with 28 μg/d cIV for 4 weeks in further cycles. At a cIV dose of 28 μg/d, the average steady-stage concentration ranges from 500–700 pg/mL.17 Pre-treatment with 20 mg of intravenous (IV) dexamethasone should be administrated 1 hour prior to initial start of the infusion, prior to dose escalation on day 8 of cycle 1, and prior to restarting treatment of any interruptions lasting 4 hours or longer. Pre-phase therapy with dexamethasone also is indicated for patients with high disease burden to prevent CRS. In the TOWER study, prephase therapy was considered mandatory for those patients with blast percentage exceeding 50% or peripheral blast count ≥15,000/µL and was recommended for those patients with LDH indicative of rapidly progressing disease or high extramedullary tumor load. Recommended dexamethasone dosing for pre-phase treatment is 10 mg/m2/day administered orally or IV given during screening and pre-phase until initiation of cycle 1. Dosage of dexamethasone can be increased to a maximum of 24 mg/day in these patients.18 As blinatumomab is a cIV, patients require an infusion pump that allows for continuous intravenous home administration. Once delivered to patients, IV bags can be stored for 8 days while refrigerated and 48 hours at room temperature.19
Adverse Drug Reactions and Management
The majority of patients receiving treatment with blinatumomab (up to 98.5%) will experience an adverse drug reaction (ADR) with approximately 61.8% experiencing a serious ADR, 19.1% experiencing a fatal serious ADR, and 12.4% experiencing an ADR leading to early termination of treatment.18 ADRs occurring in ≥10% of blinatumomab treated patients are reported in Table 1 as compared to standard of care (SOC) chemotherapy, defined as one of four different regimens: a FLAG ± anthracycline based regimen, HiDAC-based regimen, high-dose methotrexate based regimen, or a clofarabine based regimen. Two of the most significant ADRs are CRS and neurotoxicity which includes encephalopathy, convulsions, tremor, speech disturbances, confusion, and ataxia.19 All grades of neurologic toxicities occur in roughly 50% of patients, with grade ≥3 neurotoxicity occurring in approximately 15% of patients. CRS is less common with all grades occurring in 11% and grade 3 or higher occurring in 1% of patients. Other common toxicities include pyrexia, infection, and myelosuppression.19
 |
Table 1 Adverse Drug Reactions (ADRs) Reported for ≥10% of Blinatumomab Treated Patients as per Package Insert with Blinatumomab Compared to Standard of Care Chemotherapy |
Initial exposure to blinatumomab can result in supraphysiologic cytokine release ultimately leading to CRS. CRS can present with a spectrum of symptoms including flu-like symptoms, hypotension, capillary leak syndrome, and multi-organ failure. It occurs exclusively within the first cycle of treatment and coincides with the highest point of T-cell expansion and cytokine release, which occurs during the first few days of blinatumomab initiation.20 Incidence and intensity of CRS is correlated with disease burden, with increased risk and severity of CRS occurring in patients with higher disease burden. Mechanisms to minimize the severity of CRS include a two-step dose escalation as well as pre-phase and pre-treatment with dexamethasone as described above.5 Grade 1 or 2 CRS can typically be managed with observation or dexamethasone whereas grade 3 or 4 CRS require interruption of infusion. Grade 3 is managed by withholding the infusion until resolved then restarting at 9 mcg/day and grade 4 typically results in permanent discontinuation.19 Tocilizumab has also been shown to play a role in management of CRS for patients with grade 3 or 4 CRS.21
The neurologic toxicities observed following blinatumomab therapy are thought to be related to the production of neurotoxic cytokines and chemokines after the activation of T-cells which then lead to irritation of the neuroendothelium.14 Neurotoxicity typically occurs within the first 7 days of drug initiation and is managed with dexamethasone and/or interruption of the infusion.5 For grade ≥3 seizures, dose interruption is recommended along with anti-epileptic drug administration. In these cases, the infusion is typically held until symptoms are resolved to ≤ grade 1 for at least 3 days, then is restarted at 9 mcg/day. Cases of neurotoxicity that warrant permanent discontinuation of blinatumomab include grade 3 toxicities that take more than 7 days to resolve, any grade 4 toxicity, and anytime more than one seizure occurs.5
There are several other ADRs that have been documented with blinatumomab. Pyrexia is the most common ADR, occurring in approximately 55% of patients with 6% falling within grade 3 or higher. Myelosuppression is also common, with neutropenia occurring in 31% of patients, anemia in 25%, and thrombocytopenia in 21%. Infectious complications occur in >60% of patients. Edema was seen in 18% of patients with arrhythmia occurring in 14%. Transaminitis also can occur in approximately 15% of patients. Twelve percent of patients experience rash.19 Tumor lysis syndrome (TLS) is very rare in blinatumomab-treated patients. The severity and frequency of ADRs generally decrease after the first cycle, likely related to a lesser degree of cytokine release following later cycles of treatment.
