Back to Journals » Clinical Ophthalmology » Volume 15
Epithelium-on Corneal Collagen Cross-Linking with Hypotonic Riboflavin Solution in Progressive Keratoconus
Authors Beckman KA
Received 7 May 2021
Accepted for publication 9 June 2021
Published 7 July 2021 Volume 2021:15 Pages 2921—2932
DOI https://doi.org/10.2147/OPTH.S318317
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kenneth A Beckman1,2
1Comprehensive EyeCare of Central Ohio, Westerville, OH, USA; 2The Ohio State University, Columbus, OH, USA
Correspondence: Kenneth A Beckman
Comprehensive EyeCare of Central Ohio, 450 Alkyre Run Dr #100, Westerville, OH, 43082, USA
Tel +1 614 890-5692
Email [email protected]
Introduction: Epithelium-off cross-linking (epi-off CXL) has long been established as the gold standard treatment for progressive keratoconus. Several protocols for epithelium-on (epi-on) CXL have been proposed to help reduce post-operative pain and facilitate visual recovery, but there is no epi-on treatment approach that is currently approved in the United States. The hydrophilic and macromolecular characteristics of conventional epi-off riboflavin formulations may create clinical challenges for absorption through an intact epithelium. This study investigates the clinical efficacy of a dextran-free hypotonic riboflavin ophthalmic solution (Photrexa, Glaukos, Burlington, MA, USA), approved for epi-off CXL, in a novel epi-on CXL protocol.
Methods: Twenty-five eyes of 17 patients were treated in this prospective, single-arm study using a hypotonic riboflavin formulation without dextran and low irradiance UVA (3mW/cm2) for epi-on CXL. Visual acuity, as well as refractive and keratometry outcomes, were observed over 12 months.
Results: At 12 months, Kmax was stable with no clinically or statistically significant change from a mean pre-op of 55.4D to 55.9D (p=0.13). Uncorrected and best corrected logMAR visual acuity significantly improved from 0.77 to 0.62 and from 0.17 to 0.12, respectively. There were no significant adverse safety events.
Conclusion: Patients who underwent epi-on CXL with dextran-free hypotonic riboflavin demonstrated improvements in uncorrected and best corrected visual acuity with stable keratometry at 12 months post-operatively. The efficacy is consistent with other epi-on studies to date but remains lower than standard epi-off CXL. New technologies, including supplemental oxygen and transepithelial riboflavin ophthalmic solutions, are currently under clinical evaluation and may offer a path forward for epi-on CXL in the USA.
Keywords: epithelium-on, cross-linking, CXL, dextran-free, photrexa
Introduction
Keratoconus is a progressive asymmetric corneal ectatic disorder characterized by central or paracentral corneal thinning and irregular astigmatism.1 Estimates of keratoconus prevalence vary depending on geographic location, patient ethnicity, and diagnostic criteria but frequently range from 50–600 per 100,000, making the condition the most common type of corneal ectasia.2,3
Historically, treatment for keratoconus has focused on visual rehabilitation by prescribing specialty contact lenses and, for more severe cases, corneal grafting once a patient reaches contact intolerance or reports unacceptable vision.4 Intracorneal ring segments have also been utilized in attempts to improve visual function and tolerance to contact lenses in keratoconus patients; however, they do not prevent further progression of the disorder.5
In 2003, Wollensak et al described the use of riboflavin/ultraviolet-A collagen crosslinking (CXL) in 22 keratoconus patients and demonstrated halted progression in all patients included in this pilot study.6 This CXL procedure involves the removal of the corneal epithelium prior to riboflavin delivery, which is thought to aid stromal penetration and increase the overall efficiency of the photo-oxidative reactions produced by CXL. Also referred to as epithelium-off (epi-off) CXL, it has been widely adopted as the standard CXL treatment protocol due to its efficacy at stopping, or slowing, keratoconus progression.7,8 In 2016, the United States Food and Drug Administration (FDA) approved Photrexa Viscous (riboflavin 5ʹ-phosphate in 20% dextran ophthalmic solution) 0.146% and Photrexa (riboflavin 5ʹ-phosphate ophthalmic solution) 0.146% (Glaukos, Burlington, MA, USA) for use with the KXL System (Glaukos) in the treatment of progressive keratoconus and corneal ectasia following refractive surgery on the basis of three prospective, as well as randomized, controlled, clinical trials of conventional epi-off cross-linking.9–11
While epi-off conventional CXL has been proven to be safe and effective, there are several potential disadvantages, such as post-operative pain and delayed visual recovery, which are primarily attributed to the epithelial debridement.12 To address these limitations, there is significant interest in performing CXL without epithelial removal, a procedure also known as epithelium-on or epi-on CXL.
