Back to Journals » Clinical Epidemiology » Volume 10
Erythropoiesis-stimulating agents and cardiovascular events in patients with myelodysplastic syndrome and multiple myeloma
Authors Horváth-Puhó E , Suttorp MM, Frederiksen H, Hoekstra T, Dekkers OM , Pedersen L, Cannegieter SC , Dekker FW , Sørensen HT
Received 25 April 2018
Accepted for publication 29 June 2018
Published 28 September 2018 Volume 2018:10 Pages 1371—1380
DOI https://doi.org/10.2147/CLEP.S172306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Irene Petersen
Erzsébet Horváth-Puhó,1 Marit M Suttorp,2 Henrik Frederiksen,1,3 Tiny Hoekstra,2 Olaf M Dekkers,1,2 Lars Pedersen,1 Suzanne C Cannegieter,2 Friedo W Dekker,2 Henrik Toft Sørensen1
1Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark; 2Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, the Netherlands; 3Department of Haematology, Odense University Hospital, Odense, Denmark
Introduction: Erythropoiesis-stimulating agent (ESA) treatment has been associated with an increased risk of venous thromboembolism (VTE) in patients with solid tumors and with an increased risk of cardiovascular events in patients with chronic kidney disease. The ESA-related risk in patients with hematological neoplasms remains unclear. We, therefore, aimed to assess the ESA-related risk of VTE, myocardial infarction (MI), and stroke in patients with multiple myeloma (MM) and myelodysplastic syndrome (MDS).
Materials and methods: We conducted a population-based cohort study in Denmark, using medical databases to identify 2,114 MDS patients and 3,105 MM patients diagnosed in 2004–2013. Incidence rates per 1,000 person-years and hazard ratios (HRs) with 95% confidence intervals (CIs) for VTE, MI, and stroke associated with ESA treatment were computed. HRs were calculated in time-dependent Cox regression and adjusted for age, sex, MDS prognosis group, comorbidities, and treatments.
Results: Incidence rates per 1,000 person-years for VTE, MI, and stroke were 10.8, 8.2, and 16.0 in MDS patients, and 21.9, 10.2 and 9.9 in MM patients without ESA treatment, respectively. MDS patients with ESA treatment had a 1.6-fold increased risk of MI (HR 1.60 [95% CI 0.90–2.86]) and an almost twofold increased risk of stroke (HR 1.94 [95% CI 1.28–2.94]). Adjusted HR for VTE was 1.04 (95% CI 0.57–1.89) compared with MDS patients without ESAs. In MM patients with ESAs compared with patients without ESAs, HRs were 1.41 (95% CI 0.96–2.08) for VTE, 1.23 (95% CI 0.68–2.20) for MI, and 1.63 (95% CI 0.96–2.77) for stroke.
Conclusion: ESA use was associated with stroke in MDS patients. Among MM patients, ESA treatment was associated with a higher risk of all cardiovascular events, although all CIs included equivalence.