Blinatumomab Treatment Indications
Blinatumomab for R/R ALL
Blinatumomab now plays a key role in the treatment of patients with R/R B-ALL after a series of clinical trials demonstrated improvement in outcomes compared to traditional salvage chemotherapy. A summary of prior trials for treatment of B-ALL with blinatumomab is shown in Table 2. The initial trial that led to FDA approval in 2014 was a large multicenter, single-arm Phase II study (MT103-211, NCT01466179)5 in which 189 patients with Ph- R/R B-ALL were enrolled to evaluate the clinical efficacy of blinatumomab. To minimize CRS and neurotoxicity, a step-wise dose escalation was utilized, starting at 9 µg/day for the first week followed by 28 µg/day for the subsequent 3 weeks. Treatment was given in 6 week cycles with 4 weeks of treatment and 2 weeks off. After two cycles of treatment, 43% of patients achieved a CR/CR with partial hematologic recovery (CRh), with 33% obtaining a CR and 10% a CRh. Median relapse free survival (RFS) was 5.9 months with a median follow-up of 8.9 months and the median OS was 6.1 months with a median follow-up of 9.8 months. Of the patients that achieved a CR/CRh, 40% underwent allogeneic hematopoietic stem cell transplant (allo-HSCT). Twenty-percent of patients who received blinatumomab in this study were alive after two years, which was superior to historical data with traditional chemotherapy. Based on this study, the estimated long-term survival (at least 60 months) was 12.4% as compared to 5.4% with salvage chemotherapy. These findings suggested that blinatumomab may have superior outcomes in patients with R/R B-ALL when compared to salvage chemotherapy and thus led to future randomized trials to further evaluate blinatumomab in comparison to salvage chemotherapy.
 |
Table 2 Summary of Prior Trials for Treatment of B-ALL with Blinatumomab |
The landmark randomized Phase III TOWER study directly evaluated the efficacy of blinatumomab compared to four commonly employed salvage chemotherapy regimens.18 The study enrolled 405 Ph- B-ALL patients who were randomized in a 2:1 ratio with 271 patients receiving blinatumomab and 124 patients receiving salvage chemotherapy. Patients received up to 2 cycles of induction and those patients who achieved a morphologic remission then received up to 3 cycles of consolidation, with those with continued morphologic remission subsequently receiving up to 12 months of maintenance. The dosage schedule for both induction and maintenance blinatumomab was similar to in MT103-211 with maintenance treatment given as a 4 week cIV dosed every 12 weeks. Patients randomized to receive salvage chemotherapy were eligible to receive one of four physicians choice regimens. Forty-nine patients (45%) received high-dose cytosine arabinoside and granulocyte colony stimulating factor with or without anthracycline, 19 patients (17%) received high-dose cytosine arabinoside-based regimens, 22 patients (20%) received methotrexate-based regimens, and 19 patients (17%) received clofarabine-based regimens. In comparison to patients receiving salvage chemotherapy, blinatumomab-treated patients had improved CR rates (34% vs 16%, P<0.001) and OS (7.7 months versus 4 months, P=0.01) in R/R B-ALL patients. This study confirmed that treatment with blinatumomab results in significantly longer OS in adult patients with R/R B-ALL as compared to conventional chemotherapy.18
Additional trials have also focused on evaluating the role of blinatumomab in treatment of patients with Ph+ R/R B-ALL. A Phase II, single arm, multicenter study of Ph+ B-ALL patients by Martinelli et al evaluated the efficacy of blinatumomab for treatment of Ph+ B-ALL patients who progressed after failure of TKI based therapy.22 The study included 45 patients with Ph+ B-ALL who had relapsed while on a TKI or had been refractory to at least one second generation TKI. Of the 45 patients, 36% achieved a CR/CRh after the first two cycles and 88% of the CR/CRh responders achieved a complete MRD response with 44% of responders proceeding with allo-HSCT. Median OS was 7.1 months and median RFS was 6.7 months. Blinatumomab has also been evaluated in combination with TKIs for treatment of R/R Ph+ ALL. A retrospective study by King et al evaluated the utility of blinatumomab in combination with an oral TKI as consolidation therapy for patients with Ph+ B-ALL. This study demonstrated that Blinatumomab + TKI resulted in eradication of MRD in the majority of patients with Ph+ ALL and this therapy overall was well tolerated.23 Another retrospective study by Assi et al assessed Blinatumomab + TKI for treatment of Ph+ positive patients. The study included 12 patients, 9 with Ph+ ALL and 3 with chronic myeloid leukemia (CML) in blast crisis. Of these patients, 75% (9/12) achieved complete molecular response and the treatment was safe.24 Additional prospective studies are needed to further characterize the role of Blinatumomab in Ph+ B-ALL compared to conventional chemotherapy as well as in combination with TKIs.