There have been numerous independent studies focusing on epi-on CXL using a variety of transepithelial riboflavin formulations specifically designed to improve permeability of the epithelium, either through the addition of permeability enhancers such as benzalkonium chloride (BAC) and/or ethylenediaminetetraacetic acid (EDTA),13 the addition of vitamin E,14 or the use of a sterile sponge to improve epithelial permeability.15 A recent systematic review and meta-analysis demonstrated that, while the studies of various epithelium-on techniques demonstrated reduced rates of postoperative complications, they were also associated with an increased rate of post-operative disease progression.16
While several transepithelial riboflavin formulations are available outside of the US, to date, there is no US FDA approved riboflavin formulation indicated for use in epi-on CXL.17 A proposed alternative to these transepithelial riboflavin formulations is the application of a standard riboflavin solution in combination with a topical anesthetic (proparacaine with 0.01% benzalkonium chloride) which is designed to facilitate epithelial permeability.18 Additionally, it has been suggested that the use of hypotonic, dextran-free, formulations may facilitate penetration through the epithelium.18 A single-center trial of dextran-free, hypotonic riboflavin, applied via a pledget sponge following pre-operative application of topical proparacaine, showed a trend towards improved Kmax,19 albeit with a lesser degree of flattening than is typically observed with traditional epi-off protocols.
The aim of this study is to evaluate the outcomes of a modified epithelium-on CXL procedure performed with topical proparacaine, a hypotonic 0.146% riboflavin formulation currently available in the United States (Photrexa, Glaukos), and conventional 3 mW/cm2 UVA irradiance delivered with the KXL System.
Patient Selection and Methods
Patients
This prospective single center study (NCT03245853) included 25 eyes of 17 patients with keratoconus. One patient (26th eye) was initially included but received an incorrect formulation of riboflavin during the operation and was excluded from the study after consideration by the institutional review board. All patients provided written informed consent and this study was ethically approved and overseen by an institutional review board (Western Institutional Review Board) which abided by FDA regulations. All procedures were in accordance with the 1964 Helsinki declaration and its later amendments and a parent or legal guardian provided informed consent for any participant under 18 years of age.
Patient inclusion criteria included: an age range of between 14 and 40 years of age, a diagnosis of keratoconus confirmed with Scheimpflug tomography (Pentacam, Oculus, Germany), a minimum pre-debridement corneal pachymetry of 350 microns, and an ability to attend future follow-up visits. Exclusion criteria included a past ocular history of other corneal diseases (eg, herpes simplex, herpes zoster keratitis, recurrent erosion syndrome, corneal melt, and corneal dystrophy) or any clinically significant corneal scarring in the CXL treatment zone. Patients with nystagmus or any other condition that would prevent a steady fixation during CXL treatment or other clinical measurements, as well as those who were pregnant or lactating, were also excluded from the current study.
Pre-Operative Examination
Pre-operative examination took place up to 45 days before the day of the treatment. During this visit, a complete history including ocular, medical, and medication history was obtained. Ocular examination included corneal tomography (Pentacam, Oculus), monocular uncorrected (UCVA) and best corrected visual acuity (BCVA), and manifest refraction. A comprehensive ophthalmological examination, including a dilated retinal exam, was also performed.
Contact-lens wearers were instructed to discontinue the use of contact lenses for at least 1 week prior to the pre-operative examination. Additionally, contact lens wearers were required to have a stable refraction, defined as a maximum difference of 0.75D between two manifest refraction (MSRE) measurements taken at least 7 days apart. If the difference was greater than 0.75D then patients were either excluded from the study or underwent additional screening visits until a stable refraction (<0.75D difference in MSRE) was obtained.
Treatment Protocol
Topical anesthetic, proparacaine with BAC 0.01%, was instilled three times at intervals of 20 seconds. The corneal surface was prepared with a drop of proparacaine, followed by the placement of a lid speculum, before further proparacaine was administered. The epithelium was wiped with approximately 3 to 4 vertical and horizontal strokes of Weck-Cel Cellulose Eye Spears (Beaver-Visitec International, Waltham, MA, USA).
In this study, we used a hypotonic riboflavin solution, Photrexa (hypotonic riboflavin 5ʹ-phosphate 0.146% ophthalmic solution), approved by the US FDA for use in epi-off CXL. It contains an osmolality of 157–177mOsm/Kg compared with the osmolality of human tears which has been estimated at 303.7mOsm/Kg.20 Riboflavin was administered at a rate of 1–2 drops every 2 minutes for 30 minutes with additional intervening topical anesthetic every 2 minutes for 10 minutes or longer if required. Homogenous riboflavin saturation throughout the entire corneal depth was confirmed at slit lamp before proceeding with recording of corneal pachymetry. If saturation had not been achieved after 30 minutes, riboflavin application was continued until adequate saturation was achieved.
When riboflavin uptake into the corneal stroma had been confirmed, ultrasound pachymetry was then used to measure tissue thickness at the thinnest corneal point as approximated by the surgeon.
The eye was then aligned under ultraviolet light (KXL System, Glaukos), which emits continuous UVA radiation at a wavelength of 365 nm at an intensity of 3 mW/cm2. The light has a fixed aperture to produce a circular irradiation pattern with a diameter of 9.5 mm. It also emits alignment laser marks that can be used to aid the physician in properly optimizing the UVA beam profile on the corneal plane. The wireless remote is also used to further provide fine adjustment and maintain optimal positioning of the UVA energy beam, both before and during the irradiation phase of the procedure.
Once UVA light alignment was achieved, the UVA irradiation was initiated while continuing the administration of Photrexa riboflavin at a rate of 1 drop every 2 minutes and topical anesthetic every 5 minutes. After 30 minutes of UVA treatment (5.4 J/cm2), the light source was programmed to automatically switch off. All procedure details, including topical anesthetic, riboflavin administration, irradiance settings, and duration of irradiation exposure, were recorded.
All patients were administered Gatifloxacin 0.3% 4 times per day for one week and Prednisolone Acetate 1% 4 times per day in the first week, 3 times per day in the second week, twice per day in the third week, and once per day in the final week. Patients were also given Bromfenac 0.07% once daily as needed for post-operative pain for up to 3 to 4 days. All patients were fitted with a bandage contact lens which was removed at day 1. If patients were still in discomfort, the bandage contact lens would have been replaced and removed at day 7. This was not required for any patients.
Post-Operative Follow-Up
Patients were followed up post-operatively at day 1, week 1, as well as at months 3, 6, 9, and 12. At each visit, UCVA and BCVA were measured and anterior segment examination was performed. Moreover, Pentacam measurements and any changes in patient history or medication were documented. All adverse events were recorded.
Outcomes and Statistical Methods
The primary efficacy outcome was defined as a change in maximum keratometry (Kmax in diopters) from baseline over time. Secondary efficacy outcomes were UDVA and CDVA. Safety was assessed by monitoring the occurrence of adverse events, such as the loss of 2 or more lines in BCVA or an increase in Kmax greater than 2D, and slit-lamp examination.
Outcome measures were assessed using descriptive statistics at months 1, 3, 6, and 12. A one-group t-test comparing the mean change in Kmax from the baseline value to the target value of 0.75 D was performed. Mean change in visual acuity and refractive data were analyzed using ANOVA with Tukey’s correction for the comparison of multiple means. Graphic data is shown using error bars which represent the 95% confidence interval. Intention to treat analysis was intended to account for any patients who withdrew after commencing the study, but was ultimately not required.
Results
Patients and Demographics
In total, 25 eyes of 17 patients (15 males, 2 females) were recruited in this study. Patients had a mean age of 30 years old. Mean pre-operative corneal thickness was 478 ± 46.3 µm. One patient was not able to attend the follow-up visit at the 3-month time point; UCVA was not available at baseline for one patient; all other patients attended all post-operative examinations.
Analysis of Kmax (maximum keratometry) showed no statistically significant difference between the pre-operative visit (55.4 ± 7.3 D) and 12 months postoperatively (55.9 ± 7.8 D) (p=0.13) (Table 1, Figure 1). Figure 2 shows the mean change in Kmax; the positive value indicates a small steepening on average across eyes. As expected, the maximum steepening was at 1 month, with a small improvement (flattening) at 3, 6, and 12 months. Considering individual eyes at 12 months, there was a change of less than, or equal to, 1.00D in 16 eyes (64%), a flattening between 1.01D and 2D in 4 eyes (16%), and a steepening between 1.01D and 2D in 4 eyes (16%) (Figure 3). The 25th eye (4%) showed an increase in Kmax of more than 2D (7D), but the Pentacam flagged its baseline measurement for poor data quality; nevertheless, it was included in the statistical analysis.
 |
Table 1 Summary Statistics of Keratometry and Refractive Results Obtained at Post-Operative Follow-Up Visits (95% Confidence of Interval) |
 |
Figure 1 Kmax value (in diopters) taken from Pentacam readings at the pre- as well as 1-month, 3-months, 6, and 12-months post-operative visits. Error bars represent 95% confidence intervals. |
 |
Figure 2 Mean change in Kmax in dioptres at each post-operative visit compared to baseline. |
 |
Figure 3 Kmax distribution change in dioptres, from baseline to 12 months. |
There was no statistically significant change in other keratometric parameters: K1 (D), K2 (D) Kmean (D), and keratometric astigmatism defined as the difference between K1 and K2 in diopters.
There were no statistically significant differences in refractive outcomes for sphere and for cylinder, as well as for minimum corneal thickness (Table 1).
Visual acuity was measured at each visit in Snellen and then converted in logMAR for statistical analysis. A summary of these statistics is provided in Table 2. There was a significant improvement in UCVA between the pre-operative visit (0.77 logMAR) and the 1, 3, and 12-month time points (0.62 logMAR at 12-months) (p<0.001) (Figure 4). BCVA showed a similar significant improvement between pre-operative and 3- and 12-month visits (Figures 5 and 6), decreasing from 0.17 logMAR preoperatively to 0.12 logMAR at 12 months (p=0.0012).
 |
Table 2 Summary Statistics of UCVA and BCVA Data Obtained at Post-Operative Follow-Up Visits (95% Confidence of Interval) |
 |
Figure 4 Mean Uncorrected Distance Visual Acuity (UCVA) in LogMAR at baseline as well as 1-month, 3-months, 6, and 12-months post-operative visits. Error bars represent 95% confidence intervals. |
 |
Figure 5 Mean Best-Corrected Distance Visual Acuity (BCVA) measured at baseline as well as 1-month, 3-month, 6, and 12-months post-operative visits. Error bars represent 95% confidence intervals. |
 |
Figure 6 Mean change in BCVA and UCVA at post-operative visits. |
Looking at individual eyes at 12 months, there was no change in UCVA in 8 eyes (33%), a gain of 2 lines in 5 eyes (21%), a gain of 3 lines in 3 eyes (13%), a gain of 4 lines in 2 eyes (8%), a gain of 5 lines in 1 eye (4%), a gain of 6 lines in 2 eyes (8%), and a loss of 1 line in 3 eyes (13%). The 25th eye had no preoperative UCVA measurement and was not included in the percentage calculation. For BCVA, all eyes except one showed no change or a gain; there was no change in 13 eyes (52%), a gain of 1 line in 8 eyes (32%), a gain of 2 lines in 2 eyes (8%), a gain of 4 lines in 1 eye (4%), and a loss of 1 line in 1 eye (4%) (Figure 7). None of the eyes with steepening showed any associated loss of vision except one and this one eye with a minor steepening of 0.30D presented a 0.12 logMAR loss of UCVA.
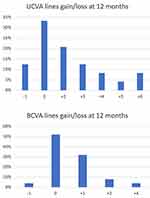 |
Figure 7 Visual acuity distribution gain or loss of lines, from baseline to 12 months. |
Safety
Safety outcome data was taken from the pooled data set of all patient visits. It was assessed by monitoring for any epithelial defect, corneal infection, use of bandage contact lens after 1 week, and any other ocular side effects.
At day one, 5 eyes showed epithelial defects that resolved spontaneously within the first week with no sequelae. No superficial punctate keratitis or photophobia was observed. Two eyes developed corneal scars that were still present at the 12-month timepoint and these were considered as minor adverse events since they were not associated with loss of vision.
No eye presented a reduction of 2 lines or more of UCVA or BCVA. One eye showed an increase in Kmax more than 2 diopters but with corresponding gains in both UCVA and BCVA at 12 months.
