Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Enhanced Activation of mTOR Signaling Pathway Was Found in the Hypertrophic and Nodular Lesions of Port Wine Stains
Authors Xu MN, Wang Q, Wang M, Xu Y, Yuan SM
Received 15 January 2022
Accepted for publication 27 March 2022
Published 13 April 2022 Volume 2022:15 Pages 643—651
DOI https://doi.org/10.2147/CCID.S358612
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Meng-Nan Xu,1,* Qian Wang,1,* Min Wang,1 Yuan Xu,1 Si-Ming Yuan1,2
1Department of Plastic Surgery, Jinling Hospital, Medical School of Nanjing University, Nanjing, Jiangsu, 210002, People’s Republic of China; 2Department of Plastic Surgery, Jinling Hospital, Nanjing School of Clinical Medicine, Southern Medical University, Nanjing, Jiangsu, 210002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Si-Ming Yuan, Department of Plastic Surgery, Jinling Hospital, Medical School of Nanjing University, Nanjing, Jiangsu, 210002, People’s Republic of China, Email [email protected]
Background: Port wine stain (PWS) is a congenital skin lesion involving capillary malformations. Most PWS lesions will gradually become hypertrophic and appear nodular in contour. Current research shows that rapamycin, an mTOR inhibitor, is probably a promising adjunctive therapy for PWS, which suggests that the mTOR signaling pathway may play an important role in its pathological process.
Methods: From January 2013 to January 2019, 13 samples were obtained during the surgical excision. Each sample was divided into 3 parts according to the type of lesion, namely, the flat, hypertrophic and nodular lesions. Pathologic structures of each type were observed under the microscope after HE staining. The expression of mTORC1, p70S6, p-p70S6, eIF4EBP1 and p-eIF4EBP1 was examined by immunohistochemical staining and western blotting. The location of the expression of mTORC1, p-p70S6 and p-elF4EBP1 was further detected by immunofluorescence staining.
Results: Large amounts of dilated and malformed vessels were observed in all types of PWS lesions. Abundant hyperplastic hair follicles/glands were shown in the hypertrophic or nodular lesions. Phosphorylation level of p70S6 and elF4EBP1 in PWS was significantly higher than those in normal skin and increased accordingly in the progression of PWS. Activated molecules in mTOR signaling pathway were mostly located in the endothelium of malformed vessels. They were also located in the hyperplastic hair follicles/glands of hypertrophic and nodular lesions.
Conclusion: The mTOR signaling pathway was increasingly activated during the progression of PWS. Enhanced activation of mTOR signaling pathway may contribute to the hypertrophy and nodularity of PWS. The results provide preliminary evidence for treating PWS and related syndromes by inhibiting mTOR signaling pathway.
Keywords: port wine stain, mTOR signaling pathway
Introduction
Port wine stain (PWS) is a congenital capillary malformation that occurs in 3 to 5 per 1000 newborns.1 Most of the lesions are in the head and neck, less frequently in the trunk and extremities. The lesions initially appear as flat, pink patches,1,2 most of which will gradually grow into red to purple, hypertrophic or nodular lesions, severely affecting the appearance.3,4
It has been reported that the pathogenesis of PWS involves gene mutation. Variants (c.548G→A, p.Arg183Gln; c.547C>G, p.Arg183Gly) in GNAQ was found in PWS.5 Somatic mutations of RASA1 and PIK3CA were also found in PWS. Aberrant activation of AKT, PI3K and PLC-g in hypertrophic and nodular PWS lesions suggests that PIK3CA and its downstream signaling pathway may play an important role in the development of hypertrophy and nodularity in PWS.6,7
Mammalian target of rapamycin (mTOR) is a downstream protein of PI3K/AKT signal transduction pathway. p70S6 Kinase 1 (S6K1) and the eukaryotic initiation factor 4E binding protein 1 (eIF4EBP1), the two typical substrates of mTORC1, are phosphorylated after activation of mTORC1, exerting a wide effect on protein synthesis and angiogenesis.8 Rapamycin, an mTORC1 inhibitor, has been reported to be used as an adjunctive therapy for PWS and that it can help maintain and improve the therapeutic effect of pulsed dye laser.9,10
Therefore, we suppose that the mTOR signaling pathway is involved in the development and progression of PWS. In this study, we summarized the pathological characteristics of flat, hypertrophic and nodular PWS lesions, detected the expression and location of mTOR signaling pathway including p70S6, phospho-p70S6 (p-p70S6), elF4EBP1 and phospho-elF4EBP1 (p-elF4EBP1), and measured the phosphorylation level of these proteins. The results provide some clues for the pathogenesis and a possible new target for the treatment of PWS.