Blinatumomab in MRD+ Disease
Eradication of MRD is another active area of research in ALL. MRD describes low-level disease that is unable to be detected by conventional cytomorphology and is only detectable by more sensitive techniques including flow cytometry, polymerase chain reaction assays, and next-generation sequencing. It has become increasingly clear that MRD positivity is one of the most significant independent prognostic factors in B-ALL patients and that achievement of MRD negativity is crucial for improving patient outcomes.25–27
Several clinical trials have evaluated the efficacy of blinatumomab in MRD-positive B-ALL patients. Topp and colleagues conducted a phase II study in which blinatumomab was administered to 21 patients in first CR after induction, and at least one round of consolidation chemotherapy, who had detectable MRD defined as ≥1 x 10−4. The dose of blinatumomab administered to MRD-positive patients was lower (15 µg/day, 4 weeks on and 2 weeks off) compared to the dosing used for salvage therapy. Of the 20 evaluable patients, 16 (80%) achieved MRD negativity, all of which occurred after one cycle of blinatumomab.28 Patients with a matched donor were permitted to undergo allo-HSCT at any time after the first cycle of blinatumomab therapy, but the study was not powered to assess the impact of HSCT after blinatumomab. Forty-five percent of the evaluable patients went on to receive a transplant. An interim efficacy analysis conducted after a median follow up of 33 months found an overall RFS rate of 61%. The RFS rate was 65% among the 9 patients who had undergone transplant and 60% among the 11 patients who had not undergone transplant.29 Patients completed follow-up visits for up to 5 years, and the final analysis was conducted after a median follow up of 50.8 months. Fifty percent of the 20 total evaluable patients remained in remission at the final analysis. Stratifying the results according to transplant status showed that 56% (5/9) of patients who had received a transplant were in remission and 45% (5/11) of patients who had not received a transplant remained in remission.6
This study was followed by the landmark phase II BLAST trial led by Gökbuget and colleagues, in which blinatumomab was administered for up to 4 cycles in patients with B-ALL in first or later hematologic CR and with persistent or recurrent MRD ≥10−3 after at least 3 cycles of intensive chemotherapy. The dosing schedule for each cycle of blinatumomab in this trial was also 15 µg/day, 4 weeks on and 2 weeks off. A total of 116 patients were enrolled, and eligible patients were permitted to undergo allo-HSCT at any time after cycle 1 of blinatumomab therapy. Of the 113 evaluable patients for the primary endpoint of MRD response, 78% achieved MRD negativity after one cycle of blinatumomab. Two additional patients achieved MRD negativity after cycle 2 with no additional patients achieving MRD negativity after cycle 3 or 4. Secondary end point analyses involving survival and transplantation were conducted in 110 patients with Ph- disease. Median RFS and OS were 18.9 months and 36.5 months, respectively, with a median follow up of 30 months. Notably, patients achieving MRD negativity after cycle 1 of blinatumomab had significantly better survival outcomes compared to MRD non-responders with a median RFS of 23.6 vs 5.7 months (P=0.002) and median OS of 38.9 vs 12.5 months (P=0.002) for MRD responders and MRD non-responders, respectively. These data thereby demonstrated a direct patient benefit for the conversion of MRD positivity to MRD negativity. Importantly, patients in second or third CR had significantly inferior RFS and OS compared to patients in first CR despite having similar rates of MRD response to blinatumomab, suggesting that administering blinatumomab for MRD eradication in first CR is more beneficial compared to using it in subsequent remissions. Sixty-seven percent (74/110) included in the secondary endpoint analysis underwent allo-HSCT. At a median follow up of 24 months, 25% (9/36) of patients who had not received HSCT or chemotherapy after blinatumomab remained in CR whereas 49% (36/74) of patients who had undergone HSCT remained in CR. This study, too, was not powered to assess the impact of allo-HSCT after blinatumomab.30 However, in light of the obvious survival benefit conferred through conversion to MRD-negativity, blinatumomab received FDA approval in 2018 for patients with B-ALL in CR with MRD 10−3 (0.1%) or greater.