Discussion
Standard epi-off CXL for keratoconus involves debridement of the corneal epithelium to facilitate absorption of riboflavin into the corneal stroma.21 While proven to be safe and effective, removal of the epithelium is associated with several potential disadvantages such as postoperative pain, delayed visual recovery and, rarely, infectious keratitis.22 Epi-on CXL, provided that sufficient clinical efficacy can be obtained, presents several potential advantages for patient recovery and the surgical workflow. Currently, there are no FDA-approved riboflavin formulations or UVA delivery devices indicated for epi-on CXL in the United States.17 Our study aimed to evaluate the use of the currently available epi-off CXL products for use in a modified epi-on procedure.
Hypo-osmolar riboflavin solutions with low salt content have been proposed to improve stromal riboflavin absorption through the corneal epithelium. The multi-layered epithelium with tight junctions serves as an effective barrier to inhibit the entry of any relatively large-sized and/or hydrophilic molecules. Due to the relatively large molar mass of Riboflavin (376.36 g/mol), as well as its hydrophilic characteristics, the paracellular epithelial permeability is understandably limited for riboflavin compounds. On the other hand, a hypotonic riboflavin solution can set up an osmotic gradient that favors the influx of water towards the stroma and, subsequently, this fluid dynamic can lower resistance of tight junctions and enhance an inward conductance of external particulates. The migration of salt ions from the apex to the corneal periphery will also compliment this directional flow of extracellular fluid and other molecules. The presence of dextran, however, will function to offset such osmotic gradient and redirect fluid dynamic to draw water contents out of the stroma.23 Thus, of the two riboflavin formulations that are indicated for epi-off CXL in the United States, we selected the dextran-free hypotonic solution (Photrexa) to evaluate this novel epi-on CXL.
While the osmotic gradient derived from hypotonic riboflavin solution may improve diffusivity of riboflavin, Raiskup et al demonstrated higher transepithelial riboflavin permeability with added benzalkonium chloride (BAC) when comparing two equal concentrations of hypo-osmolar riboflavin solution. It has been suggested that BAC acts as an epithelial permeabilization agent by disrupting epithelial tight junctions;13,24 and, therefore, the presence of BAC may complement hypotonic riboflavin solution in elevating the transportation of riboflavin across the epithelial barrier. Outside the US, there are commercially available riboflavin formulations that incorporate permeability enhancers, such as ParaCel (Riboflavin 0.25%, BAC, EDTA, Trometamol, HPMC).25 Since there is no FDA-approved riboflavin ophthalmic solution in the US that contains BAC, prior to instillation of the riboflavin formulation in the current study, we first applied proparacaine hydrochloride 0.5% ophthalmic solution with 0.01% BAC, followed by preparation of the corneal surface with Weck-Cel cellulose eye spears. Both modifications aimed to partially disrupt the corneal epithelium and further improve epithelial permeability to riboflavin. After confirming riboflavin absorption in the slit lamp, UVA was then applied according to the conventional protocol.
In our prospective study, 25 eyes of 17 patients were treated using this modified protocol. Prior to initiating UVA, we confirmed riboflavin loading in the corneal stroma through the slit lamp. At 12 months, we observed no statistically significant change in keratometry or refraction from baseline, but a significant improvement in visual acuity. Kmax remained stable15 within 1D of baseline in 64% of eyes, a flattening of Kmax greater than 1D occurred in 16% of the eyes, and a steepening greater than 1D and less than 2D in 16% of eyes. One eye (4%) showed a more acute increase in Kmax (7D), but this may have been due to a borderline pre-operative measurement flagged by the Pentacam, as also suggested by the patient’s gain of 6 lines of UCVA from baseline to 12-month postoperative. Only minor adverse events were observed and none were visually significant.
Interestingly, none of the eyes with steepening showed any associated loss of vision except one, excluding a correlation in this study between loss of vision and an increase in Kmax. In fact, the large majority of eyes demonstrated improved visual acuity. We observed a statistically significant improvement in 12-month UCVA (p<0.001) and BCVA (p=0.0012) compared with baseline vision, with a gain of one and half lines of mean UCVA and half a line of mean BCVA. Overall, UCVA improved or remained stable in 88% of eyes and BCVA improved, or remained stable, in 96% of eyes.
