Back to Journals » International Journal of Nanomedicine » Volume 15
Engineering of Aerogel-Based Biomaterials for Biomedical Applications
Authors Zheng L , Zhang S, Ying Z, Liu J, Zhou Y , Chen F
Received 10 November 2019
Accepted for publication 25 February 2020
Published 3 April 2020 Volume 2020:15 Pages 2363—2378
DOI https://doi.org/10.2147/IJN.S238005
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Longpo Zheng,1,* Shaodi Zhang,1,* Zhengran Ying,1 Junjian Liu,1 Yinghong Zhou,2– 4 Feng Chen1,4
1Department of Orthopedics, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai 200072, People’s Republic of China; 2The Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, QLD 4059, Australia; 3Key Laboratory of Oral Medicine, Guangzhou Institute of Oral Disease, Stomatology Hospital of Guangzhou Medical University, Guangzhou 510140, People’s Republic of China; 4The Australia-China Centre for Tissue Engineering and Regenerative Medicine (ACCTERM), Queensland University of Technology (QUT), Brisbane, QLD 4000, Australia
*These authors contributed equally to this work
Correspondence: Feng Chen Email [email protected]
Abstract: Biomaterials with porous structure and high surface area attract growing interest in biomedical research and applications. Aerogel-based biomaterials, as highly porous materials that are made from different sources of macromolecules, inorganic materials, and composites, mimic the structures of the biological extracellular matrix (ECM), which is a three-dimensional network of natural macromolecules (e.g., collagen and glycoproteins), and provide structural support and exert biochemical effects to surrounding cells in tissues. In recent years, the higher requirements on biomaterials significantly promote the design and development of aerogel-based biomaterials with high biocompatibility and biological activity. These biomaterials with multilevel hierarchical structures display excellent biological functions by promoting cell adhesion, proliferation, and differentiation, which are critical for biomedical applications. This review highlights and discusses the recent progress in the preparation of aerogel-based biomaterials and their biomedical applications, including wound healing, bone regeneration, and drug delivery. Moreover, the current review provides different strategies for modulating the biological performance of aerogel-based biomaterials and further sheds light on the current status of these materials in biomedical research.
Keywords: aerogels, wound healing, bone regeneration, drug delivery, biomedical application
Introduction
With the enormous progress achieved in clinical science and technology, the requirements on advanced biomaterials with designable structures and functions have become more and more urgent. The use of biomimetic principles and methods is an important and feasible strategy in preparing high-performance biomaterials. Therefore, it is extremely important to explore and understand the natural environment of living cells, which are helpful in the preparation of biomaterials with biomimetic structures and components. In human body, various cells are living in a dynamic extracellular matrix (ECM) environment with a three-dimensional (3D) structure. The formation of 3D structure in ECM is due to the collagen-based self-assembly process which is regulated in part by glycosaminoglycans, proteoglycans, and other proteins.1 ECM not only provides physical support for tissue integrity and elasticity but also regulates adhesion, migration, proliferation, apoptosis, and differentiation of surrounding cells, by serving as ligands for cell receptors such as integrin.2–4 The use of native ECM is of special interest for cell culture and tissue engineering applications, due to the presence of intrinsic regulatory structure and bioactive factors.5 Up to now, many efforts have been made to develop the ECM-like biomaterials through mimicking the biological structures and chemical properties of natural ECM.