In an effort to determine whether blinatumomab provides the same survival benefit for Ph+ B-ALL patients, Richard-Carpentier et al sought to address this question in their ongoing phase II study in which blinatumomab was administered to Ph- and Ph+ B-ALL patients with persistent or recurrent MRD of ≥10−4. In this trial, patients were able to receive up to 5 cycles of blinatumomab therapy followed by allo-HSCT any time after cycle 1. Of note, Ph+ B-ALL patients were treated with physician’s choice TKI in addition to blinatumomab. Of the 25 patients who were enrolled between December 2015 and June 2019, MRD negativity was achieved in 75% of patients with Ph+ disease and 80% of patients with Ph- disease (P=1.00), and there was no significant difference in 2-year RFS or OS according to Ph status. Results of longer follow up will be informative, particularly in regards to the outcomes for patients with Ph+ disease who are treated with blinatumomab plus TKI therapy.31
Future Study Directions
Although blinatumomab has earned its place as an effective and well tolerated treatment option for R/R B-ALL patients and those with MRD+ disease, several key questions remain that are currently being evaluated in ongoing clinical trials, as described below and summarized in Table 3.
 |
Table 3 Summary of Ongoing Trials for Treatment of B-ALL with Blinatumomab |
What is the Optimal MRD Level in Which to Use Blinatumomab?
Blinatumomab is currently FDA approved for patients with MRD of 10−3 or greater based on the trial conducted by Gökbuget and colleagues.30 However, other clinical trials studying blinatumomab in the MRD+ setting have used different MRD thresholds for blinatumomab administration.28,31 This speaks to the controversial nature of MRD thresholds and the lack of standardization regarding the level of MRD that should be used to inform clinical decisions. Improvement in MRD detection techniques has also allowed for increased sensitivity. For example, the FDA-approved ClonoSeq technology uses next-generation sequencing of immunoglobulin receptor genes as well as regions of the genome that are frequently translocated. This assay is able to detect residual disease at a level of 10−6.32 Continued technologic improvements will likely continue to allow us to detect increasingly small amounts of MRD. It will therefore be important to continue to assess whether patients with increasingly low levels of detectable MRD benefit from treatment with blinatumomab and to continue to refine our understanding of the most appropriate MRD threshold for treatment.
Do All Patients Who Achieve MRD-Negativity Following Blinatumomab Benefit from Transplant?
At present, allo-HSCT is recommended for ALL patients who have detectable MRD following standard frontline induction therapy.33,34 However, it remains unknown whether MRD+ patients who clear their MRD after being treated with blinatumomab derive benefit from transplant. For instance, Topp and colleagues reported that 45% of patients who had not undergone transplant remained in remission at median follow up of 50.8 months compared to 56% of the patients who had received a transplant.28 This was further supported in the BLAST study in which 25% of the patients who had not undergone transplant remained in CR at a median follow up of 24 months compared to 49% who had undergone transplant. Taken together, these data suggest that not all MRD responders necessarily require a transplant. However, these trials were not powered to assess the impact of transplant after blinatumomab, which will be an important consideration to address in future studies. For MRD responders who are ineligible for allo-HSCT, future studies designed to determine the optimal number of cycles of blinatumomab in addition whether or not combination therapies are needed will also be important to address. For transplant recipients, the role of blinatumomab in the post-transplant maintenance setting is also an important issue, which is currently under investigation.35
What is the Optimal Place of Blinatumomab in the Context of Other Immunological Therapeutic Strategies?
