Back to Journals » International Medical Case Reports Journal » Volume 12
Enlarging acute tentorial subdural hematoma evacuated by surgery
Authors Kim J
Received 24 December 2018
Accepted for publication 17 March 2019
Published 10 April 2019 Volume 2019:12 Pages 103—107
DOI https://doi.org/10.2147/IMCRJ.S198708
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Jiha Kim1,2
1Department of Neurosurgery, Kangwon National University School of Medicine, Chuncheon-Si, Gangwon-Do, South Korea; 2Department of Neurosurgery, Kangwon National University Hospital, Chuncheon-Si, Gangwon-Do, South Korea
Abstract: Acute intracranial subdural hematomas (SDHs) of tentorial type generally pose no serious clinical threats, and unlike other variants of SDH, rarely require surgical intervention. Herein, we present an exceedingly rare case of tentorial SDH, marked by gradual enlargement and eventually calling for surgical evacuation. A 55-year-old man presented to the emergency department after sustaining head trauma. Initially, he was alert, fully oriented, and neurologically stable. Although computed tomography (CT) of the brain revealed an acute SDH scantily distributed along right tentorium, brain swelling or midline shift was negligible. On the following day, he became confused, but pupil size and light reflex remained normal. A follow-up CT scan showed considerable enlargement of the acute SDH, with midline shift. In a matter of hours, he deteriorated to a stuporous state, as the SDH enlarged even more. We performed a craniotomy and completely evacuated the SDH on an emergency basis. As a result, the midline shift improved, and he again became alert, soon recovering without any new neurologic deficit. This illustrative case demonstrates that even a tentorial SDH may ultimately deteriorate, forcing surgical evacuation. We, therefore, feel that close observation is mandatory for such events, even if the initial volume is small.
Keywords: tentorial subdural hematoma, surgical evacuation
Introduction
Acute traumatic subdural hematoma (SDH) is a common event in neurosurgical practice,1,2 presenting in 12–29% of the patients admitted with severe traumatic brain injuries.2 Most involve the convexity of the brain,1,3,4 and rarely encroach on tentorium cerebelli (tentorial SDH).3,4
Although acute traumatic SDH has traditionally been regarded as a lesion that should be treated surgically,2 tentorial SDH is by nature clinically much more benign, seldom enlarging or progressing to the point of neurologic deterioration.1,5 Thus, the need to surgically evacuate a tentorial SDH would be highly unusual.
Given their rarity and the innocuous clinical course displayed, less attention has been paid to such lesions. The few available reports published in English are confined to isolate cases or limited series of patients.4 Little is thus known about tentorial SDH, despite the many prognostic factors, treatment strategies, and outcomes documented for those involving the brain convexity.1,6,7
Herein, we describe a 55-year-old man who sustained head trauma and thereafter developed an acute tentorial SDH. To our knowledge, delayed surgical evacuation of an enlarging tentorial SDH has yet to be reported. However, the extent of enlargement that we witnessed made the present case unique.
Case report
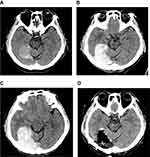
The case review was conducted according to all guidelines outlined in the Declaration of Helsinki. Written informed consent for publication of this case report was obtained from the patient. The patient, a 55-year-old man, presented to the emergency department with a headache and a scalp laceration. He had reportedly fallen from his wheelchair while traveling down the street. Upon arrival at the hospital, he was alert, fully oriented, and showed no new neurologic deficits when examined. Aside from occipital scalp laceration (5 cm) and a chin abrasion, there were no other cuts or deep wounds elsewhere on his body. Computed tomography (CT) of the brain disclosed an acute SDH, scantily distributed along right tentorium (Figure 1A). Evidence of brain swelling or midline shift was negligible, and no skull fracture was detected. Bony injuries in other areas of the body were also excluded radiographically.
He had hypertension and antiphospholipid syndrome (APS) as underlying disorders. Furthermore, several related episodes of cerebral infarction, resulted from APS had imposed a right hemiparesis (grade 4) long before the head trauma occurred, and for 2 years he had received warfarin as prophylaxis. By immediately administrating vitamin K, his initially prolonged clotting time (international normalized ratio [INR]) was reversed in a matter of hours (3.22->1.1) and remained within normal range. Conservative management was then initiated within the intensive care unit, anticipating that surgery was avoidable.
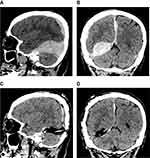
On the following day, the patient abruptly became confused, although pupil size and light reflex were normal, and there was no change in his neurologic assessment since admission. Follow-up CT images of the brain showed noticeable enlargement of an acute SDH, compressing and elevating the occipito-temporal base (Figures 1B and 2A, B). Midline shift had also progressed, albeit with sparing of cisterns around the brain stem. Several hours later, his mental state worsened. He was stuporous, and the acute SDH had enlarged substantially (Figure 1C). We, therefore, performed an emergency craniotomy to evacuate the SDH using a frameless navigation system.