Methods
Statistics
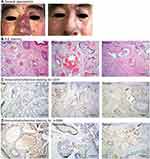
The research was approved by the Ethics Committee of Jinling Hospital (registration number: 2013NZKY-011-01) and the study complies with the Declaration of Helsinki. Informed consent was achieved by all patients. From January 2013 to January 2019, 13 samples were obtained from 10 patients who received surgical removal of PWS lesions. Unused normal skin samples, harvested from a full thickness skin grafting operation, were grouped into the controls. According to the clinical manifestation, the PWS samples were divided into flat, hypertrophic and nodular types. Flat lesions (n = 2) manifest as flat red macules, hypertrophic lesions (n = 6) manifest as hypertrophic red soft tissues and nodular lesions (n = 5) as red nodules developed from hypertrophic lesions. The general appearance of each type is shown in Figure 1. Black arrows show the lesions. Demographics of all patients are listed in Table 1.
 |
Table 1 Demographics of Patients |
Hematoxylin–Eosin Staining and Immunohistochemical Analysis
The sections were fixed in 4% paraformaldehyde, embedded in paraffin wax, cut into 5 μm sections, de-waxed through xylene, dehydrated through graded alcohols and were prepared for hematoxylin–eosin staining (HE) or immunohistochemical analysis. Antigen retrieval was performed using citrate buffer (pH 6.0) and high-power steam. Tissue sections were then incubated overnight with the primary antibody solution (diluted according to manufacturer’s recommendation) at 4°C. Primary antibodies include mouse anti-human CD31 (Ab24590, Abcam, a typical marker for vascular endothelial cells), alpha-smooth muscle actin (α-SMA, BM0002, BOSTER), rabbit-anti-human mTORC1 (Ab115330, Abcam), p70S6 (Ab32359, Abcam), eIF4EBP1 (Ab2606, Abcam), p-p70S6 (Thr 389, #9234, Cell Signaling Technology), and p-eIF4EBP1 (Thr70, #9455, Cell Signaling Technology) antibodies. The sections were then incubated with secondary antibody for 2 h and DAB Horseradish Peroxidase Color Development Kit (DAB, Dako Code K5007, Agilent Technologies, Santa Clara, CA) and were performed at room temperature. The sections were observed under a light microscope.
Immunofluorescence Assay
The assay was conducted by using the same sections and microwave antigen retrieval techniques. Co-staining of CD31 with mTOR/p-p70S6/p-eIF4EBP1 and α-SMA with mTOR/p-p70S6/p-eIF4EBP1were performed. The sections were blocked for 30 minutes in 5% serum and incubated with mouse-anti-human CD31 or α-SMA antibody, with the co-staining of rabbit anti-human p-p70S6, p-eIF4EBP1, and mTORC1 antibodies overnight at 4°C respectively, and followed by the second antibodies Alexa Fluor 555 donkey anti-mouse IgG (H+L) highly cross-adsorbed antibody (A-31570, Thermo Fisher Scientific) and Alexa Fluor 488 donkey anti-rabbit IgG (H+L) highly cross-adsorbed antibody (A-21206, Thermo Fisher Scientific). The isotype controls of rabbit polyclonal IgG (ab27478, Abcam) and mouse IgG1 (ab18448, Abcam) were used in the control staining. The sections were counterstained with DAPI. Fluorescent images were taken with a fluorescence microscope (BX51, OLYMPUS), equipped with a digital microscope camera (ProgRes MFcool, JENOPTIK) and an image analysis system (IMSTAR KARYO, IMSTAR).