This improvement in visual acuity observed in our study cohort was consistent with the findings of a previous US multicenter study of epi-on CXL by Stulting et al, where the authors investigated epi-on CXL on 512 eyes utilizing a proprietary dextran-free transepithelial riboflavin formulation.15 Two years after the treatment, their study showed a Kmax decrease of 0.48D as well as a significant improvement in UCVA and BCVA of 1 and 1.5 Snellen lines, respectively (n=133 at 24 months). The authors defined success for epi-on CXL based on the lack of post-treatment progression, where progression of keratoconus after treatment was defined as an increase in Kmax of more than 1D associated with a loss of more than 1 line of BCVA in the same eye.15 Both the present study and the study by Stulting et al met this definition of success. However, neither study demonstrated a clinically significant (≥1 D) mean flattening with respect to baseline and a common limitation of both our single center study and the multicenter study of Stulting et al is the absence of a control group. Therefore, the results of these two epi-on CXL studies cannot be evaluated against the gold standard for treatment success as established by the pivotal trials for epi-off CXL performed in the United States, namely a ≥1 D difference in change from baseline between treated and untreated control eyes.9,10
Although the outcomes of the current study seem promising, studies to date suggest that while epi-on CXL may reduce progression in keratoconus, fewer patients show keratometric stabilization and flattening than reported with standard epi-off CXL.8 In a recent meta-analysis of randomized control trials comparing overall epi-on and ep-off CXL results, Nath et al found that current epi-on CXL protocols generally demonstrate lower efficacy in manifesting post-operative topographic improvements and the clinical trend of such keratometric differences between epi-on and epi-off appear to increase over time.16 Despite this observed discrepancy in topographical metrics between standard epi-off CXL and the current epi-on CXL protocols, the same systematic review by Nath et al also confirmed that epi-on CXL, to date, produced UCVA and BCVA outcomes comparable to those obtained with epi-off CXL and also offered potential advantages in terms of providing better patient comfort, quicker post-operative recovery, and reduced incidences of adverse events.16
A major limitation of current approaches to epi-on CXL, including the present study, may stem from the impedance of the epithelial barrier on more than just riboflavin diffusion, but also on the transmission of UVA and oxygen, two other key components of cross-linking photochemistry.26–28 In addition to optimized riboflavin formulations, advanced epi-on treatment protocols may include pulsed, accelerated, UVA irradiation protocols to reduce the rate of oxygen consumption29,30 and, most recently, the addition of supplemental oxygen at the corneal surface to increase the rate of oxygen diffusion to the corneal stroma in an attempt to improve the photochemical efficiency of the procedure.
A promising technical modification to increase stromal oxygen availability applies supplemental oxygen, at high concentrations, over the surface of the eye (>90%) throughout the procedure in combination with the use of specially designed oxygen goggles, transepithelial riboflavin ophthalmic solution, and pulsed UVA. Ex vivo studies have demonstrated that a highly oxygenated environment increases the rate of stromal oxygen diffusion and helps to maintain a steady state of oxygen bioavailability despite its constant consumption during CXL.31 A recently completed US Phase III multicenter clinical trial incorporated these procedural advancements in a protocol for epi-on, pulsed, accelerated, oxygen-supplemented CXL (NCT03442751).32 While final study data has not been published from this phase III multicenter trial, the results obtained from a 12-month-long single center French study utilizing this novel approach (N=34) showed that the Kmax decreased by 1.56 D,31 with no significant adverse events. Other studies also point to oxygen as a crucial factor in maximizing epi-on CXL outcomes in patients with progressive keratoconus33 as well as refractive applications.34,35
We have identified two potential limitations of this study. Firstly, the follow-up period was limited to 12 months and a study which also investigates the long-term safety profile and effectiveness is required. Secondly, the study was designed to include both eyes of the same patient, if deemed appropriate under the disclosed patient selection criteria. As such, the efficacy of the treatment on one eye may have a correlation with the efficacy on the other eye and thus has an impact on the statistical outcome.
Conclusion
In our study, progressive keratoconus patients who received epi-on CXL using a standard hypotonic riboflavin ophthalmic solution demonstrated mean stability in Kmax at 12 months and a small, but statistically significant, improvement in both best corrected and uncorrected visual acuity. Adverse events were minor and not visually significant. While the results of this current study produced outcomes similar to other epi-on CXL studies, such as stable keratometry and improved UCVA and BCVA, it did not produce the keratometric flattening typically expected from epi-off CXL treatments. Given the absence of change in keratometry and the small sample size, a larger, controlled, clinical study is necessary to confirm these findings. However, a new epi-on drug and device combination with supplemental oxygen that is currently under clinical evaluation may offer promise for the future of epi-on CXL in the United States.
