Back to Journals » Drug, Healthcare and Patient Safety » Volume 7
Environmental and host-related determinants of tuberculosis in Metema district, north-west Ethiopia
Authors Tesema C, Tadesse T, Gebrehiwot M, Tsegaw A, Weldegebreal F
Received 2 February 2015
Accepted for publication 2 April 2015
Published 27 May 2015 Volume 2015:7 Pages 87—95
DOI https://doi.org/10.2147/DHPS.S82070
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
Cheru Tesema,1 Takele Tadesse,2 Mulat Gebrehiwot,2 Azanaw Tsegaw,3 Fitsum Weldegebreal4
1College of Medical and health science, Debremarkos Universitty, Debremarkos, 2Institute of Public Health, College of Medical and Health Sciences, University of Gondar, 3College of Health and Medical Sciences, University of Gondar, Gondar, 4Haramaya University, College of Health and Medical Sciences, Department of Medical laboratory Science, Harar, Ethiopia
Background: Each year, one third of the world's population is estimated to be infected with tuberculosis (TB). Globally in 2011, there were an estimated 8.7 million TB cases that resulted in 1.4 million deaths. In Ethiopia, TB is the leading cause of morbidity and the third most common cause of hospital admission. The aim of this study is to assess environmental and host-related determinants of TB in Metema district, north-west Ethiopia.
Methods: A community-based unmatched case-control study was conducted from March 12 to April 5, 2013. The study population included 655 subjects (218 cases and 437 controls in a ratio of 1:2). Cases were TB patients selected from a total of 475 cases registered and treated from March 2012 to February 2013 at the Metema District Hospital DOTS (direct observation therapy, short-course) clinic and selected randomly using a lottery method. Controls were people who had had no productive cough for at least 2 weeks previously and were selected from the community.
Results: A total of 655 respondents (218 cases and 437 controls) participated in the study. In multivariate analysis, being illiterate (adjusted odds ratio [AOR] 3.65, 95% confidence interval [CI] 2.31–5.76), households containing more than four family members (AOR 3.09, 95% CI 2.07–4.61), living space <4 m2 per person (AOR 3.11, 95% CI 2.09–4.63), a nonseparated kitchen (AOR 3.27, 95% CI 1.99–5.35), history of contact with a TB patient (AOR 2.05, 95% CI 1.35–3.12), a house with no ceiling (AOR 1.46, 95% CI 1.07–2.21), and absence of windows (AOR 4.42, 95% CI 2.46–7.95) were independently associated with the development of TB.
Conclusion: This study identified that the number of family members in the household, educational status, room space per person, history of contact with a TB patient, availability and number of windows, location of kitchen facilities within the house, and whether or not the house had a ceiling were independently associated with contracting TB. Every community should construct houses with the kitchen separated from the main living room, and include a ceiling and more than one window. Cigarette smoking should be avoided since this also contributed to the risk of transmission of TB. Further research focusing on coinfection with human immunodeficiency virus, helminth burden, and malnutrition is important for the control and prevention of TB.
Keywords: determinants, tuberculosis, Metema district, north-west Ethiopia
Background
Tuberculosis (TB) is a major public health problem throughout the world.1 One third of the world’s population is estimated to be infected with tubercle bacilli and at risk of developing active TB.2,3 According to a 2013 World Health Organization report, in 2012 there were 8.6 million new TB cases and 1.3 million TB deaths. Most TB cases and deaths occur among men, but the burden of the disease is also high in women. In 2012, there were an estimated 2.9 million TB cases and 410,000 TB deaths in women, as well as an estimated 530,000 cases and 74,000 deaths among children. The African region had approximately one quarter of the world’s cases, and the highest rates of cases and deaths.4
According to the 2012 World Health Organization TB report, Ethiopia ranks ninth among the 22 high-burden countries in the world, and is one of the top five in Africa with regard to the prevalence of TB. According to the same report, the incidence and prevalence of TB is 261/100,000 population and 394/100,000 population, respectively. The TB-related mortality rate for the same year is 35/100,000 population.5
In Ethiopia, according to the Ministry of Health hospital statistics data, TB is the leading cause of morbidity, the third cause of hospital admission (after obstetric deliveries and malaria), and the second cause of death after malaria.2
The first Ethiopian national population-based survey conducted in July 2007 showed that the prevalence of TB was 161/100,000, with the prevalence of males and females being 193/100,000 and 133/100,000, respectively. The survey also showed a higher prevalence of smear-positive TB in males (123 [75–171]) than in females (88 [44–122]) per 100,000, with the highest rates seen among pastoralists (170/100,000) and the lowest rates in urban areas (77/100,000). The rural prevalence was 109 (67–151)/100,000, and close to the national prevalence.6 In Gondar, Ethiopia, the death rate from TB is still high when compared with other diseases. A population-based cross-sectional study done in Dabat district, north Gondar, Ethiopia, showed that pulmonary TB accounted for 36.0% of all deaths.7 According to the 2012 Metema District Hospital annual report, TB was the ninth, third, and fifth of the top ten leading causes of outpatient visits, admissions, and deaths, respectively.8
The aim of this study is to assess environmental and host-related determinants of TB in Metema district in north-west Amhara, Ethiopia.
Methods and materials
Description of study area and study design
This community-based unmatched case-control study was conducted between March 2012 and April 5, 2013. The study subjects were clients from the Metema District Hospital DOTS (direct observation therapy, short-course) clinic. A systematic random sampling technique was used.
Study area
Metema district is located 900 km north-west of Addis Ababa, the capital city of Ethiopia, and approximately 180 km west of Gondar township. The woreda has an international boundary of more than 60 km between Ethiopia and Sudan. Metema is located north of Quarra and Alefa, west of Chilga, south of Tach Arma Choho woredas, and east of the Sudan border. It is one of the 24 woredas in north Gondar. According to the 2007 census, the total population living in the district is approximately 127,000. The district has 21 kebele, with only one district hospital, ie, Metema District Hospital, which provides all types of services (curative, preventive, and rehabilitative, for communicable and noncommunicable diseases), and comprises six health centers and 24 health posts. The altitude in Metema ranges from as low as 550 m to 1,608 m, while the minimum annual temperature ranges between 22°C and 28°C. The daytime temperature becomes very high from March to May, reaching up to 43°C. Nearly all of the land in the woreda is in the lowlands, except some mountain tops which fall outside. At the time of this study, the temperature was approximately 36°C. Metema is one of the districts in the country where the climate is harsh, and the government allows a 30% hardship allowance. According to available digital data, the mean annual rainfall for the region ranges from approximately 850 mm to 1,100 mm. Approximately 90% of the district receives a mean annual rainfall of 850–1,000 mm. Metema has a unimodal rainfall. The rainy months extend from June until the end of September; however, most of the rainfall occurs in July and August.9,10
Study variables
The dependent variable was pulmonary TB. The independent variables were:
- Environmental factors – housing conditions (ventilation, lighting), number of household residents, room size, house size (number of rooms), type of house, family size, history of TB contact, availability of solid waste disposal site, sanitation in the compound, window size per room, and water source
- Sociodemographic factors – sex, age, educational status, occupational status, marital status, income
- Host-related factors – cigarette smoking, chewing khat, drinking alcohol
- Comorbidities – malnutrition, diabetes mellitus.