Aerogels were firstly produced by Kistler with supercritical drying method in the early 1930s, which is not referred to as a specific material, but a group of materials with extraordinary characteristics.6 The definition of aerogel has been given by IUPAC (international union of pure and applied chemistry) as a “gel comprised of a microporous solid in which the dispersed phase is a gas”.7 With the rapid development in materials science, the types of aerogel recognized as matters with special characteristics have been largely extended, and the method for drying process is not necessary for the identification of aerogels. More materials with porous structure (e.g., xerogel and cryogel) are recognized as aerogel. For instance, the carbon nanotubes aerogels can be directly synthesized by catalytic chemical vapor deposition.8 Therefore, aerogel can be regarded as a state of matter with similar porous structure to solid networks of a gel with gas or vacuum in-between the skeletons.9 Due to the outstanding physicochemical properties, diverse aerogels have been developed and recognized as promising candidates for various applications, such as thermal insulation,10 photocatalysts,11 sensor,12,13 supercapacitor,14,15 and water treatment.16
Presently, clinical treatments have impressively requirements on the generation of functional biomaterials with designable structures. Generally, aerogel can be facilely manufactured by replacing the liquid portion of wet gel materials with gas, by using proper drying technologies. The wet gels prepared by the sol–gel method usually have interconnected nanostructures formed by the assembling process of nanoparticles, polymer, or other nanostructural materials. By this strategy, the microstructures and physical characteristics of aerogels can be obtained; meanwhile, the sizes and shapes of aerogels are preserved from the initial wet gel materials. The resulting solid materials of aerogel are porous and ultralight, which are usually constructed with 3D cross-linked networks and partially resemble the physical characteristics of natural ECM. The porous microstructure in aerogel-based biomaterials is extremely favorable for the absorption and preservation of biological fluids, and features properties of fluid and molecule transport. Meanwhile, aerogel-based biomaterials may interact with adjacent cells, and then modulate diverse cellular functions (e.g., attachment, proliferation, migration, and differentiation).17,18 Especially, aerogel-based biomaterials with a high surface-to-volume ratio can provide 3D porous structure and partial mechanical support for homogeneous/isotropic growth of cells.19 Some specially designated aerogel-based biomaterials can provide biological or chemical/physical stimulation to direct cell growth for tissue engineering and regenerative medicine, which have presented with ideal properties in biomedical research and clinical applications.20–22
Various aerogel-based biomaterials made from macromolecules, inorganic materials, and organic-inorganic composite materials have been developed. Natural macromolecules are ideal choices and have been widely used for the preparation of aerogel-based biomaterials, including cellulose, collagen, gelatin, silk fibroin, chitosan, and so on.23 Meanwhile, the inorganic materials and their composites have also been used for aerogel-based biomaterials, such as hydroxyapatite, silica, graphene.21–24 It is easy to achieve a good biocompatibility by selecting the composition of aerogel-based biomaterials. For example, many aerogel-based biomaterials originated from decellularized tissues have shown the porous structure and high biocompatibility, and have been used to facilitate tissue regeneration in clinical applications.25,26
Over the past decades, the aerogel-based biomaterials that provide similar condition to the ECM environments for cell attachment, proliferation, and differentiation have attracted much interest and represented as a promising class of biomaterials for various biomedical applications, due to their porosity, permeability, high surface area, biocompatibility, and biomimetic structures (Figure 1). This review presents an up-to-date overview of the advances in aerogel-based biomaterials with high porous structure and highlights their major applications in wound healing, bone regeneration, and drug delivery.
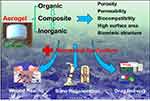 |
Figure 1 The diagrammatic sketch illustrates the aerogel-based biomaterials for biomedical applications. |
Aerogel-Based Biomaterials for Wound Healing
Wound healing is a complex process involving several stages and a range of cells, cytokines, and genes.27–29 There are three phases in the repair of wound, including initial inflammatory phase, proliferative/repair phase, and remodeling phase.30 In the first phase, inflammation cells are rapidly recruited to wounded tissue. The inflammatory phase is critical to wound healing process, which is essential in cleaning contaminating bacteria and creating the facilitated environment for subsequent tissue repair or regeneration.31 In injured tissue, leukocytes (e.g., neutrophils, monocytes, and macrophages) not only remove the damaged cells and clots but also release different growth factors and cytokines which affect the following proliferative/repair phase.32 In proliferative/repair phase, the related cells can grow into the wound gap and displace the residual clots. Then, the process of wound healing could gradually complete after remodeling the ECM in injured tissue.