Data Sharing Statement
The author will not share individual deidentified participant data.
Disclosure
The author is a clinical investigator for Glaukos and has received funding for this study.
References
1. Mas Tur V, MacGregor C, Jayaswal R, O’Brart D, Maycock N. A review of keratoconus: diagnosis, pathophysiology, and genetics. Surv Ophthalmol. 2017;62(6):770–783. doi:10.1016/j.survophthal.2017.06.009
2. Hofstetter HW. A keratoscopic survey of 13,395 eyes. OptometryVision Sci. 1959;36(1):3–11. doi:10.1097/00006324-195901000-00002
3. Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267–273. doi:10.1016/0002-9394(86)90817-2
4. Jamali H, Gholampour AR. Indications and surgical techniques for corneal transplantation at a tertiary referral center. J Ophthalmic Vis Res. 2019;14(2):125–130. doi:10.4103/jovr.jovr_92_18
5. Sakellaris D, Balidis M, Gorou O, et al. Intracorneal ring segment implantation in the management of keratoconus: an evidence-based approach. Ophthalmol Ther. 2019;8:5–14. doi:10.1007/s40123-019-00211-2
6. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi:10.1016/S0002-9394(02)02220-1
7. Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol. 2006;17(4):356–360. doi:10.1097/01.icu.0000233954.86723.25
8. Soeters N, Wisse RPL, Godefrooij DA, Imhof SM, Tahzib NG. Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: a randomized controlled trial. Am J Ophthalmol. 2015;159(5):821–828.e3. doi:10.1016/j.ajo.2015.02.005
9. Hersh PS, Stulting RD, Muller D, et al. U.S. multicenter clinical trial of corneal collagen crosslinking for treatment of corneal ectasia after refractive surgery. Ophthalmology. 2017;124(10):1475–1484. doi:10.1016/j.ophtha.2017.05.036
10. Hersh PS, Stulting RD, Muller D, et al. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology. 2017;124(9):1259–1270. doi:10.1016/j.ophtha.2017.03.052
11. Khaimi MA, Dvorak JD, Ding K. An analysis of 3-year outcomes following canaloplasty for the treatment of open-angle glaucoma. J Ophthalmol. 2017;2017. doi:10.1155/2017/2904272.
12. Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35(8):1358–1362. doi:10.1016/j.jcrs.2009.03.035
13. Alhamad TA, Dps O, Nal O, Meek KM. Evaluation of transepithelial stromal riboflavin absorption with enhanced riboflavin solution using spectrophotometry. J Cataract Refract Surg. 2012;38(5):884–889. doi:10.1016/j.jcrs.2011.11.049
14. Caruso C, Ostacolo C, Epstein RL, Barbaro G, Troisi S, Capobianco D. Transepithelial corneal cross-linking with Vitamin E-enhanced riboflavin solution and abbreviated, low-dose UV-A: 24-month clinical outcomes. Cornea. 2016;35(2):145–150. doi:10.1097/ICO.0000000000000699
15. Stulting RD, Trattler WB, Woolfson JM, Rubinfeld RS. Corneal crosslinking without epithelial removal. J Cataract Refract Surg. 2018;44(11):1363–1370. doi:10.1016/j.jcrs.2018.07.029
16. Nath S, Shen C, Koziarz A, et al. Transepithelial versus epithelium-off corneal collagen cross-linking for corneal ectasia: a systematic review and meta-analysis. Ophthalmology. 2021. doi:10.1016/j.ophtha.2020.12.023
17. Belin MW, Lim L, Rajpal RK, Hafezi F, Gomes JAP, Cochener B. Corneal cross-linking: current USA status report from the cornea society. Cornea. 2018;37(10):1218–1225. doi:10.1097/ICO.0000000000001707
18. Koppen C, Wouters K, Mathysen D, Rozema J, Tassignon MJ. Refractive and topographic results of benzalkonium chloride-assisted transepithelial crosslinking. J Cataract Refract Surg. 2012;38(6):1000–1005. doi:10.1016/j.jcrs.2012.01.024
19. Hersh PS, Lai MJ, Gelles JD, Lesniak SP. Transepithelial corneal crosslinking for keratoconus. J Cataract Refract Surg. 2018;44(3):313–322. doi:10.1016/j.jcrs.2017.12.022
20. Craig JP, Simmons PA, Patel S, Tomlinson A. Refractive index and osmolality of human tears. OptometryVision Sci. 1995;72(10):718–724. doi:10.1097/00006324-199510000-00004
21. Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34(5):796–801. doi:10.1016/j.jcrs.2007.12.039
22. Boxer Wachler BS, Pinelli R, Ertan A, Chan CCK. Safety and efficacy of transepithelial crosslinking (C3-R/CXL). J Cataract Refract Surg. 2010;36(1):186–188. doi:10.1016/j.jcrs.2009.08.019
23. Greenstein S, Fry KL, Hersh PS, et al. Corneal thickness effects using riboflavin/dextran versus hypotonic riboflavin during corneal collagen crosslinking. J Cataract Refract Surg. 2011;2011(1):149–160.