Operational definitions
- Cases – patients with pulmonary TB who registered at the health facility
- Controls – people who had had no productive cough for at least 2 weeks previously and were selected from the community
- Good lighting – a house was considered well lit if it is possible to read documents written in pencil in the center of the house
- Overcrowding – a house was deemed to be overcrowded when the area of the room per person was less than 4 m2
- New sputum smear-positive pulmonary TB case – presence of at least one acid-fast bacillus in at least one sputum sample in countries with a well-functioning external quality assurance system
- New sputum smear-positive pulmonary TB case with human immunodeficiency virus (HIV) – if HIV was detected in the bloodstream of a new sputum smear-positive pulmonary TB case
- New sputum smear-positive pulmonary TB case without HIV – if HIV was not found in the bloodstream of a new sputum smear-positive pulmonary TB case or patient
- Smear-negative pulmonary TB case: if both sputum specimens were smear-negative, but a chest X-ray suggested TB, a diagnosis of smear-negative TB could be made if the clinician decided to treat with a full course of TB treatment and monitor closely for response
- Extrapulmonary TB – involving organs other than the lungs, eg, pleura, lymph nodes, abdomen, genitourinary tract, skin, joints and bones, or meninges
- Kebele – the smallest administrative unit.
Sample size estimation and data collection
The sample size was calculated using EPI Info™ StatCalc version 3.5.1 statistical software for an unmatched case-control study design. The control group exposure in the household group of greater than ten (5%) were considered from previous study as the main exposure. A control to case ratio of 2:1, an odds ratio of 2.59, a 95% confidence interval (CI), a study power of 80%, and a 10% nonresponse rate were assumed.
Based on the above assumptions, it was calculated that 197 cases and 394 controls were needed, giving a total of 591 study subjects. Adding 10% for nonresponse in both groups, the final sample size recruited was 655 (218 cases and 437 controls). Table 1 shows how the sample size was determined.
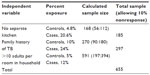  | Table 1 Determination of sample size |
Procedure for data collection
Two days of intensive training was provided for the data collectors and supervisors to familiarize them with the questionnaire, consent form, ethical clearance, and the aim of the study. The questionnaires were gone through question by question, with instruction provided on the art of interviewing and data collection. The data were collected by face-to-face interview using the structured questionnaire after it had been pretested in 5% of the total sample at another kebele to determine whether it was easily understood or not. The questionnaire was first prepared in English and translated into the local language (Amharic) for data collection and translated back to English for consistency. The data were collected by nine diploma nurses and supervised by two BSc nurses and the principal investigator. Materials like a meter, weight scale, and MUAC tape were used to assess the nutritional status of the respondents at the field level. Height and weight were usually used to calculate body mass index for males, while the MUAC tape was used for females.
Selection of cases
Cases were recruited from patients who had been on follow-up treatment at the DOTS clinics in the district.
Selection of controls
Two control households in the community were selected from the five dwellings to the immediate left and right of each case household. For each case, two healthy controls were randomly selected. First two household one from right and the other from left direction among the first five immediate neighborhood of the case’s household were randomly selected. After explaining the aim of the study to the members of each selected household, one healthy control was selected at random using a lottery method. If the head of the household refused to take part in the study, the procedure was repeated to select another household in the neighborhood. Information was collected from cases and controls on a wide range of potential environment and host-related determinants for TB.
Quality assurance
A structured questionnaire was used to keep the quality of data standardized. The questionnaire was first pretested on 33 (5%) of the study participants for accuracy and consistency and to avoid unclarity prior to actual data collection in the neighboring woreda, Negade Bahir (the pilot area). During this period, the data collectors received daily feedback from the supervisor and principal investigator before starting the actual data collection. The completeness, accuracy, and clarity of the collected data were checked carefully. The data were double-entered into EPI Info version 3.5.1 to check for quality, consistency, and completeness, then exported to Statistical Package for the Social Sciences version 20 software (SPSS Inc., Chicago, IL, USA) for analysis.
Data analysis
Completed questionnaires were categorized into cases (with TB) and controls (without TB). The data were then entered into Epi Info version 3.5.1 software. The strength of associated variable was determined using the odds ratio with the 95% CI. Further analysis was also performed using binary logistic regression with Statistical Package for the Social Sciences version 20 software to assess the relative effect of possible explanatory variables on the outcome variable by controlling the effect of confounders. A multivariate analysis model was run by selecting only those variables that appeared to be statistically significant (P<0.2) in the bivariate analysis, and reported in the results of this study.