In this process, the conditions such as hypoxia, pH changes, and microbial growth in the injured tissue may negatively affect treatment outcome of wound healing.33 It is suggested that the suitable biomaterials will be significant to accelerate wound healing, by reducing exudation, encouraging clot formation, and enhancing the anti-microbial activity.34–36 Therefore, biomaterials for wound dressing are expected to absorb exudate and toxic substances, allow gas exchange, and prevent the invasion of microorganisms. The large surface area, highly porous structure, and great permeability make the aerogel-based biomaterials capable of absorbing large amounts of aqueous fluids and providing an adequate moisture balance and pH in the wound site. A range of aerogels made from natural materials have been developed for wound healing, such as nanocellulose, alginate, collagen, and chitin/chitosan. These aerogels with high hydration abilities can minimize the amount of matter applied to wound area, reduce the electrostatic potential of the material, decrease metabolic stress over wounds, and provide specific properties to promote wound healing.37
Nanocellulose-based biomaterials display excellent biocompatibility, biodegradability, and low cytotoxicity. Nanocelluloses that are usually prepared from plants or bacteria are economically competitive biomaterials, including cellulose nanocrystals (CNC) and cellulose nanofibrils (CNF). These nanocellulose-based materials have been suggested for various biomedical applications, such as orthopedic and dental implants, drug carriers, vascular grafts, and wound dressings.38 Recently, a number of studies have reported the fabrication of nanocellulose-based aerogels with different functions.39,40 For example, Nordli et al41 reported the preparation of ultrapure CNF-based aerogels using sodium hydroxide followed by TEMPO-mediated oxidation. The CNF aerogel has a low endotoxin level (45 EU/g) and great water absorbing capacity (4–5 times higher than that of alginate-based wound dressings). It has been widely accepted that the biomaterials used for human should be free of endotoxin.42 The results of cytotoxic assessments towards human epidermal keratinocytes and fibroblasts indicated that the CNF-based aerogels are equally safe as the commercially wound dressing (AquaCel®). These studies confirm that the CNF-based aerogels are excellent candidates for wound dressing.
Collagen is the main structural protein of the ECM in connective tissues. Various collagen and its composite-based biomaterials have been developed for wound healing. Recently, some molecules have been used as cross-linkers or functional additive agents for collagen-based biomaterials to achieve the excellent characteristic for wound healing and tissue regeneration.43 Dharunya et al44 reported a curcumin cross-linked collagen aerogel system with enhanced physical and mechanical properties, anti-proteolytic activity, and pro-angiogenic efficacy. The curcumin cross-linked collagen aerogel displayed a 3D microstructure, and the addition of curcumin did not influence the structure of aerogel. These porous structures not only enhanced the permeability and water-retaining ability but also promoted cell adhesion and proliferation. The pro-angiogenic properties and controlled anti-proteolytic activity of collagen-curcumin aerogels augmented their applications in biomedical field including restoration of damaged tissues. Govindarajan et al45 reported the progress of the wheat grass bioactive compounds-reinforced collagen-based aerogel system as an instructive scaffold for collagen turnover and angiogenesis for wound healing applications. As shown in Figure 2, the study illustrated the therapeutic efficacy of bioactive compounds from wheat grass for wound healing. Wheat grass extract contains several bioactive constituents and has been reported to display potent antibacterial and antioxidant effects, which are beneficial for wound healing.45,46 The bioactive compounds from wheat grass enhance the physicochemical and biomechanical properties of collagen aerogels with a 3D porous architecture similar to natural ECM. The sustained transportation of bioactive compounds across the aerogels to the cellular microenvironment displays antimicrobial and pro-angiogenic properties for enhanced wound healing.
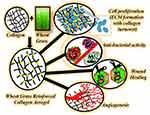 |
Figure 2 The wheat grass bioactive compounds-reinforced collagen-based aerogel system as an instructive scaffold for collagen turnover and angiogenesis for wound healing applications. Note: Reprinted with permission from Govindarajan D, Durabandy N, Sriyatsan KV, et al. Fabrication of hybrid collagen aerogels reinforced with wheat grass bioactives as instructive scaffolds for collagen turnover and angiogenesis for wound healing applications. ACS Appl Mater Interfaces. 2017;9(20):16940–16951. Copyright (2017) American Chemical Society.45 |
Chitosan, as a natural linear polysaccharide, can be obtained from shells of shrimp and other crustaceans and is abundant, widely available, and inexpensive materials. Chitosan-derived biomaterials have shown a promising commercial potential in wound healing, due to its adhesive nature and antimicrobial activity. A recent study has demonstrated that chitosan can avoid gram-negative and gram-positive bacteria growth, accelerate blood coagulation, and promote tissue regeneration.47 Radwan-Praglowska et al48 reported a microwave-assisted strategy for the synthesis of antioxidant chitosan aerogels which are further functionalized with Tilia platyphyllos extract. The as-prepared chitosan aerogels were demonstrated to significantly promote cell proliferation and inhibit the growth of Staphylococcus aureus. López-Iglesias et al49 reported chitosan-based aerogels as a potential approach to treat chronic wounds and to prevent infection, which have loaded with vancomycin. The chitosan aerogels showed fibrous and porous microstructures with high porosities (> 96%) and large surface areas (> 200 m2/g). Chitosan aerogels with vancomycin presented remarkable antibacterial effects, whereas, no significant negative effects on collagenase activity and the normal physiological process of wound healing. Concha et al37 reported highly neutralized, porous, and ultralight polymeric aerogels which were prepared from aqueous colloidal suspensions of chitosan and chondroitin sulfate (CS/ChS) nanocomplexes. The CS/ChS aerogels were light and soft, with facilitated mechanical properties for the application in open wounds. The CS/ChS aerogels were easily adapted to the wound contour due to their low toughness and hydration abilities. It was demonstrated that the CS/ChS aerogels did not affect the in vitro metabolic activity of 3T3 fibroblasts, and the rapid adaptation to the wound bed can significantly induce the early closure of open wounds (Figure 3). The applications of CS/ChS aerogels for would healing were based on their safety, low cost, and easiness to handle. The CS/ChS aerogels could also improve wound bed quality, granulation tissue, and suppress pain.