Two other promising immunological therapies that are now FDA approved for R/R B-ALL patients are inotuzumab ozogamicin and CAR-T cell therapy. Inotuzumab ozogamicin is an anti-CD22 antibody that is attached to the cytotoxic agent calicheamicin. An early phase II trial by Kantarjian et al showed a CR/CRi rate of 57% with 63% of these responders achieving a complete molecular response (CMR).36 An additional phase I/II trial by DeAngelo et al had similar response rates with 68% of patients achieving a CR/CRi and 84% of these responders achieving MRD negativity.37 A subsequent phase III trial, the INO-VATE study, comparing inotuzumab to SOC chemotherapy showed a significantly higher CR/CRi rate with inotuzumab (80.7% vs 29.4%). Patients randomized to SOC chemotherapy received either FLAG (fludarabine, cytarabine, and granulocyte colony-stimulating factor), cytarabine plus mitoxantrone, or HiDAC. Overall survival was also superior in the inotuzumab group (7.7 vs 6.7 months P=0.04). This survival difference has remained durable over time with the 2-year OS rate of 22.8% versus 10% with SOC; P=0.01.38 Common toxicities of inotuzumab included myelosuppression as well as increased risk for veno-occlusive disease (VOD) post-allo-SCT. The rate of VOD was as high as 23% in the initial phase II trial but was lower at 11% in the INO-VATE study, in which dual-alkylating therapy was the only significant covariate for development of VOD.36–38
Chimeric antigen receptor T cell therapy (CAR-T) has also recently emerged as an option for treatment of patients with R/R B-ALL. CAR-T cells are auto-reactive T-cells that have been engineered to recognize and target cells expressing a specific marker. The primary CAR-T cells that are studied for B-ALL patients are CD19 targeted CAR-T cells. A landmark phase II trial of CAR-T cell therapy for R/R B-ALL patients was conducted in 75 patients aged 3–21 years old. These patients received tisagenlecleucel (Kymriah), an anti-CD19 CAR-T cell therapy. Of those patients who received tisagenlecleucel infusion, 81% of patients achieved MRD-negativity within 3 months. At 12 months, the rate of event free survival and OS were 50% and 76%, respectively. Median duration of remission was not reached. Most common toxicities were CRS, which occurred in 77% of patients with 48% of patients requiring tocilizumab, as well as neurologic events, which occurred in 40% of patients.39
The optimal sequence for these therapies is not always clear, but there are several important considerations when deciding which of these treatments to use and when. CAR-T cell therapy is currently only approved for patients aged 21 years old and younger although there are ongoing trials to assess its role in adult patients (NCT01044069, NCT01865617, NCT02614066). When deciding between blinatumomab and inotuzumab in patients with leukemia that is both CD19 and CD22 positive, blinatumomab is preferred for patients that will be proceeding to allo-HCT due to increased risk of VOD with inotuzumab. However, if a patient has high tumor burden including extramedullary disease, inotuzumab is often preferred because response rates with inotuzumab do not correlate with disease burden, whereas blinatumomab has lower response rates in the setting of high tumor burden.5,37 Inotuzumab is also favorable to blinatumomab for patients with CNS disease due to risk of neurotoxicity with blinatumomab.19 Blinatumomab is preferable for patients with MRD-positive disease, as inotuzumab currently is not approved for these patients.30
Does Blinatumomab Have a Place in the Frontline Setting for B-ALL Patients?
Given the encouraging results seen with blinatumomab in the R/R and MRD+ setting, it is under active investigation in a number of frontline clinical trials. ECOG E1910 (NCT02003222) is a randomized phase III study comparing chemotherapy plus blinatumomab to chemotherapy alone in adults with newly diagnosed Ph- B-ALL. This trial has completed enrollment and results are expected to be available in the near future. SWOG S1318 (NCT02143414), is an open-label phase II clinical trial for older adults (≥65 years) with untreated Ph- or Ph+ B-ALL in which blinatumomab plus POMP (prednisone, vincristine, methotrexate, and 6-mercaptopurine) is used as induction therapy. Ph+ patients also receive dasatinib as part of their treatment as well. Interim analysis of untreated Ph- patients showed that blinatumomab plus POMP as frontline therapy was associated with a CR/CRi rate of 66% and that 92% of these responders with available MRD data had achieved MRD negativity after one cycle of blinatumomab.40 The D-Alba trial is an ongoing phase II multicenter study combining blinatumomab with dasatinib for frontline therapy in patients with Ph+ B-ALL. Patients were treated with dasatinib monotherapy for induction for 85 days with blinatumomab added for consolidation for those patients who achieved a complete hematologic response with dasatinib induction. Interim analysis showed a molecular response in 80% of patients after 4 cycles of blinatumomab with a 12-month OS and disease-free survival of 94.2% and 87.8%, respectively.41
Another unique ongoing frontline phase II study is Alliance A041703 (NCT03739814) in which older adults with untreated CD22-positive B-ALL are treated with inotuzumab induction, followed by blinatumomab consolidation therapy. Richard-Carpentier and colleagues are currently conducting a phase II study evaluating treatment of adults with newly diagnosed B-ALL with sequential hyper-CVAD and blinatumomab followed by maintenance with POMP and blinatumomab. Interim analysis of 27 patients has shown a 100% CR rate with 96% of patients achieving MRD of 10−4 or less and a 12 month estimated RFS of 76% and OS of 89%.42 Lastly, ALLG ALL8 (ACTRN12617000084381) is a phase II study of blinatumomab alternating with “part B” cycles of hyper-CVAD for adults with newly diagnosed B-ALL. Of the first 10 patients treated on this study, 7 achieved MRD of 10−4 or greater by completion of one consolidation.43
What are the Mechanisms of Resistance to Blinatumomab?
Another important question to consider is mechanisms of resistance to blinatumomab. One potential mechanism of resistance to CD19-directed therapy is target antigen loss, which is seen in a significant subset of patients undergoing CD19-directed CAR-T cell therapy.44 This phenomenon is known to occur in patients treated with blinatumomab, but appears to be a less common mechanism of resistance compared to CAR-T cell therapy.45 Treatment of KMT2A (formerly MLL)-rearranged B-ALL with blinatumomab has also been reported to be associated with subsequent lineage switch to AML, which is another potential mechanism of resistance.46–49 Additionally, as the efficacy of blinatumomab is dependent on the activity of T-cells, down-regulation of T-cell activity has also been studied as a potential mechanism of resistance to blinatumomab. Studies have shown that markers of T-cell exhaustion including PD-L1 and PD1 are increased in the setting of blinatumomab therapy thereby decreasing T-cell activity.50,51 The addition of immune checkpoint inhibitors to blinatumomab therapy may therefore enhance its efficacy, and there are several ongoing trials assessing the efficacy and safety of this approach in both adult patients (NCT02879695, NCT03160079, NCT03512405) and pediatric patients (NCT03605589).
Blinatumomab in the Real World
Another question to consider is the outcomes for patients who receive blinatumomab in the “real world” outside of the context of clinical trials. Badar and colleagues conducted a retrospective, multicenter trial analyzing outcomes for patients with R/R or MRD+ disease who were treated with blinatumomab between 12/2014 and 5/2019. They found that blinatumomab was well tolerated with similar rates of grade 3–4 CRS and neurotoxicity as seen in clinical trials. They also found encouraging results with respect to efficacy. Rate of CR/CRi for patients with R/R disease was 61% with 44% having CR with MRD negativity. Median OS for patients with R/R disease was 12.7 months. For patients in remission but with MRD prior to treatment, 75% achieved MRD negativity and median OS was 34.7 months for this group of patients.52 These results support the safety and utility of blinatumomab administered outside of clinical trials. It will be important to continue to monitor patient outcomes over time as more patients are treated with blinatumomab in routine clinical practice.
Conclusion
This is an exciting time for research in ALL with several targeted and immune therapies recently approved and currently under investigation. Blinatumomab is one such therapy that has led to improved outcomes for patients with R/R and MRD+ B-ALL. There are several ongoing areas of study in relation to blinatumomab that will help to further refine our understanding of blinatumomab and its place in therapy. This will continue to enhance our ability to use blinatumomab as effectively as possible and maximize its therapeutic potential.
Disclosure
Bhavana Bhatnagar reports advisory board honorarium from Novartis, Astellas, Cell Therapeutics, Pfizer, Kite, and research support from Karyopharm Therapeutics and Cell Therapeutics, outside the submitted work.
The authors report no other potential conflicts of interest in this work.
These authors contributed equally to the preparation of the manuscript: Audrey M Sigmund, Kieran D Sahasrabudhe.
References
1. Hoelzer D, Bassan R, Dombret H, et al. Acute lymphoblastic leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v69–v82. doi:10.1093/annonc/mdw025