Keywords: cohort study, epidemiology, erythropoietin, multiple myeloma, myelodysplastic syndromes, myocardial infarction, stroke, pulmonary embolism, venous thrombosis
Introduction
Erythropoiesis-stimulating agents (ESAs) are used to treat anemia, in order to reduce the need for red blood cell transfusions. After ESAs first proved successful in treating anemia in patients with chronic kidney disease, ESAs were also implemented in the treatment of anemia associated with malignancies.1 In the past decade, several meta-analyses, including trials of patients with mainly solid tumors, have reported an up to 1.17-fold increased mortality risk associated with ESA treatment.2–4 Also, an increased risk of venous thromboembolism (VTE) has been reported in cancer patients treated with ESAs.5 Concurrently, higher doses of ESAs and higher hemoglobin targets have also been associated with increased mortality and cardiovascular risk in patients with non-malignant chronic kidney disease.6
As a consequence, ESA treatment in patients with solid tumors is now restricted to certain patients with chemotherapy-induced anemia.1,7 However, ESA treatment is widely used in patients with the hematological neoplasms myelodysplastic syndrome (MDS) and multiple myeloma (MM). MDS patients generally are believed to be at low risk of VTE, mainly because of the high incidence of thrombocytopenia and anemia8 and a previous case-crossover study reported no increased risk of VTE in MDS patients treated with ESAs.9 However, an earlier Phase II trial in low-to-intermediate risk MDS patients was halted early because three out of seven patients developed a VTE.10 Also, a higher incidence of VTE with ESA treatment in patients with MM has been reported.11,12
The hemato-oncology literature has concentrated mainly on the effect of ESAs on VTE events.13 An effect of ESAs on cardiovascular events, as described in patients with chronic kidney disease, is infrequently reported. A meta-analysis showed a 1.7-fold increased risk of a combination of venous and arterial thrombotic events in ESA-treated patients, mainly with solid tumors, compared with cancer patients who did not receive ESA treatment.4 It is difficult to deduce the contribution of myocardial infarction (MI), and stroke. No increased risk of combined arterial and venous thrombotic events with ESA treatment was reported in an earlier study of MM patients.14 In the current study, we therefore aimed to examine the association between ESA use and cardiovascular events in patients with MM and MDS in a large population-based cohort study. To that end, we calculated the ESA-related risk of VTE, MI, and stroke separately.
Methods
We conducted a population-based cohort study in Denmark, linking individual-level records among different Danish registries using the civil personal register (CPR) number.15,16 This unique and permanent identification number is assigned to all Danish residents alive on, or born after 1968 or at the time of immigration. In Denmark, the National Health Service provides universal tax-supported health care, guaranteeing unfettered access to general practitioners and hospitals.
Data sources
The Danish Cancer Registry (DCR) contains records of all incident cases of malignant neoplasms in Denmark since 1943 and provides details on histology, morphology, and cancer stage at diagnosis.17 Throughout our study period, tumors were classified according to the tenth revision of the International Classification of Diseases (ICD-10).
The Danish National Patient Registry (DNPR) provides information on all non-psychiatric hospital inpatient admissions since 1977 and on all outpatient clinic and emergency room visits since 1995.18 Each inpatient hospital discharge or outpatient visit is recorded with one primary diagnosis and up to 19 secondary diagnoses classified according to ICD-8 through 1993 and ICD-10 thereafter. Surgical and treatment information is coded according to a Danish classification (1977 through 1995) and a Danish version of the Nordic Medico-Statistical Committee (NOMESCO) Classification of Surgical Procedures (from 1996 on).
The National Health Service Prescription Database (NHSPD) includes information on reimbursed prescriptions redeemed at community and outpatient pharmacies since January 1, 2004.19 Each time a prescription is redeemed at a pharmacy, the date, the types, and quantity of the prescribed drug (according to the Anatomical Therapeutic Chemical Classification System [ATC]) is transmitted to the database.
The Danish Civil Registration System (CRS), established in 1968, contains information on gender, residence and date of death and emigration, with daily updates.16
Study cohort
The study included all adult patients (age ≥18 years) with a first-time diagnosis of MDS or MM in the DCR during the period July 1, 2004–December 31, 2013 (codes in Table S1). Non-Danish born patients were excluded due to possible incomplete data on comorbidities (see Figure 1). MDS was in this study categorized as “standard prognosis,” “poor prognosis,” and “other”, based solely on the ICD-10 diagnoses (codes in Table S1).
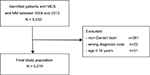  | Figure 1 Flow chart of study population. Abbreviations: MM, multiple myeloma, MDS, myelodysplastic syndrome. |
Exposure
ESA treatment was defined as a time-dependent exposure according to data in the DNPR (codes in Table S1). Start of ESA treatment was defined as the first date on which the specific ESA treatment code appeared (Table S1). Generally, MDS and MM patients would be provided with free-of-charge ESAs from the hospital departments that treat their diseases. However, in order to investigate if any patients were prescribed ESA treatment through community pharmacies, we also performed a cross-check with the NHSPD (codes in Table S1). The cross-check did not yield additional patients who were treated with ESAs. Patients and person-years were considered unexposed before starting ESA treatment and exposed from start of ESA treatment until end of follow-up.
Covariates
Sex and age were extracted from CRS. Diabetes mellitus, chronic kidney disease, and cardiovascular disease were identified from the DNPR. In order to increase the sensitivity of diagnoses of diabetes mellitus, and cardiovascular diseases, we also searched the NHSPD for any previous dispensing of diabetic medication and medication of cardiovascular diseases. Statin use was also obtained from the NHSPD. Antithrombotic therapy was also identified from the NHSPD and checked with a treatment code from the DNPR. Antithrombotic therapy was defined as vitamin K antagonists, platelet aggregation inhibitors, and others (ie, heparins, direct thrombin inhibitors, direct factor Xa inhibitors). VTE prophylaxes in MM patients was with platelet aggregation inhibitors during and shortly after treatment with immunomodulating therapy, or with low molecular weight heparin if there is a history of venous thrombosis or other risk factors. Data on chemotherapy, treatment with azacitidine, thalidomide or lenalidomide, any other immunomodulating or bone modulating therapy and radiation therapy were obtained from the DNPR (all codes and definitions are provided in Table S1). Pomalidomide was not in clinical use during the study period.
Follow-up and outcomes
The DNPR was also used to identify the outcomes of interest: VTE, MI, and stroke. Patients diagnosed with VTE, MI, or stroke only in the emergency room setting were excluded from the analysis, due to the expected low positive predictive value of the working diagnoses. For each analyzed event, patients who were diagnosed with that specific event before start of follow-up were excluded from the analyses. For the primary analysis, follow-up started on the MDS or MM diagnosis date. Patients were followed until the first date of the specific outcome, emigration, death, or end of follow-up on December 31, 2014, whichever came first. Mortality data were obtained from the CRS.16
Statistical analyses
Baseline characteristics were stratified for MDS and MM patients with and without ESA treatment. Age was expressed as median with interquartile range, and categorical data as percentages. Rates of VTE, MI, and stroke were calculated and expressed as the number of events per 1,000 person-years. Time-dependent Cox regression analysis was performed to compute crude and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs). Analyses were adjusted for sex, age, MDS risk group (only for analyses including MDS patients), diabetes mellitus, chronic kidney disease, cardiovascular disease, statin, and antithrombotic agent use at baseline. Additional adjustment (adjustment b) was made for time-updated concurrent treatments: chemotherapy, azacitidine, thalidomide or lenalidomide, immunomodulating therapy, and radiation therapy. In addition, we included use of Filgrastim and/or thrombopoietin analogs in regression models adjusting for time-dependent concurrent treatments (all codes and definitions are provided in Table S1).
Three sensitivity analyses were performed to check the robustness of our results. First, as ESA treatment is most often started shortly after diagnosis, and the duration of ESAs’ effect is under debate, we did a sensitivity analysis where follow-up was restricted to the first 2 years following MDS or MM diagnosis. The second sensitivity analysis was restricted to patients receiving chemotherapy, since this would create a more homogeneous group of patients. In this sensitivity analysis, follow-up was started on the date of chemotherapy initiation. Furthermore, in order to address a potential effect of patients in current anti-myeloma treatment we did a sensitivity analysis excluding patients without chemotherapy or immunomodulating treatment.
All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). The study was approved by the Danish Data Protection Agency (record number 2011-41-5755).
Results
Descriptive characteristics of the study population
In total, 2,114 patients with MDS and 3,105 patients with MM were identified. Baseline characteristics are shown in Table 1. Slightly more patients in both groups were male and median age was 76 years in MDS patients and 71 years in MM patients. ESA-treated patients were more likely to have a previous diagnosis of cardiovascular disease, diabetes mellitus, or chronic kidney disease. Antithrombotic agent use at baseline was similar in patients with and without ESA treatment. As expected, most MM patients were treated with chemotherapy during follow-up.