 |
Figure 3 Preparation and application of aerogel in the closure of experimental wounds. (A) Step‐by‐step addition of equimolar amounts of both CS/ChS as model of complexation; (B) colloidal suspensions; and (C) aerogel after freeze-drying. (D) Wound closure in an animal model. The representative images demonstrated the progress of experimental wound closure. Open wounds of 0.80 cm2 in diameter made on the back of a rabbit received CS/ChS aerogels (right) and normal saline solution (left). The wounds were subsequently photographed on the days indicated in the figure.Notes: Reproduced with permission from Concha M, Vidal A, Giacaman A, et al.Aerogels made of chitosan and chondroitin sulfate at high degree of neutralization: biological properties toward wound healing. J Biomed Mater Res B. 2018;106(6):2464–2471. © 2018 Wiley Periodicals, Inc.37 |
Apart from the natural macromolecules-based biomaterials, inorganic and composite aerogels have also been prepared and applied for wound healing. It has been reported that inorganic and composite aerogels have remarkable absorption capacity and hemostatic performance. Quan et al50,51 reported that cross-linked graphene aerogels could not only absorb plasma quickly but also induced rapid blood coagulation by absorbing erythrocytes and platelets. The cross-linked graphene aerogels promoted clotting by immediately absorbing plasma (<40 ms) and forming a blood cell layer. Thereafter, the enrichment of hemocytes and platelets on the wound surface leads to fast blood coagulation. Mellado et al52 prepared dry and stable composite aerogels with graphene oxide (GO) and polyvinyl alcohol (PVA) by a sol-gel method, and incorporated the natural extracts of País grape into the aerogels (Figure 4). The PVA strengthened the porous structure of the composite aerogels with grape extracts. The aerogels exhibited great stability and rapid water absorption capacity, showing excellent coagulation and bioactive compounds capacity. Considering the remarkable hemostatic performance, simple preparation process, low cost, and nontoxicity, these new GO-based aerogels have displayed great potential in the management of trauma bleeding, wound healing and dermal delivery.
Wound healing involves complex mechanisms in the human body, such as multiple cellular pathways and different molecules. Biomaterials with single constituent or function are difficult to successfully solve all the problems occurred in the wound healing process. The reparative process in wound requires different functions of biomaterials in different phases, such as protection, absorption of exudate, encouraging clot formation, microbial resistance, and so on. Therefore, the applications of drug-loaded and composite aerogels are considered promising strategies for wound healing. The aerogels can provide a fast local administration of the growth factors, antibiotics, and other drugs at the wound site to promote tissue regeneration and prevent infections after wound debridement. On the other hand, inorganic nanoparticles (e.g., mesoporous silica nanoparticles and silver-based nanomaterials) with good biological properties have been widely used for wound healing.53–56 The mesoporous silica nanoparticles have a porous structure which is suitable for drug loading and release. The silver-based nanomaterials are common antibacterial materials, which have displayed favorable effects in inhibitingboth gram-negative and gram-positive bacteria. Therefore, the applications ofdrug-loaded mesoporous silica nanoparticles and silver-based nanomaterials may obviously shorten the wound healing time and inhibit the pathological inflammation.56
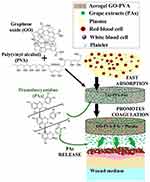 |
Figure 4 Composite aerogels from graphene oxide (GO) and polyvinyl alcohol (PVA) incorporated with natural extracts of País grape seed and skin by a sol-gel method. These porous GO-reinforced PVA aerogels with the inclusion of PAs promoted the coagulation on the wound surface, showing great potential in dermal, hemostatic, and wound healing applications. Note: Reprinted with permission from Mellado C, Figueroa T, Baez R, et al. