Back to Journals » OncoTargets and Therapy » Volume 11
ERCC1 rs3212986 A/C polymorphism is not associated with chemotherapy treatment outcomes in gastric cancer patients: evidence from 11 publications in Chinese populations
Authors Tang WW, Wang H, Wang YM, Wang XW
Received 3 August 2017
Accepted for publication 14 November 2017
Published 20 December 2017 Volume 2018:11 Pages 1—8
DOI https://doi.org/10.2147/OTT.S148214
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ingrid Espinoza
Weiwei Tang,1,* Hanjin Wang,1,* Yuemei Wang,2 Xiaowei Wang3
1Department of General Surgery, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 2Department of Operation Anesthesiology, Huai’an First People’s Hospital, Nanjing Medical University, Huai’an, Jiangsu, People’s Republic of China; 3Department of Medical Oncology, Huai’an First People’s Hospital, Nanjing Medical University, Huai’an, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Background: A number of studies have investigated the roles of excision repair cross-complementation group 1 (ERCC1) gene rs3212986 polymorphisms as potential biomarkers in gastric cancer (GC). However, the results were inconsistent. Here, we performed a meta-analysis to explore ERCC1 rs3212986 polymorphisms in the chemotherapy response and clinical outcome of GC.
Methods: PubMed, Embase, and Web of Science were searched up to July 28, 2017, for studies on the association between ERCC1 rs3212986 A/C polymorphisms and response to chemotherapy as well as overall survival time of GC. A fixed-effect or random-effect model was used to calculate the pooled odds ratios (ORs) based on the results from the heterogeneity tests.
Results: The result revealed that there was no significant association between the ERCC1 rs3212986 A/C polymorphism and response to chemotherapy in GC under comparison models (AA + CA versus CC, OR 0.95, P=0.80, AA versus CA, OR 0.85, P=0.55, AA versus CC, OR 0.74, P=0.47). Further identification suggested that ERCC1 rs3212986 A/C polymorphisms were not linked with the overall survival of GC (AA + CA versus CC, OR 1.09, P=0.52, AA versus CA, OR 1.05, P=0.85, AA versus CC, OR 1.43, P=0.23).
Conclusion: Our meta-analysis indicated that the ERCC1 rs3212986 A/C polymorphism was not associated with response to chemotherapy or overall survival time in GC. Well-designed studies with larger sample sizes and more ethnic groups should be performed to further validate our results.
Keywords: ERCC1, rs3212986, cancer, polymorphism, meta-analysis, survival, prognoses
Introduction
Gastric cancer (GC) is the most common digestive system tumor and still remains the main cause of human death in developing countries.1 GC can be divided into early GC and advanced GC according to the degree of malignancy and invasion depth. However, despite advanced diagnosis and treatment have been made in GC in recent years, the survival of GC still remains poor.2 Systemic chemotherapy is the primary treatment for advanced GC, but there exists a problem of chemotherapy resistance. As is known, tumor–node–metastasis (TNM) stage and patients’ age are the most important prognostic factors for GC.3 However, patients with similar TNM stage and patients’ age still show different prognoses in GC. Therefore, identification of genetic biomarkers could be helpful in designing individualized therapy, postoperational treatment, and follow-up strategies.
The DNA repair systems play an important role in repairing the damage to DNA induced by endogenous and/or exogenous factors such as therapeutic agents. Nucleotide excision repair (NER) is a key DNA repair mechanism that can influence gene–gene rearrangement, translocation, and amplification. Previous study reported that the alternation of NER capacity could play a pivotal role in the clinical outcomes of GC patients.4 The excision repair cross-complementation group 1 (ERCC1) enzyme is an essential factor involved in DNA damage repair. It has been reported that ERCC1 genetic variation can be a predictive marker for prognostic and response to chemotherapy for patients with non-small cell lung cancer (NSCLC), colorectal cancer, and osteosarcoma, and a series of meta-analyses have been performed.5–9 Yamada et al’s study showed that low ERCC1 expression was a significant independent favorable prognostic factor in patients with advanced GC who were receiving first-line chemotherapy regardless of the treatment regimen in JCOG9912. High ERCC1 expression confers cisplatin resistance and reconstitutes the cell’s ability to remove cisplatin from cellular DNA in an animal model.10
In recent years, a number of studies have investigated the roles of ERCC1 gene rs3212986 A/C polymorphisms in the development of GC; however, the results were inconsistent. Here, we conducted a meta-analysis to explore whether rs3212986 A/C polymorphisms in ERCC1 are predictor factors for the chemotherapy response, as well as the clinical outcome of patients with GC.