Incidence rates overall and in subjects without ESA treatment
Table 2 displays the number of events and incidence rates of VTE, MI, and stroke per 1,000 person-years. In MDS patients, a total of 54 VTEs, 52 MIs, and 101 strokes were observed in at least 5,036 person-years of follow-up, depending on the event of interest. For MM patients, the total number of person years was at least 8,602, and 195 VTEs, 92 MIs, and 93 strokes were observed. The incidence rate of VTE was 10.8 per 1,000 person-years in MDS patients without ESA treatment and twice as high in MM patients without ESA treatment, at 21.9 per 1,000 person-years. The incidence rate of stroke was higher in MDS patients than in MM patients, with 16.0 per 1,000 person-years and 9.9 per 1,000 person-years in MDS and MM patients without ESA treatment, respectively.
ESA-treated patients
As displayed in Table 2, crude incidence rates of MI and stroke were higher in ESA-treated patients compared with patients without ESA treatment. The crude and adjusted HRs for VTE, MI, and stroke during the total follow-up period are shown in Table 3. While the fully adjusted HR of VTE for ESA-treated MDS patients compared to MDS patients without ESA treatment was 1.04 (95% CI 0.57–1.89), ESA-treated MDS patients had a 1.6-fold increased risk of MI (HR 1.60 [95% CI 0.90–2.86]) and an almost twofold increased risk of stroke (HR 1.94 [95% CI 1.28–2.94]). Among MM patients, ESA treatment was associated with a higher risk of all cardiovascular events, although all CIs included equivalence. Fully adjusted HRs were 1.41 (95% CI 0.96–2.08) for VTE, 1.23 (95% CI 0.68–2.20) for MI, and 1.63 (95% CI 0.96–2.77) for stroke. Overall, additional adjustment for time-updated concurrent treatments (adjustment b) affected estimates only minimally. The results were only marginally different after inclusion of Filgrastim and/or thrombopoietin analogs in the fully adjusted regression models (data not shown).
Sensitivity analyses
When the analyses were restricted to 2 years of follow-up, results for MDS patients were in line with the main results (Table 4). In MM patients, the results were also similar. Although the association between ESA treatment and VTE and MI became somewhat more pronounced, CIs were wide. When we repeated the analyses in the cohort of MDS patients treated with chemotherapy (Table 5), the association of ESA treatment with MI and stroke attenuated. In MM patients, the results were in line with the main analysis. The HR for stroke in ESA-treated MM patients with chemotherapy was 1.68 (95% CI 0.95–2.98). Exclusion of patients without chemotherapy or immunomodulating treatment from the MM cohort resulted in only marginal changes in the crude and adjusted HRs (data not shown).
Discussion
Our population-based cohort study showed an elevated risk of cardiovascular events in ESA-treated MM patients. In MDS patients, ESA use was also associated with cardiovascular events, mainly with stroke and MI.
In patients with MM, the overall incidence rate of VTE was high, ie, 22.67 per 1,000 person-years, which is about tenfold higher than in the general population. Previous studies have reported a VTE frequency ranging from 2% up to 28% in MM patients during treatment with different regimens including thalidomide, in varying timeframes up to two years,11,20 suggesting that the risk seems to depend on concurrent treatments. Especially use of the immunomodulatory agents thalidomide, lenalidomide, and dexamethasone has been associated with higher VTE risk.21,22 ESA use has also been reported as a risk factor for VTE in MM patients taking immunomodulating drugs and in newly diagnosed MM patients, with a relative risk up to 3.4.12,23 Furthermore, in MM patients taking lenalidomide and dexamethasone, concomitant ESA therapy increased the incidence from 5% to 23%.11 However, an earlier study in MM patients taking thalidomide did not show a relation between the occurrence of thrombosis and ESA treatment,14 and a pooled analyses of newly diagnosed MM patients enrolled in clinical trials in Italy and the United States showed no effect of ESA on the VTE rate.24 Our results, taking concomitant anticoagulant and immunomodulating therapy into account, indicated a 1.4-fold increase in VTE in ESA-treated MM patients.
Risk of VTE in MDS patients does not seem so much higher than that in the general population, but the contribution of ESAs or other treatments has been investigated infrequently. Available studies in MDS patients found that ESAs did not increase the VTE rate.25,26 The reported VTE rate for MDS patients receiving lenalidomide in a postmarketing surveillance study was only 0.53%, but there were some signals of an association with ESA use.27 In line with our results, a later study found an odds ratio of 1.2 with ESA treatment and concluded that the safety profile of ESAs may be different in MDS patients than in patients with solid tumors.9 Still, an increased VTE rate has been reported in MDS patients receiving ESAs in combination with thalidomide.10
Little is known about the occurrence of MI and stroke in the two patient groups, especially in relation with ESA use. However, compared to an age-matched general population, MM patients have a 1.5 to 2-fold increased risk of arterial thrombosis.28 In our study, the incidence of MI in both MDS and MM without ESA treatment is in line with the upper limit of the incidence of the general population.29–31 The incidence of stroke in MM patients without ESA is also in line with the upper limit of the incidence in the general population, but in MDS patients without ESA the incidence of stroke is about two to three times higher compared with the general population.29,32 ESA treatment has been related to cardiovascular events in general oncology patients and patients with chronic kidney disease. A meta-analysis reported a 1.67 (95% CI 1.35–2.06) increased risk of thromboembolic events (a composite of VTE, transient ischemic attack, stroke, and MI) in oncology patients treated with ESAs.4 Only a minority of the studies in this meta-analysis included MM or MDS patients, and these were mostly performed before 2002. In patients with chronic kidney disease, ESA use is related to a 1.15-fold increased risk of serious cardiovascular events, with a 1.51 fold increased risk of stroke.6 Our results also indicate a higher risk of stroke and MI with ESA use in both MDS and MM patients.
A number of mechanisms have been proposed for the increase in cardiovascular events with ESA therapy.33 The higher blood viscosity induced by ESAs could increase the risk of thrombotic events, but this is mainly reported for above-normal hematocrit levels, such as in patients with polycythemia vera.34 ESAs also have been associated with vasoconstriction, may activate vascular endothelium, and could increase blood pressure.35 In addition, ESAs have been associated with pro-thrombotic changes, including increased platelet counts, lower levels of protein C and S, and increased thrombin generation.36,37 ESA treatment probably augments the already increased risk of cardiovascular events in the two populations, due to inherent thrombosis risks of MDS and MM and shared risk factors. For instance, smoking increases the risk of both MDS and arterial thrombosis.38 Furthermore, MM increases the risk of VTE in itself, through fractures and surgeries and the aforementioned immunomodulating therapy.39
Our study is notable for the large number of patients identified from population-based registries covering the complete Danish population. Also, the accuracy of a number of ICD-10 codes and quality of VTE diagnosis in the DNPR have been validated, and the positive predictive value was found to be very high.40,41 Furthermore, any misclassification would probably be non-differential and would lead to underestimation, since ICD-10 codes and diagnosis are unlikely to differ by ESA use. A concern is that the exact end date of ESA treatment and its effects are unknown. In our analyses, we assumed that patients were exposed from the start of ESA treatment until the end of follow-up. It is noteworthy that sensitivity analyses restricting follow-up to 2 years yielded similar results.
The validity of our findings also depends on proper adjustment for confounding. Our HRs were adjusted for an array of confounding factors, including comorbidities and concurrent therapies such as antithrombotic agents and immunomodulating drugs. However, as with all observational studies, residual confounding cannot be excluded.42 Unfortunately, we lacked detailed information on laboratory parameters, including Hb and platelet values. Since the decision for ESA treatment is based on Hb levels, symptoms of anemia, and transfusion dependency, therefore all ESA-treated patients are anemic, and the effects of Hb and ESA are always strongly associated. Since anemia is a risk factor for cardiovascular events or at least for MI, ESA-treated patients would have a higher risk of MI due to their anemia. This could lead to confounding by indication and an overestimation of the ESA effect. Also, because low-risk MDS patients are most often treated with ESAs in clinical practice, we also adjusted for type of MDS in our analyses. If we did assume that higher-risk MDS confers a higher risk of cardiovascular events, the HRs calculated in our study would likely underestimate the true ESA effect in the MDS patient group. On the other hand, other symptoms not resulting in a specific diagnosis – such as suspected angina pectoris – and estimated prognosis that influence treatment decisions could result in confounding by indication and overestimated HRs. Among MM patients, patients with bone marrow failure, renal failure, and elderly frail patients not appropriate for other treatment typically are treated with ESAs. These more fragile patients are generally at higher risk of any adverse event. If any residual confounding were present, our results were probably overestimated for this group.
Conclusion
We found that ESA use was associated with stroke in MDS patients. Among MM patients, ESA treatment was associated with a higher risk of all cardiovascular events, although all CIs included equivalence. Identification of patients who will benefit most from ESA treatment, may be even combined with antithrombotic agents to prevent cardiovascular events, is an important goal for future research.
Acknowledgments
The study was supported by the Aarhus University Research Foundation, the Danish Cancer Society, and the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation. MMS, TH, and FWD were supported by the FP7-Health European Commission EpoCan grant (282551). The European Commission was not involved in the collection, interpretation, and analysis of the data, nor in the decision for writing and submitting this report for publication.
Author contributions
EH-P, MMS, TH, SCC, FWD, and HTS contributed to the research design, LP was involved in acquisition of the data, and EH-P analyzed the data. EH-P, MMS, TH, HF, FWD, OMD, and HTS discussed the interpretation of the data. MMS and EH-P drafted the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflict of interest in this work.
References
Glaspy J. Current status of use of erythropoietic agents in cancer patients. Semin Thromb Hemost. 2014;40(3):306–312. | ||
Bohlius J, Schmidlin K, Brillant C, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373(9674):1532–1542. | ||
Bohlius J, Langensiepen S, Schwarzer G, et al. Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis. J Natl Cancer Inst. 2005;97(7):489–498. | ||
Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98(10):708–714. | ||
Glaspy J, Crawford J, Vansteenkiste J, et al. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer. 2010;102(2):301–315. | ||
Palmer SC, Navaneethan SD, Craig JC, et al. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010;153(1):23–33. | ||
Oster HS, Neumann D, Hoffman M, Mittelman M. Erythropoietin: the swinging pendulum. Leuk Res. 2012;36(8):939–944. | ||
Landolfi R, Di Gennaro L. Thrombosis in myeloproliferative and myelodysplastic syndromes. Hematology. 2012;17(Suppl 1):S174–S176. | ||
Smith SW, Sato M, Gore SD, et al. Erythropoiesis-stimulating agents are not associated with increased risk of thrombosis in patients with myelodysplastic syndromes. Haematologica. 2012;97(1):15–20. | ||
Steurer M, Sudmeier I, Stauder R, Gastl G. Thromboembolic events in patients with myelodysplastic syndrome receiving thalidomide in combination with darbepoietin-alpha. Br J Haematol. 2003;121(1):101–103. | ||
Knight R, Delap RJ, Zeldis JB. Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med. 2006;354(19):2079–2080. | ||
Leleu X, Rodon P, Hulin C, et al. MELISSE, a large multicentric observational study to determine risk factors of venous thromboembolism in patients with multiple myeloma treated with immunomodulatory drugs. Thromb Haemost. 2013;110(4):844–851. | ||
Bennett CL, Spiegel DM, MacDougall IC, et al. A review of safety, efficacy, and utilization of erythropoietin, darbepoetin, and peginesatide for patients with cancer or chronic kidney disease: a report from the Southern Network on Adverse Reactions (SONAR). Semin Thromb Hemost. 2012;38(8):783–796. | ||
Galli M, Elice F, Crippa C, Comotti B, Rodeghiero F, Barbui T. Recombinant human erythropoietin and the risk of thrombosis in patients receiving thalidomide for multiple myeloma. Haematologica. 2004;89(9):1141–1142. | ||
Frank L. Epidemiology. When an entire country is a cohort. Science. 2000;287(5462):2398–2399. | ||
Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. | ||
Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 Suppl):42–45. | ||
Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. | ||
Johannesdottir SA, Horváth-Puhó E, Ehrenstein V, Schmidt M, Pedersen L, Sørensen HT. Existing data sources for clinical epidemiology: The Danish National Database of Reimbursed Prescriptions. Clin Epidemiol. 2012;4:303–313. | ||