2. Gokbuget N, Dombret H, Ribera JM, et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica. 2016;101(12):1524–1533.
3. Gokbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120(10):2032–2041. doi:10.1182/blood-2011-12-399287
4. Rowe JM. Prognostic factors in adult acute lymphoblastic leukaemia. Br J Haematol. 2010;150(4):389–405.
5. Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi:10.1016/S1470-2045(14)71170-2
6. Gokbuget N, Zugmaier G, Klinger M, et al. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica. 2017;102(4):e132–e135. doi:10.3324/haematol.2016.153957
7. Uckun FM, Jaszcz W, Ambrus JL, et al. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood. 1988;71(1):13–29. doi:10.1182/blood.V71.1.13.13
8. Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma. 1995;18(5–6):385–397. doi:10.3109/10428199509059636
9. Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93(3):290–296. doi:10.1038/icb.2014.93
10. Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–977. doi:10.1126/science.1158545
11. Kontermann RE. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4(2):182–197. doi:10.4161/mabs.4.2.19000
12. Dreier T, Baeuerle PA, Fichtner I, et al. T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3- bispecific single-chain antibody construct. J Immunol. 2003;170(8):4397–4402. doi:10.4049/jimmunol.170.8.4397
13. Schlereth B, Quadt C, Dreier T, et al. T-cell activation and B-cell depletion in chimpanzees treated with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Cancer Immunol Immunother. 2006;55(5):503–514. doi:10.1007/s00262-005-0001-1
14. Nagorsen D, Kufer P, Baeuerle PA, et al. Blinatumomab: a historical perspective. Pharmacol Ther. 2012;136(3):334–342. doi:10.1016/j.pharmthera.2012.07.013
15. Goebeler M-E, Knop S, Viardot A, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-hodgkin lymphoma: final results from a phase i study. J Clin Oncol. 2016;34(10):1104–1111. doi:10.1200/JCO.2014.59.1586
16. Zhu M, Wu B, Brandl C, et al. Blinatumomab, a bispecific T-cell engager (BiTE((R))) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. 2016;55(10):1271–1288. doi:10.1007/s40262-016-0405-4
17. Dreier T, Lorenczewski G, Brandl C, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100(6):690–697. doi:10.1002/ijc.10557
18. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–847. doi:10.1056/NEJMoa1609783
19. Blincyto ® [package insert]. Thousand Oaks, CA: Amgen Inc; 2014.
20. Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119(26):6226–6233. doi:10.1182/blood-2012-01-400515
21. Choudhry J, Parson M, Wright J. A retrospective review of tocilizumab for management of blinatumomab (a bispecific T cell engager)-induced cytokine release syndrome (CRS). Blood. 2018;132:5211. doi:10.1182/blood-2018-99-117353
22. Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase ii, single-arm, multicenter study. J Clin Oncol. 2017;35(16):1795–1802. doi:10.1200/JCO.2016.69.3531
23. King AC, Pappacena JJ, Tallman MS, et al. Blinatumomab administered concurrently with oral tyrosine kinase inhibitor therapy is a well-tolerated consolidation strategy and eradicates measurable residual disease in adults with Philadelphia chromosome positive acute lymphoblastic leukemia. Leuk Res. 2019;79:27–33. doi:10.1016/j.leukres.2019.02.009
24. Assi R, Kantarjian H, Short NJ, et al. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed philadelphia chromosome-positive leukemia. Clin Lymphoma Myeloma Leuk. 2017;17(12):897–901. doi:10.1016/j.clml.2017.08.101
25. Beldjord K, Chevret S, Asnafi V, et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123(24):3739–3749. doi:10.1182/blood-2014-01-547695
26. Berry DA, Zhou S, Higley H, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7):e170580. doi:10.1001/jamaoncol.2017.0580
27. Brüggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107(3):1116–1123. doi:10.1182/blood-2005-07-2708
28. Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–2498. doi:10.1200/JCO.2010.32.7270
29. Topp MS, Gökbuget N, Zugmaier G, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185–5187. doi:10.1182/blood-2012-07-441030
30. Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522–1531.
31. Richard-Carpentier G, Kantarjian HM, Jorgensen JL, et al. Phase II study of blinatumomab in patients with B-cell acute lymphoblastic leukemia (B-ALL) with positive measurable residual disease (MRD). Blood. 2019;134(Supplement_1):1299. doi:10.1182/blood-2019-130283
32. ClonoSEQ cleared for residual cancer testing. Cancer Discov. 2018;8(12):OF6. doi:10.1158/2159-8290.CD-NB2018-136
33. Dhedin N, Huynh A, Maury S, et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125(16):
34. Gokbuget N, Kneba M, Raff T, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120(9):1868–1876. doi:10.1182/blood-2011-09-377713
35. Kebriaei P, Banerjee PP, Ganesh C, et al. Blinatumomab is well tolerated maintenance therapy following allogeneic hematopoietic cell transplantation for acute lymphoblastic leukemia. Blood. 2019;134(Supplement_1):1298. doi:10.1182/blood-2019-125931
36. Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 2012;13(4):403–411. doi:10.1016/S1470-2045(11)70386-2
37. DeAngelo DJ, Stock W, Stein AS, et al. Inotuzumab ozogamicin in adults with relapsed or refractory CD22-positive acute lymphoblastic leukemia: a Phase 1/2 study. Blood Adv. 2017;1(15):1167–1180. doi:10.1182/bloodadvances.2016001925
38. Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, Phase 3 INO-VATE study. Cancer. 2019;125(14):2474–2487. doi:10.1002/cncr.32116
39. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448.