Methods
Data sources and searching
We searched the PubMed, Embase, and Web of Science for eligible studies assessing the association of ERCC1 polymorphisms and response to chemotherapy or overall survival time (last search updated to July 28, 2017). The search terms were “gastric cancer” in combination with “ERCC1” in combination with “rs3212986 polymorphism”. There was no restriction on time period, sample size, population, language, or type of report in order to minimize potential publication bias.
Inclusion and exclusion criteria
Studies included in this meta-analysis had to meet the following criteria: 1) case–control studies, 2) studies investigating the association between ERCC1 gene polymorphisms and response to chemotherapy or survival time, and 3) sufficient data available to calculate an odds ratio (OR) with 95% CI.
The exclusion criteria of the meta-analysis were as follows: 1) case–control studies not focusing on the correlation between ERCC1 rs3212986 polymorphisms and response to chemotherapy or survival time; 2) availability of insufficient original data for data extraction; and 3) meta-analyses, letters, reviews, and editorial articles. If more than one study was published by the same author using the same patient population, the study with the largest size of samples was included.
Data extraction
The data of eligible studies were extracted in duplicate by two investigators independently (WT and HW). The following information was recorded: name of first author, year of publication, ethnicity, number of cases and controls, chemotherapeutic drugs, outcomes, and genotype method. Ethnicity was simply categorized as Chinese. Discrepancies were resolved by consensus and by consulting the third author.
Quality assessment
The quality of the studies was modified from previous meta-analyses and independently assessed by two authors (Table 1).11,12 Quality scores ranged from 0 points (worst) to 13 points (best). Studies scoring <9 points were classified as low quality, and those scoring ≥9 points were classified as high quality.
  | Table 1 Scale for quality assessment |
Statistical analyses
Crude ORs with their corresponding 95% CIs were used to assess the strength of association between ERCC1 polymorphisms and response to chemotherapy or survival time. The Hardy–Weinberg equilibrium (HWE) in the control group was also assessed, and a P<0.05 was considered as significant disequilibrium. This meta-analysis was performed using the RevMan5.2 software. I2 statistic test was used to examine the heterogeneity. If I2>50%, it was considered with severe heterogeneity and the random-effects model would be applied, otherwise fixed-effects model was applied. The potential publication bias was assessed by using a “funnel plot” and Begg’s test. A P-value of <0.05 was considered as statistically significant.
Results
Study characteristics
A flowchart of the process of study selection is shown in Figure 1. Based on the inclusion and exclusion criteria, a total of 10 articles were included in the meta-analysis after full-text review. The main characteristics of included studies are presented in Table 2.13–22 The distribution of genotypes in the control groups of all studies was in agreement with HWE except three.18,20,21 A total of 10 studies were performed in Chinese populations. Most studies used the World Health Organization criteria (Miller et al23) as the assess criteria while only Bai’s study assessed using Response Evaluation Criteria in Solid Tumors (RECIST). In all studies, GC patients who showed complete response (CR) or partial response (PR) to chemotherapy were considered as response to chemotherapy, while patients who showed stable disease (SD) or progressive disease (PD) were considered as nonresponse to chemotherapy.
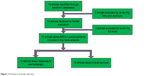  | Figure 1 Flowchart of study selection. |
Association between ERCC1 gene rs3212986 A/C polymorphisms and response to chemotherapy in GC
Great efforts have been made to identify the molecular predictive markers of chemotherapy sensitivity. In the overall analysis, we did not find any significant association between the ERCC1 rs3212986 A/C polymorphisms and response to chemotherapy in GC under three comparison models (Figure 2, AA + CA versus CC, OR 0.95, P=0.80; AA versus CA, OR 0.85, P=0.55; AA versus CC, OR 0.74, P=0.47).