Zangari M, Elice F, Fink L, Tricot G. Thrombosis in multiple myeloma. Expert Rev Anticancer Ther. 2007;7(3):307–315. | ||
Falanga A, Marchetti M, Russo L. Venous thromboembolism in the hematologic malignancies. Curr Opin Oncol. 2012;24(6):702–710. | ||
Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37. | ||
Anaissie EJ, Coleman EA, Goodwin JA, et al. Prophylactic recombinant erythropoietin therapy and thalidomide are predictors of venous thromboembolism in patients with multiple myeloma: limited effectiveness of thromboprophylaxis. Cancer. 2012;118(2):549–557. | ||
Menon SP, Rajkumar SV, Lacy M, Falco P, Palumbo A. Thromboembolic events with lenalidomide-based therapy for multiple myeloma. Cancer. 2008;112(7):1522–1528. | ||
Rose EH, Abels RI, Nelson RA, McCullough DM, Lessin L. The use of r-HuEpo in the treatment of anaemia related to myelodysplasia (MDS). Br J Haematol. 1995;89(4):831–837. | ||
Terpos E, Mougiou A, Kouraklis A, et al. Prolonged administration of erythropoietin increases erythroid response rate in myelodysplastic syndromes: a phase II trial in 281 patients. Br J Haematol. 2002;118(1):174–180. | ||
Yang X, Brandenburg NA, Freeman J, et al. Venous thromboembolism in myelodysplastic syndrome patients receiving lenalidomide: results from postmarketing surveillance and data mining techniques. Clin Drug Investig. 2009;29(3):161–171. | ||
Kristinsson SY, Pfeiffer RM, Björkholm M, et al. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population-based study. Blood. 2010;115(24):4991–4998. | ||
Malki N, Koupil I, Eloranta S, et al. Temporal trends in incidence of myocardial infarction and ischemic stroke by socioeconomic position in Sweden 1987-2010. PLoS One. 2014;9(8):e105279. | ||
van Oeffelen AA, Agyemang C, Stronks K, Bots ML, Vaartjes I. Incidence of first acute myocardial infarction over time specific for age, sex, and country of birth. Neth J Med. 2014;72(1):20–27. | ||
Schmidt M, Jacobsen JB, Lash TL, Bøtker HE, Sørensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ. 2012;344:e356. | ||
Kissela BM, Khoury JC, Alwell K, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79(17):1781–1787. | ||
Smith KJ, Bleyer AJ, Little WC, Sane DC. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59(3):538–548. | ||
Kwaan HC, Wang J. Hyperviscosity in polycythemia vera and other red cell abnormalities. Semin Thromb Hemost. 2003;29(5):451–458. | ||
Lippi G, Franchini M, Favaloro EJ. Thrombotic complications of erythropoiesis-stimulating agents. Semin Thromb Hemost. 2010;36(5):537–549. | ||
Malyszko J, Malyszko JS, Borawski J, et al. A study of platelet functions, some hemostatic and fibrinolytic parameters in relation to serotonin in hemodialyzed patients under erythropoietin therapy. Thromb Res. 1995;77(2):133–143. | ||
Taylor JE, McLaren M, Henderson IS, Belch JJ, Stewart WK. Prothrombotic effect of erythropoietin in dialysis patients. Nephrol Dial Transplant. 1992;7(3):235–239. | ||
Björk J, Albin M, Mauritzson N, Strömberg U, Johansson B, Hagmar L. Smoking and myelodysplastic syndromes. Epidemiology. 2000;11(3):285–291. | ||
Cesarman-Maus G, Braggio E, Fonseca R. Thrombosis in multiple myeloma (MM). Hematology. 2012;17(Suppl 1):S177–S180. | ||
Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. | ||
Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6(11):e012832. | ||
Sørensen HT, Lash TL, Rothman KJ. Beyond randomized controlled trials: a critical comparison of trials with nonrandomized studies. Hepatology. 2006;44(5):1075–1082. |
Supplementary material
  | Table S1 ICD codes, treatment, and ATC codes used in the study. Abbreviations: ATC, anatomical therapeutic chemical classification system; ICD, International Classification of Diseases. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