Western Blotting
Samples stored in liquid nitrogen were thawed on ice and washed 3 times with PBS. The total protein was extracted using a KeyGEN Protein Assay Kit (KGP250, KeyGENBioTECH, Nanjing, China). Protein concentration range was measured with BCA Protein Assay Kit (RL242684, Thermo Scientific, Rockford, USA). 30 ug protein lysates of each sample were resolved by SDSPAGE and transferred to PVDF membrane (IPVH00010, Millipore, Billerica, MA, USA). After blocking with TBS/0.1% Tween 20/5% BSA, the membranes were incubated at 4°C with primary antibodies including mTORC1, p70S6, p-p70S6, eIF4EBP1 and p-eIF4EBP1. Then, the membranes were washed 3 times with 0.1% Tween 20/TBS for 10 minutes. Immuno-reactive proteins were identified with secondary antibody (10285-1-AP, Santa Cruz Biotechnology, CA, USA) coupled to HRP antibody at room temperature, and visualized by ECL solution (180–501, Tanon, Shanghai, China). Quantitative analysis of the electropherogram was done with Image J software.
Statistical Analysis
Statistical analysis was conducted with GraphPad 8.0, using a two-tailed paired Student’s t-test to evaluate the statistical significance of the results. The data was expressed as mean ± SD. A P-value less than 0.05 was considered statistically significant.
Results
Pathological Characteristics and Expression of Vascular Specific Antigens in PWS
Massive dilated vessels with irregular shapes and thin sinusal vascular channels were observed in all PWS lesions. The vessels’ walls were thin with flat, elongated endothelium lined and few pericytes surrounded. Malformed vessels mostly exist in the papillary layer and upper reticular dermis, rarely extending into deep dermis or subcutaneous fat layer. The differences in pathological structures were also obvious among different types. In flat lesions, vessels were slightly dilated and were mainly post-capillary venules. Lymphocytic infiltration was found in deep dermis surrounding capillaries. The thickness of the epidermis layer was normal without proliferative secretory glands or hair follicles (Figure 1B). In hypertrophic lesions, thicker epidermis, hypertrophic hair follicles and hyperplastic glands were observed (Figure 1B). While in nodular lesions, vessels displayed features of arterioles with thickened walls. The lymphocytic infiltration, looser stroma, significantly proliferated hair follicles and glands also appeared in some nodule samples (Figure 1B). Immunohistochemical studies showed the positive expression of CD31 in endothelium of vessel walls (Figure 1C). Staining of α-SMA was weak and scattered. The results revealed the absence or sparse distribution of smooth muscle cells in the ectatic vessel walls (Figure 1D). Increased or abnormally dilated vessels were not observed in normal skin samples.
Expression of mTOR Signaling Pathway in PWS
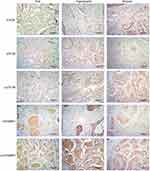
Immunohistochemical staining showed expression of mTORC1, p70S6, p-p70S6, eIF4EBP1 and p-eIF4EBP1 in endothelium. Phosphorylation of p70S6 and eIF4EBP1 progressively increased in normal skin, flat, hypertrophic and nodular lesions, which positively correlated with the pathological progression of PWS. P-p70S6 and p-eIF4EBP1 could also be observed in a small portion of secretory glands and hair follicles in the hypertrophic and nodular lesions (Figure 2).