Association between ERCC1 gene rs3212986 A/C polymorphism and overall survival of GC
In the overall analysis, we did not find any significant association between the ERCC1 rs3212986 polymorphisms and overall survival of GC in comparison models (Figure 3, AA + CA versus CC, OR 1.09, P=0.52; AA versus CA, OR 1.05, P=0.85; AA versus CC, OR 1.43, P=0.23).
Publication bias
Begg’s test was used to assess the publication bias (Figures 4 and 5). The heterogeneity was significantly observed in some comparison models, which might have resulted from differences in ethnicity, country, and genotype methods, so the random-effects model was used. For other polymorphism models, no significant publication bias was observed. This result showed that this meta-analysis was meaningful and the conclusion of this meta-analysis had high credibility.
Discussion
Patients with GC always show individualized response to platinum-based chemotherapy, which may result from hereditary factors by increasing the cell activity of biotransformation, the accumulation of intracellular, and the weakened capacity of DNA repairing.24
In recent years, a number of studies have investigated the roles of ERCC1 gene rs3212986 polymorphisms and serving as potential biomarkers for prognosis in GC. However, the results were inconsistent.
In this study, we described the meta-analysis findings of associations between ERCC1 gene rs3212986 and treatment outcomes of GC patients receiving chemotherapy. Our study identified that there was no significant association between the ERCC1 rs3212986 A/C polymorphism and response to chemotherapy in GC. This was inconsistent with the conclusion of some previous studies. Zheng et al13 have reported that the AA genotype of ERCC1 rs3212986 was associated with lower rates of complete remission and partial remission following chemotherapy in GC patients. Similarly, Bai et al16 discovered that patients carrying the GT and TT genotypes of rs3212986 showed a significantly poorer response to chemotherapy than did those carrying the GG genotype. In contrast to them, Zhong et al’s17 research showed no significant association between ERCC1 rs3212986 polymorphism and GC, which was consistent with our conclusions. Considering the reason, this may result from the complicated and multistep process and NER pathway factors might play an important role in functioning jointly to alter clinical outcome of GC. We further identified ERCC1 gene rs3212986 polymorphism and treatment outcomes of chemotherapy in GC. We did not find any significant association between the ERCC1 rs3212986 A/C polymorphisms and overall survival of GC. This was inconsistent with the results of abovementioned studies and the main reasons might be the differences in studies populations, study design, and sample size as well as by chance. To confirm or refute this result, well-designed studies with larger sample sizes and more ethnic groups are suggested performing to validate our conclusion.
Several limitations should not be ignored when interpreting the results. First, all eligible studies were from Chinese populations and our results were limited to this population. Therefore, more studies containing the full range of possible ethnic differences are needed to avoid the bias. Second, we had insufficient data to evaluate such interactions for the independent role of ERCC1 rs3212986 polymorphisms in GC in this study. Third, we did not perform subgroup analysis by sex, age, or different stages of cancer with limited data in primary studies. Last but not least, publication bias may have occurred due to only published studies included in this study. As a result, further biological and functional evidence is needed to confirm the genetic effects of ERCC1 rs3212986 A/C polymorphisms on GC.
Conclusion
This meta-analysis indicated that the ERCC1 rs3212986 A/C polymorphism was not associated with response to chemotherapy or overall time of GC in Chinese populations. This result should be interpreted cautiously. To confirm or refute this result, well-designed studies with larger sample sizes and more ethnic groups should be further performed to validate.
Acknowledgment
We are particularly thankful for the support from W Shi.