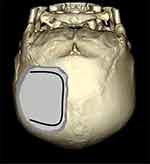
Under general anesthesia, the patient was placed in a prone position and immobilized with skull fixation. After checking the location of the superior sagittal sinus (SSS) and transverse sinus (TS) using the navigation system, we performed a right occipital craniotomy. The craniotomy site was almost square and measured approximately 7.5 cm×7.5 cm; the distances between the medial margin of the craniotomy and the SSS and the lower margin of the craniotomy and the TS were approximately 1.5 cm and 1 cm, respectively (Figure 3). Although there was scalp laceration, the scalp contusion was not severe and there was no skull fracture. The epidural hemorrhage was minimal and the dura was tense. Upon opening the dura, the brain surface was initially obscured by a 0.5-cm-thick SDH. After removing this, the large SDH between the occipital base and the tentorium was exposed. We evacuated the entire SDH and confirmed that there was no residual hematoma using the navigation system.
Intraoperatively, we found no active bleeding focus or vascular abnormality to account for the SDH. The SDH was partially attached to normal brain tissue and tentorium, but it was still distinguishable from normal structures. There was no cortical discoloration or overt damage to brain cortex. Once the tentorial SDH was completely evacuated, brain compression improved (Figures 1D and 2C, D). The patient regained alertness soon after surgery and showed no new neurologic deficits.
Discussion
Acute SDH is a common consequence of head trauma,1,2 but purely tentorial SDH is rarely encountered.3,4 Among 5,066 patients hospitalized due to head trauma, Takeuchi et al,4 identified only 32 (0.6%) with tentorial SDH, and most of those were accompanied by other intracranial lesions such as falx or convexity SDH. Only eight patients had purely tentorial lesions.4
Acute SDH of the tentorial region sometimes develops at birth3 as a distinctly different entity from that seen in adults. Lesions of this sort are attributable to tears in the tentorium itself, caused by cranial deformation during delivery.3 In adults, the basis of traumatic tentorial SDH is still unclear, but it is likely that in the process of linear brain acceleration during trauma, bridging veins adjacent to the tentorium are torn.3,4 The findings of Takeuchi et al,4 offer support for this mechanism, including a high incidence of impact in occipital or frontal regions and a low incidence of the skull fracture. Our patient similarly had sustained occipital impact, without suffering a skull fracture.
Whereas convexity lesions may become life-threatening and are ready surgical targets,2,6,8 the need for surgical evacuation of a purely tentorial SDH is extremely rare. In an analysis of clinical outcomes in 210 patients with acute traumatic SDH, Sweis et al,1 found that while 47% needed surgical evacuation, none of 27 patients with falcine/tentorial lesions require surgical evacuation, and, in contrast to convexity SDH, outcomes of falcine/tentorial SDH were all favorable in terms of residual neurologic deficits (32% vs 0%, respectively). Similarly, Bajsarowicz et al,2 examined the clinical courses of 646 patients with acute SDH who were initially treated conservatively. Overall, 6.5% progressed to the point of requiring surgery, but not one tentorial SDH (n=75) required subsequent surgical evacuation. Furthermore, the poor outcomes (44%) reported by Takeuchi et al,4 in patients with tentorial SDH did not involve pure tentorial lesions. All patients in that study were treated conservatively for tentorial SDH, with some undergoing surgery for concomitant intracranial pathologies.4 Devulapalli et al,5 likewise followed 80 patients with CT findings of isolated SDH of the falx or tentorium, and found that all of the lesions were either unchanged or diminished at follow-up CT, with no sign of new intracranial hemorrhage. The limited number of bridging veins and the lesser tension exerted at the tentorial location are thought to contribute to these favorable outcomes,2 while the fact that there is less counter-pressure from the brain in the convexity, and more frequent coexistence of cortical contusion, may predispose SDH in that region to expansion.2,9
APS is an autoimmune antibody-mediated disorder marked by thrombotic events.10,11 To date, antithrombotic therapy is key in its management,10, and warfarin is regarded as the standard preventative treatment.11 Due to several episodes of cerebral infarction, our patient was taking warfarin as prophylaxis, which may have influenced his clinical course. Some reports have implicated coagulation abnormality in such events,1,6,7 whereas others have shown that there is no heightened risk of deterioration attached.2,12 Nevertheless, the patient's INR was immediately normalized by administrating vitamin K, and it remained within normal range. Even had warfarin predisposed to this SDH, the effect on its eventual enlargement is believed to be very limited.