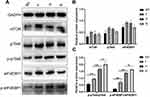
Western blotting showed the expression of mTORC1, p70S6, p-p70S6, eIF4EBP1 and p-eIF4EBP1 in PWS lesions and normal skin. Semiquantitative analysis showed that there was no significant difference in the expression of mTORC1, p70S6, and eIF4EBP1 between normal skin samples and PWS lesions (P > 0.05), but the expression of p-p70S6 and p-eIF4EBP1 in PWS lesions was significantly higher than that in normal skin samples (P < 0.05). In addition, the expression of p-p70S6 and p-eIF4EBP1 in hypertrophic and nodular lesions was higher than that in flat lesions. However, there was no significant difference between hypertrophic and nodular lesions (Figure 3).
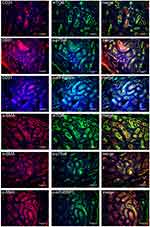
Co-Expression of CD31/α-SMA and mTORC1/p-p70S6/p-eIF4EBP1 in PWS
To verify the expression sites of mTOR signaling pathway, co-staining of CD31/α-SMA and mTORC1/p-p70S6/p-eIF4EBP1 was performed. The expression of CD31 showed a vascular distribution. Co-expression of CD31/mTORC1, CD31/p-p70S6, and CD31/p-eIF4EBP1 was found in the cytoplasm and nuclei in CD31 positive cells. Expression of α-SMA in smooth muscle cells or pericytes was weak and distributed dispersedly around the expression of mTORC1/p-p70S6/p-eIF4EBP1 (Figure 4). The results showed that the activation of mTOR signaling pathway was in the endothelium in the malformed vessels of PWS lesions.
Discussion
Port wine stain (PWS) is a congenital disease involving capillary malformation. The birthmarks usually first appear as flat, red patches and then more than half of them will develop into hypertrophic and nodular lesions.11,12 In our study, we found that PWS consisted of massive ectatic vessels with irregular lumen and thin walls. Morphological differences were apparent in three types of PWS lesions. Hyperplastic secretory glands and hair follicles were observed in hypertrophic lesions. Arterioles with thickened walls were found in nodular lesions. Positive expression of mTOR signaling was observed in endothelium and in some secretory glands or hair follicles. The relevance between aberrant activation of mTOR signaling and the progression of PWS indicates that mTOR signaling may participate in the pathological process.
Current studies show that the mutation of GNAQ, RASA1and PIK3CA may be associated with most PWSs. Mutation of GNAQ (R183Q) was primarily located within the vessels in PWS lesions and some connective tissues and hair follicles/glands. The finding suggested that enrichment of GNAQ (R183Q) in PWS might induce abnormal proliferation of blood vessels and hair follicles/glands, thus contributing to the hypertrophic or nodular lesions.13 Consecutive activation of downstream signaling pathway including ERK and MAPK induced by GNAQ (R183Q) may cause disordered proliferation of endothelial cells and further lead to the dysplasia and remodeling of capillary plexus. Activation of PI3K signaling may be the direct or indirect result of GNAQ or RASA1 mutations.14
mTOR is a serine/threonine protein kinase that belongs to PI3K related kinase family and takes an important part in regulating cell cycle and protein synthesis. mTOR was found in two protein complexes including mTORC1 and mTORC2. The activity of mTORC1 is sensitive to rapamycin, while mTORC2 is contrary. Activated mTOR has regulatory effects on the transcription and translation of various angiogenesis-related factors like VEGF or PDGF, which may promote angiogenesis and modulate the chemotaxis of endothelial cells to pericytes by binding to surface-specific receptors on endothelial cells. S6K1 and eIF4EBP1 are direct regulatory substrates of mTOR. The phosphorylated forms have greater impacts on the promotion of mRNA translation, thereby further affecting cell growth and proliferation through the modulation of ribosomal proteins and translation regulatory proteins.15,16