Ethical considerations
The study was reviewed and approved by the institutional review board, University of Gondar, Ethiopia. Data were collected after informed consent was obtained from each study subject. Individual records were coded and accessed only by research staff.
Results
Sociodemographic variables
A total of 655 subjects (218 cases and 437 controls) participated in the study, giving a response rate of 100%. Of these respondents, 388 (59.2%) were male and 267 (40.8%) were female. The mean age was 34.64±11.29 years and 33.33±10.12 years for cases and controls, respectively (Table 2).
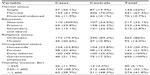  | Table 2 Sociodemographic characteristics of study participants according to health status |
Environmental determinants
Of the 218 cases and 437 controls, 181 (80.3%) and 332 (76.0%) respondents, respectively, had only one household in the compound, while 43 (19.7%) remaining cases and 105 (24.0%) remaining controls had more than one household in the compound. Most of the respondents (339 [51.8%]), comprising 145 (66.5%) cases and 194 (44.4%) controls, lived in a household containing up to four family members, while the rest (316 [48.2%]), comprising 73 (33.5%) cases and 243 (55.6%) controls, lived in a household containing more than four family members. The majority (548 [83.7%]) of respondents, comprising 162 (74.3%) cases and 386 (88.3%) controls, had no previous history of TB, while the rest (107 [16.3%]), comprising 56 (25.7%) cases and 51 (11.7%) controls did, had a history of TB (Table 3).
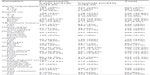  | Table 3 Environmental conditions of study participants according to health status |
Behavioral factors
The majority of respondents (547 [83.5%]), comprising 183 (83.9%) cases and 364 (83.3%) controls, had never smoked cigarettes, while 108 (16.5%), comprising 35 (16.1%) cases and 73 (16.7%) controls, had smoked cigarettes. The majority of respondents (505 [77.1%]), comprising 164 (75.2%) cases and 341 (78.0%) controls, were able to eat three and more times per day, while the rest (150 [22.9%]), comprising 54 (24.5) cases and 96 (22.0%) controls, could eat two and fewer times per day.
Host-related determinants
The majority of respondents (378 [58%]), comprising 105 (54%) cases and 273 (62%) controls, had normal nutritional status, while 207 (32%), comprising 64 (29%) cases and 143 (33%) controls, were classified as having moderate malnutrition, while the remainder (70 [11%]), comprising 49 (22%) cases and 21 (5%) controls, were classified as having severe malnutrition. Of the total number of respondents who participated in the study (652 [99.05%]), 216 (99.08%) cases and 436 (99.8%) controls did not have diabetes mellitus disease/disorder, while the remainder (three [0.5%]), comprising two (0.3%) cases and one (0.2%) control, did have diabetes mellitus disease/disorder.
Bivariate logistic regression identified that age, sex, educational status, income, number of adults in household, family size, room size per person, history of TB, presence of a separate kitchen, contact history, marital status, religion, house with a ceiling, a floor in the house, a house with a window, a latrine, ventilation, cigarette smoking, alcohol consumption, and meal frequency had a significant association with the outcome variable (Table 4). Multivariate logistic regression identified that educational status, family size, room size per person, history of previous TB, presence of a separate kitchen, contact history, house with a ceiling, house with a floor, and house with a window had a significant association with the outcome variable (Table 5).
Discussion
This community-based unmatched case-control study investigated determinants of TB in a healthy adult community and in TB patients from the DOTS clinic in Metema district, north-west Ethiopia. Among the sociodemographic variables, educational status and family size showed a significant association with TB when tested in a multivariate model. Our findings showed that people who were illiterate were four times more likely to develop TB than those who had a secondary or higher level of education (adjusted odds ratio [AOR] 3.65, 95% CI 2.31–5.76). Other studies performed in south-west Ethiopia, South India, and rural Bangladesh have also reported that illiterate people are more likely to develop the disease than those with higher education.11–13 This might be because literate people have a better quality of life relative to those who are illiterate, and this may decrease the risk of developing the disease. Household family size also had an impact on risk of transmission of pulmonary TB. We identified that people living with more than four family members per household were three times more likely to develop pulmonary TB than those living with fewer than four family members per household (AOR 3.09, 95% CI 2.07–4.61). This is in agreement with a study conducted in Bissau which found that people living in households containing more than four family members had a greater chance of developing TB than those households containing fewer than four family members.14 This might be due to overcrowded rooms increasing the risk of transmission of TB.