Despite the substantial volume of the SDH in our patient, neither his clinical course nor his postoperative neurologic outcome was particularly ominous, perhaps thanks to the inherently advantageous characteristics of tentorial SDH. First, the thick tentorial or falcine membrane serves to minimize the mass effect on the contralateral cerebral hemisphere or brain stem. Although moot when the SDH is small, the protection thus provided limits direct compression as the SDH expands, thereby buffering the subsequent mass effect and preventing damage to brain tissue and the bridging veins over the contralateral tentorium or falx. Indeed, maintenance of microcirculation and venous drainage was especially critical in this patient, who had ceased warfarin and could have been highly vulnerable to brain infarction. In addition, the direction of brain compression proved favorable in this patient, averting brain stem damage. Rather than forcing the cerebral hemisphere medially and downward to result in cerebral herniation (as a convexity SDH may readily do), compression by the tentorial SDH had elevated the occipito-temporal base. Irreversible brain damage (ie, brain stem compression from cerebral herniation) was thus avoided. The preoperative CT scan confirmed that cisterns around the brain stem were unaffected. This was also corroborated clinically by normal pupil size and intact light/stem reflexes.
In interhemispheric SDH, laceration of brain cortex is a poorer prognosticator than bridging vein laceration.9 However, we did not find an active bleeding focus or damaged tissue intraoperatively.
Conclusion
Unlike most instances of traumatic tentorial SDH, which are clinically innocuous, the deteriorating circumstances detailed herein underscore the potential for such lesions to become life-threatening and require surgical evacuation. Hence, we feel that close observation is mandatory in the event of a tentorial SDH, even if initially small and neurologically stable.
Acknowledgments
This study was supported by 2016 Research Grant from Kangwon National University. This case review was approved by the Institutional Review Board of the Kangwon National University Hospital (2018-12-007)
Disclosure
The author reports no conflicts of interest in this work.
References
1. Sweis RT, Ouyang B, Lopez GA, Bleck TP, Busl KM. Falcine and tentorial subdural hematomas may not routinely require transfer to a tertiary care center. J Emerg Med. 2015;49(5):679–685. doi:10.1016/j.jemermed.2015.06.055
2. Bajsarowicz P, Prakash I, Lamoureux J, et al. Nonsurgical acute traumatic subdural hematoma: what is the risk? J Neurosurg. 2015;123(5):1176–1183. doi:10.3171/2014.10.JNS141728
3. Matsumoto K, Houri T, Yamaki T, Ueda S. Traumatic acute subdural hematoma localized on the superior surface of the tentorium cerebelli–two case reports. Neurol Med Chir (Tokyo). 1996;36(6):377–379.
4. Takeuchi S, Takasato Y, Masaoka H, et al. Traumatic peritentorial subdural hematomas: a study of 32 cases. Turk Neurosurg. 2012;22(3):305–308. doi:10.5137/1019-5149.JTN.5178-11.2
5. Devulapalli KK, Talbott JF, Narvid J, et al. Utility of repeat head CT in patients with blunt traumatic brain injury presenting with small isolated falcine or tentorial subdural hematomas. AJNR Am J Neuroradiol. 2018;39(4):654–657. doi:10.3174/ajnr.A5557
6. Powers AY, Pinto MB, Aldridge AM, et al. Factors associated with the progression of conservatively managed acute traumatic subdural hemorrhage. J Crit Care. 2018;48:243–250. doi:10.1016/j.jcrc.2018.09.014
7. Seddighi AS, Motiei-Langroudi R, Sadeghian H, et al. Factors predicting early deterioration in mild brain trauma: a prospective study. Brain Inj. 2013;27(13–14):1666–1670. doi:10.3109/02699052.2013.830333
8. Cui V, Kouliev T. Isolated oculomotor nerve palsy resulting from acute traumatic tentorial subdural hematoma. Open Access Emerg Med. 2016;8:97–101. doi:10.2147/OAEM.S117687
9. Wang Y, Wang C, Cai S, et al. Surgical management of traumatic interhemispheric subdural hematoma. Turk Neurosurg. 2014;24(2):228–233. doi:10.5137/1019-5149.JTN.8377-13.0
10. Fleetwood T, Cantello R, Comi C. Antiphospholipid syndrome and the neurologist: from pathogenesis to therapy. Front Neurol. 2018;9:1001. doi:10.3389/fneur.2018.01001
11. Ohnishi N, Fujieda Y, Hisada R, et al. Efficacy of dual antiplatelet therapy for preventing recurrence of arterial thrombosis in patients with antiphospholipid syndrome. Rheumatology (Oxford). 2018. doi:10.1093/rheumatology/key340
12. Kim BJ, Park KJ, Park DH, et al. Risk factors of delayed surgical evacuation for initially nonoperative acute subdural hematomas following mild head injury. Acta Neurochir (Wien). 2014;156(8):1605–1613. doi:10.1007/s00701-014-2151-4
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.