We suppose that during the process of PWS, the somatic mutation mediated activation of PI3K/AKT/mTOR signaling pathway. Multiple factors are involved in disrupting angiogenesis and vessel maturation in PWS. Chemotactic activity of smooth muscle cells and the ultra-structure of pericytes are abnormal. Changes in blood stream dynamics may eventually lead to blood vessels dilation or malformation and form flat PWS lesions. Chronic activation of molecules in mTOR signaling pathway, including p70S6 and eIF4EBP1, further regulate transcription and promotes protein translation. Consequently, new and abnormal blood vessels grow and expand. Besides, the activation of mTOR signaling pathway in glands/hair follicles accelerates cell proliferation, leading to the hyperplasia and hypertrophy. These results together contribute to the progress of hypertrophic and nodular PWS lesions. This is consistent with previous studies that GNAQ mutation occurred in both blood vessels and hair follicles/glands.13
There are several treatment options for PWS, including laser therapy, photodynamic therapy (PDT) and surgical therapy.17,18 These therapies are effective in patients with a certain dermal vascular phenotype. Complications like skin necrosis and scar hypertrophy not only affect the appearance but also contribute to psychological trauma.19 There is no effective treatment or targeted biotech drugs towards extensive PWSs. New methods are needed to solve this problem. Rapamycin, as an adjunctive therapy, has achieved good results in the treatment of PWS although there was much debate about the treatment effect.9,10,20 Current studies have indicated that rapamycin exerts strong inhibitory effect on p70S6 and eIF4EBP1 and can interfere with the cell cycle. Rapamycin can also inhibit proliferation, migration and tubular morphogenesis of endothelial cells in vitro.21 Another study showed that rapamycin can induce cell apoptosis and inhibit proliferation of endothelial progenitor cells.22 A higher potency in terms of anti-angiogenesis was shown in rapamycin.23 Our results further provide a theoretical basis for treating PWS by using rapamycin or other mTOR inhibitors.
There are some limitations in this research. The number of the cases and samples was relatively small. In addition, we only conducted qualitative and semi-quantitative experiments in this research. Further research with more samples and quantitative experiments are needed to better demonstrate this point.
In summary, our research preliminarily revealed that downstream regulators of mTOR signaling including p70S6 and eIF4EBP1 was abnormally activated in PWS, which might contribute to its hypertrophy and nodularity. The results provide a research basis for developing mTOR-targeted therapies against PWS.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (No. 81272989, awarded to Dr. Si-Ming Yuan), “333 Project” of Jiangsu Province, China (No. BRA2020416, awarded to Dr. Si-Ming Yuan), and the Foundation for Key Medical Specialty of Nanjing (SZDZK202001, awarded to Dr. Si-Ming Yuan).
Disclosure
None of the authors declared any conflicts of interest.
References
1. Barsky SH, Rosen S, Geer DE, et al. The nature and evolution of port wine stains: a computer-assisted study. J Invest Dermatol. 1980;74(3):154–157. doi:10.1111/1523-1747.ep12535052
2. Martinez-Lopez A, Salvador-Rodriguez L, Montero-Vilchez T, et al. Malformations syndromes: an update. Curr Opin Pediatr. 2019;31(6):747–753. doi:10.1097/MOP.0000000000000812
3. Galligan ER, Baselga E, Frieden IJ, et al. Characterization of vascular stains associated with high flow. J Am Acad Dermatol. 2021;84(3):654–660. doi:10.1016/j.jaad.2020.06.985
4. Lee MS, Liang MG, Mulliken JB. Diffuse capillary malformation with overgrowth: a clinical subtype of vascular anomalies with hypertrophy. J Am Acad Dermatol. 2013;69(4):589–594. doi:10.1016/j.jaad.2013.05.030