Among the potential environmental determinants of TB, room space/area per person, location of kitchen, history of contact with TB, ceiling of the house, and availability and number of windows showed a significant association with TB on multivariate logistic regression. People with a personal living space of less than 4 m2 were almost three times more likely to be infected with TB than those with a larger personal living space (AOR 3.11, 95% CI 2.09–4.63). Again, these findings are consistent with those in the report from Bissau,14 and could reflect the fact that a decreased personal living space indicates overcrowding and poor air circulation.
The likelihood of people who do not had kitchen and those with kitchen but not separated from the living room develop TB was (AOR 3.27, 95% CI 1.99–5.35) and (AOR 1.75, 95% CI 1.11–2.78) times more likely to develop TB when compared with those who have separated or open kitchen. This finding is consistent with a study from rural Bangladesh reporting that people who live in households without a separate kitchen were (AOR=3.66) times more likely to develop TB than those who had a separate kitchen.13 This might be because use of the main living area as a kitchen increases exposure to dust and gaseous particles that increase the prevalence of TB.
We also found that people who had a history of contact with TB patients had a twofold increased risk of contracting TB than those with no contact history (AOR 2.05, 95% CI 1.35–3.12). This finding is in line with other reports from south-west Ethiopia, West Africa, Gambia, South India, Thailand, and rural Bangladesh with regard to factors associated with pulmonary TB.11–13,15–18
Another environmental determinant that showed a significant association with TB was whether there was a ceiling in the house. People living in a house without a ceiling were 1.46 times more likely to develop TB than those living in a house with a ceiling (AOR 1.46, 95% CI 1.07–2.21). This finding is similar to that of a study done in Gambia, which reported that living in a house without a ceiling was associated with a twofold increased risk of developing TB (AOR 2.27, 95% C1.07–4.83).16 This might be because a room with a ceiling might have result high refraction power of radiation than room without ceiling. So the radiation will kill the causative agent of TB which might be found suspended in the room.
Multivariate analysis also showed that the availability and number of windows in the house was associated with TB, ie, the likelihood of developing TB in a house without a window or with only one window was increased by 2.0 and 4.4 times, respectively, when compared with living in a house with more than one window. This finding is in agreement with the report from Bissau (AOR 4.42, 95% CI 2.46–7.95) and (AOR 1.91, 95% CI 1.25–2.92),14 possibly reflecting the fact that ventilation removes the organism that causes TB if it is present in the room.
Study strengths
The present study assessed the socioeconomic, sociodemographic, environmental, behavioral, and host-related characteristics of TB patients and healthy controls living in the community. Identifying the most important risk factors for TB may allow more effective allocation of our limited resources. Multiple factors were considered in this study, and were analyzed using a multiple logistic regression model. This technique helped to control mediating and potentially confounding factors, and identified the most important risk factors for appropriate intervention.
Study limitations
This study has all the drawbacks associated with a case-control design. An important limitation was lack of confirmation of the participants’ HIV status because data were collected from their dwellings, where it was difficult to integrate HIV testing into the data collection process. The other main limitation is that the controls were recruited from neighbors of cases, so their TB status could be assessed only by history of cough, with no laboratory confirmation.
Conclusion
Family size in the household, educational status, room space, history of contact with TB, number of windows, location of kitchen, and existence of a ceiling were the major factors associated with risk of developing TB. To reduce the transmission of TB, we recommend that the kitchen should be separate from the main living room and that houses should have a ceiling and more than one window. Cigarette smoking contributes to transmission of TB, so should be avoided. Coinfection with HIV, helminth burden, and malnutrition is important for TB control and prevention, and further research is needed in TB coinfection with HIV, helminth burden, and malnutrition is important for TB control and prevention.