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. | ||
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. | ||
Costa RM, Chiganças V, Galhardo Rda S, Carvalho H, Menck CF. The eukaryotic nucleotide excision repair pathway. Biochimie. 2003;85(11):1083–1099. | ||
Vilmar A, Sørensen JB. Excision repair cross-complementation group 1 (ERCC1) in platinum-based treatment of non-small cell lung cancer with special emphasis on carboplatin: a review of current literature. Lung Cancer. 2009;64(2):131–139. | ||
Gan Y, Li XR, Chen DJ, Wu JH. Association between polymorphisms of XRCC1 Arg399Gln and XPD Lys751Gln genes and prognosis of colorectal cancer in a Chinese population. Asian Pac J Cancer Prev. 2012;13(11):5721–5724. | ||
Biason P, Hattinger CM, Innocenti F, et al. Nucleotide excision repair gene variants and association with survival in osteosarcoma patients treated with neoadjuvant chemotherapy. Pharmacogenomics J. 2012;12(6):476–483. | ||
Tan LM, Qiu CF, Zhu T, et al. Genetic polymorphisms and platinum-based chemotherapy treatment outcomes in patients with non-small cell lung cancer: a genetic epidemiology study based meta-analysis. Sci Rep. 2017;7(1):5593. | ||
Liu X, Zhang Z, Deng C, Tian Y, Ma X. Meta-analysis showing that ERCC1 polymorphism is predictive of osteosarcoma prognosis. Oncotarget. 2017;22;8(37):62769–62779. | ||
Yamada Y, Boku N, Nishina T, et al. Impact of excision repair cross-complementing gene 1 (ERCC1) on the outcomes of patients with advanced gastric cancer: correlative study in Japan Clinical Oncology Group Trial JCOG9912. Ann Oncol. 2013;24(10):2560–2565. | ||
Thakkinstian A, D’Este C, Eisman J, Nguyen T, Attia J. Meta-analysis of molecular association studies: vitamin D receptor gene polymorphisms and BMD as a case study. J Bone Miner Res. 2004;19(3):419–428. | ||
Qian T, Zhang B, Qian C, He Y, Li Y. Association between common polymorphisms in ERCC gene and glioma risk: a meta-analysis of 15 studies. Medicine. 2017;96(20):e6832. | ||
Zheng DL, Tang GD, Chen YN, Zhang T, Qin MB. Genetic variability of ERCC1 and ERCC2 genes involved in the nucleotide excision repair pathway influences the treatment outcome of gastric cancer. Genet Mol Res. 2016;15(2). | ||
Mo J, Luo M, Cui J, Zhou S. Prognostic value of ERCC1 and ERCC2 gene polymorphisms in patients with gastric cancer receiving platinum-based chemotherapy. Int J Clin Exp Pathol. 2015;8(11):15065–15071. | ||
Yu W, Wang YX, Guo JQ, Wang YL, Zheng JS, Zhu KX. Genetic variability of ERCC1 and ERCC2 influences treatment outcomes in gastric cancer. Genet Mol Res. 2015;14(4):17529–17535. | ||
Bai Y, Wang L, Li G, Fang X, Li Y, Yang S. Genetic variability of ERCC1 genes in NER pathway influences the treatment outcome of gastric cancer. Int J Clin Exp Pathol. 2015;8(10):13367–13373. | ||
Zhong G, Li HK, Shan T, Zhang N. Genetic variability of DNA repair mechanisms in chemotherapy treatment outcome of gastric cancer patients. Genet Mol Res. 2015;14(4):17228–17234. | ||
Ding C, Zhang H, Chen K, Zhao C, Gao J. Genetic variability of DNA repair mechanisms influences treatment outcome of gastric cancer. Oncol Lett. 2015;10(4):1997–2002. | ||
Xue MH, Li GY, Wu XJ, Zhang CX, Zhang CF, Zhu KX. Genetic variability of genes in NER pathway influences the treatment outcome of gastric cancer. Int J Clin Exp Pathol. 2015;8(5):5563–5569. | ||
Yu H, Wu X, Zhang Y, Jin Z, Li G, Zhao H. Genetic variability of DNA repair mechanisms influences chemotherapy outcome of gastric cancer. Int J Clin Exp Pathol. 2015;8(4):4106–4112. | ||
Liu L, Li CH, Jin TF, Xu DY. Study on the ERCC1 gene polymorphism response to chemotherapy and prognosis of gastric cancer. Genet Mol Res. 2014;13(4):8722–8728. | ||
Li J, Zuo X, Lv X, Kong F, Xu W, Yang S. Association of DNA repair gene polymorphisms with response to chemotherapy and prognosis of gastric cancer in a Chinese population. Tumor Biol. 2014;35(5):7569–7574. | ||
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. | ||
Chung TT, Pan MS, Kuo CL, et al. Impact of RECK gene polymorphisms and environmental factors on oral cancer susceptibility and clinicopathologic characteristics in Taiwan. Carcinogenesis. 2011;32(7):1063–1068. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