24. Raiskup F, Pinelli R, Spoerl E. Riboflavin osmolar modification for transepithelial corneal cross-linking. Curr Eye Res. 2012;37:3. doi:10.3109/02713683.2011.637656
25. Rubinfeld RS, Stulting RD, Gum GG, Talamo JH. Quantitative analysis of corneal stromal riboflavin concentration without epithelial removal. J Cataract Refract Surg. 2018;44(2):237–242. doi:10.1016/j.jcrs.2018.01.010
26. Hill J, Liu C, Deardorff P, et al. Optimization of oxygen dynamics, UV-A delivery, and drug formulation for accelerated epi-on corneal crosslinking. Curr Eye Res. 2020;45(4):450–458. doi:10.1080/02713683.2019.1669663
27. Torres-Netto EA, Kling S, Hafezi N, Vinciguerra P, Bradley Randleman J, Hafezi F. Oxygen diffusion may limit the biomechanical effectiveness of iontophoresis-assisted transepithelial corneal cross-linking. J Refract Surg. 2018;34(11):768–774. doi:10.3928/1081597X-20180830-01
28. Seiler TG, Komninou MA, Nambiar MH, Schuerch K, Frueh BE, Büchler P. Oxygen kinetics during corneal cross-linking with and without supplementary oxygen. Am J Ophthalmol. 2021;223:368–376. doi:10.1016/j.ajo.2020.11.001
29. Park YM, Kim HY, Lee JS. Comparison of 2 different methods of transepithelial corneal collagen cross-linking: analysis of corneal histology and hysteresis. Cornea. 2017;36(7):860–865. doi:10.1097/ICO.0000000000001229
30. Sun L, Li M, Zhang X, et al. Transepithelial accelerated corneal collagen cross-linking with higher oxygen availability for keratoconus: 1-year results. Int Ophthalmol. 2018;38(6):2509–2517. doi:10.1007/s10792-017-0762-5
31. Matthys A, Cassagne M, Galiacy SD, et al. Transepithelial corneal cross-linking with supplemental oxygen in keratoconus: 1-year clinical results. J Refract Surg. 2021;37(1):42–48. doi:10.3928/1081597X-20201111-01
32. Study to Evaluate the Safety and Efficacy of Epi-on Corneal Cross-linking in Eyes With Progressive Keratoconus. identifier: NCT03442751. Available from: ClinicalTrials.gov.
33. Kamiya K, Kanayama S, Takahashi M, Visual SN. Topographic improvement with epithelium-on, oxygen-supplemented, customized corneal cross-linking for progressive keratoconus. J Clin Med. 2020;9(10):3222. doi:10.3390/jcm9103222
34. Näslund S, Fredriksson A, Alm A, Rehnman JB, Behndig A. Treatment effect with 2 photorefractive intrastromal cross-linking protocols in low-grade myopia through 24-month follow-up. Acta Ophthalmol. 2020. doi:10.1111/aos.14669
35. El Hout S, Cassagne M, Sales de Gauzy T, Galiacy S, Malecaze F, Fournié P. Transepithelial photorefractive intrastromal corneal crosslinking versus photorefractive keratectomy in low myopia. J Cataract Refract Surg. 2019;45(4):427–436. doi:10.1016/j.jcrs.2018.11.008
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