5. Frigerio A, Wright K, Wooderchak-Donahue W, et al. Genetic variants associated with port-wine stains. PLoS One. 2015;10(7):e0133158. doi:10.1371/journal.pone.0133158
6. Luks VL, Kamitaki N, Vivero MP, et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J Pediatr. 2015;166(4):
7. Lee KT, Park JE, Eom Y, et al. Phenotypic association of presence of a somatic GNAQ mutation with port-wine stain distribution in capillary malformation. Head Neck. 2019;41(12):4143–4150. doi:10.1002/hed.25962
8. du Rusquec P, Blonz C, Js F, et al. Targeting the PI3K/Akt/mTOR pathway in estrogen-receptor positive HER2 negative advanced breast cancer. Ther Adv Med Oncol. 2020;12:1–12. doi:10.1177/1758835920940939
9. Musalem HM, Alshaikh AA, Tuleimat LM, et al. Outcome with topical sirolimus for port wine stain malformations after unsatisfactory results with pulse dye laser treatment alone. Ann Saudi Med. 2018;38(5):376–380. doi:10.5144/0256-4947.2018.376
10. Marqués L, Núñez-Córdoba JM, Aguado L, et al. Topical rapamycin combined with pulsed dye laser in the treatment of capillary vascular malformations in Sturge-Weber syndrome: Phase II, randomized, double-blind, intraindividual placebo-controlled clinical trial. J Am Acad Dermatol. 2015;72(1):151–8.e1. doi:10.1016/j.jaad.2014.10.011
11. Finley JL, Noe JM, Arndt KA, et al. Port-wine stains. Morphologic variations and developmental lesions. Arch Dermatol. 1984;120(11):1453–1455. doi:10.1001/archderm.1984.01650470059013
12. van Drooge AM, Beek JF, van der Veen JP, et al. Hypertrophy in port-wine stains: prevalence and patient characteristics in a large patient cohort. J Am Acad Dermatol. 2012;67(6):1214–1219. doi:10.1016/j.jaad.2012.05.027
13. Tan W, Nadora DM, Gao L, et al. The somatic GNAQ mutation (R183Q) is primarily located within the blood vessels of port wine stains. J Am Acad Dermatol. 2016;74(2):380–383. doi:10.1016/j.jaad.2015.09.063
14. Pérez-Alea M, Vivancos A, Caratú G, et al. Genetic profile of GNAQ-mutated blue melanocytic neoplasms reveals mutations in genes linked to genomic instability and the PI3K pathway. Oncotarget. 2016;7(19):28086–28095. doi:10.18632/oncotarget.8578
15. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi:10.1016/j.cell.2012.03.017
16. Mercurio L, Albanesi C, Madonna S. Recent updates on the Involvement of PI3K/AKT/mTOR molecular cascade in the pathogenesis of hyperproliferative skin disorders. Front Med. 2021;8:665647. doi:10.3389/fmed.2021.665647
17. Mathes EF, Frieden IJ. Early use of laser for port-wine stains: timing, efficacy, and shared decision making. JAMA Dermatol. 2019;155(4):421–423. doi:10.1001/jamadermatol.2018.5189
18. Lee JW, Chung HY. Capillary malformations (port-wine stains) of the head and neck: natural history, investigations, laser, and surgical management. Otolaryngol Clin North Am. 2018;51(1):197–211. doi:10.1016/j.otc.2017.09.004
19. Bae YC, Alabdulrazzaq H, Brauer JA, et al. Treatment of recalcitrant port-wine stains (PWS) using a combined pulsed dye laser (PDL) and radiofrequency (RF) energy device. J Am Acad Dermatol. 2017;76(2):321–326. doi:10.1016/j.jaad.2016.03.004
20. Greveling K, Prens EP, van Doorn MB. Treatment of port wine stains using pulsed dye laser, erbium YAG laser, and topical rapamycin (sirolimus)-A randomized controlled trial. Lasers Surg Med. 2017;49(1):104–109. doi:10.1002/lsm.22548
21. Manning BD. Game of TOR - The target of rapamycin rules four kingdoms. N Engl J Med. 2017;377(13):1297–1299. doi:10.1056/NEJMcibr1709384
22. Zhang P, Han G, Gao P, et al. Protective effect of silymarin against rapamycin-induced apoptosis and proliferation inhibition in endothelial progenitor cells. Nat Prod Commun. 2015;10(2):263–266.
23. Kratzsch T, Piffko A, Broggini T, et al. Role of mTOR and VEGFR inhibition in prevention of metastatic tumor growth in the spine. Front Oncol. 2020;10:174. doi:10.3389/fonc.2020.00174
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.