Acknowledgment
We are grateful to all the subjects who participated in this study, which was financially supported by the College of Health and Medical Sciences, Gondar University, Ethiopia.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Central Statistical Agency. Ethiopian Demographic Health Survey. Addis Ababa, Ethiopia, 2005. Available from: http://dhsprogram.com/pubs/pdf/FR179/FR179[23June2011].pdf. Accessed April 21, 2015. | |
Federal Ministry of Health. Tuberculosis, leprosy and TB/HIV prevention and control programme manual. Addis Ababa, Ethiopia, 2008. Available from: http://www.who.int/hiv/pub/guidelines/ethiopia_tb.pdf. Accessed April 21, 2015. | |
World Health Organization. WHO global tuberculosis report. Geneva, Switzerland: World Health Organization; 2012. Available from: http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf. Accessed April 21, 2015. | |
World Health Organization. Global tuberculosis report. Geneva, Switzerland: World Health Organization; 2013. Available from: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. Accessed April 21, 2015. | |
World Health Organization. Global tuberculosis control: WHO Report 2011. Geneva, Switzerland: World Health Organization; 2011. Available from: whqlibdoc.who.int/publications/2011/9789241564380_eng.pdf. Accessed April 21, 2015. | |
Federal Ministry of Health. First Ethiopian national population-based survey, Addis Ababa, Ethiopia; 2011. Available from: http://www.who.int/tb/advisory_bodies/impact_measurement_taskforce/meetings/tf5doc06_keyresultsprevalencesurveyethiopia2010.pdf. Accessed April 21, 2015. | |
Tadesse T, Tadesse S. Evaluating the performance of interpreting Verbal Autopsy 3.2 model for establishing pulmonary tuberculosis as a cause of death in Ethiopia: a population-based cross-sectional study. BMC Public Health. 2012;12:1039. | |
Metema Hospital. Metema Hospital Annual Report. 2012. | |
Central Statistical Agency. National Central Statistical Agency Report. 2008. | |
Metema District Annual Health Report. 2012. | |
Taha M, Deribew A, Tessema F, Assegid S, Duchateau L. Risk factors of active tuberculosis in people living with HIV/AIDS in Southwest Ethiopia. Ethiop J Health Sci. 2011;21:131–139. | |
Shetty N, Shemko M, Vaz M. An epidemiological evaluation of risk factors for tuberculosis in South India: a matched case control study. Int J Tuberc Lung Dis. 2006;10:80–86. | |
Karima MR, Rahmana MA, Mamunb SAA, Alama MA, Akhterc S. Risk factors of childhood tuberculosis: a case control study from rural Bangladesh. WHO SouthEast Asia Journal of Public Health. 2012;1:76–84. | |
Gustafson P, Victor F, Cesaltina S, et al. Tuberculosis in Bissau: incidence and risk factors in an urban community in sub-Saharan Africa. Int J Epidemiol. 2004;33:163–172. | |
Lienhardt C, Fielding K, Sillah JS, et al. Investigation of the risk factors for tuberculosis: a case–control study in three countries in West Africa. Int J Epidemiol. 2005;34:914–923. | |
Hill PC, Jackson-Sillah D, Donkor SA, Otu J, Adegbola RA, Lienhardt C. Risk factors for pulmonary tuberculosis: a clinic-based case control study in The Gambia. BMC Public Health. 2006;6:7. | |
Tornee S, Kaewkungwal J, Fungladda W, Silachamroon U, Akarasewi P. Risk factors for tuberculosis infection among household contacts in Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2004; 35:375–383. | |
Lienhardt C, Fielding K, Sillah J, et al. Risk factors for tuberculosis infection in Sub-Saharan Africa. Am J Respir Crit Care Med. 2003;168:448–455. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