40. Advani AS, Moseley A, O’Dwyer KM, et al. Results of SWOG 1318: a phase 2 trial of blinatumomab followed by pomp (prednisone, vincristine, methotrexate, 6-mercaptopurine) maintenance in elderly patients with newly diagnosed philadelphia chromosome negative B-cell acute lymphoblastic leukemia. Blood. 2018;132(Supplement 1):33. doi:10.1182/blood-2018-99-111992
41. Chiaretti S, Bassan R, Vitale A, et al. Dasatinib-blinatumomab combination for the front-line treatment of adult Ph+ ALL patients. Updated results of the gimema LAL2116 D-alba trial. Blood. 2019;134(Supplement_1):740. doi:10.1182/blood-2019-128759
42. Richard-Carpentier G, Kantarjian HM, Short NJ, et al. Updated results from the phase ii study of hyper-CVAD in sequential combination with blinatumomab in newly diagnosed adults with B-cell acute lymphoblastic leukemia (B-ALL). Blood. 2019;134(Supplement_1):3807. doi:10.1182/blood-2019-129657
43. Fleming S, Venn N, Reynolds J, et al. Preliminary minimal residual disease analysis of the australasian leukaemia & lymphoma group (ALLG) ALL8 study of front-line blinatumomab with chemotherapy in adults with Ph negative B-cell acute lymphoblastic leukaemia. Blood. 2019;134(Supplement_1):1300. doi:10.1182/blood-2019-132048
44. Ruella M, Maus MV. Catch me if you can: leukemia escape after CD19-directed T cell immunotherapies. Comput Struct Biotechnol J. 2016;14:357–362. doi:10.1016/j.csbj.2016.09.003
45. Jabbour E, Dull J, Yilmaz M. Outcome of patients with relapsed/refractory acute lymphoblastic leukemia after blinatumomab failure: no change in the level of CD19 expression. Am J Hematol. 2018;93(3):371–374. doi:10.1002/ajh.24987
46. Haddox CL, Mangaonkar AA, Chen D, et al. Blinatumomab-induced lineage switch of B-ALL with t(4:11)(q21;q23) KMT2A/AFF1 into an aggressive AML: pre- and post-switch phenotypic, cytogenetic and molecular analysis. Blood Cancer J. 2017;7(9):e607. doi:10.1038/bcj.2017.89
47. He RR, Nayer Z, Hogan M, et al. Immunotherapy- (blinatumomab-) related lineage switch of KMT2A/AFF1 rearranged B-lymphoblastic leukemia into acute myeloid leukemia/myeloid sarcoma and subsequently into B/myeloid mixed phenotype acute leukemia. Case Rep Hematol. 2019;2019:7394619. doi:10.1155/2019/7394619
48. Wolfl M, Rasche M, Eyrich M, et al. Spontaneous reversion of a lineage switch following an initial blinatumomab-induced ALL-to-AML switch in MLL-rearranged infant ALL. Blood Adv. 2018;2(12):1382–1385. doi:10.1182/bloodadvances.2018018093
49. Zoghbi A, Zur Stadt U, Winkler B, et al. Lineage switch under blinatumomab treatment of relapsed common acute lymphoblastic leukemia without MLL rearrangement. Pediatr Blood Cancer. 2017;64(11):e26594. doi:10.1002/pbc.26594
50. Feucht J, Kayser S, Gorodezki D, et al. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget. 2016;7(47):76902–76919. doi:10.18632/oncotarget.12357
51. Kohnke T, Krupka C, Tischer J, et al. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J Hematol Oncol. 2015;8(1):111. doi:10.1186/s13045-015-0213-6
52. Badar T, Szabo A, Advani AS, et al. Real world outcomes of adult B-cell acute lymphocytic leukemia patients treated with blinatumomab. Blood. 2019;134(Supplement_1):3809. doi:10.1182/blood-2019-125466
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.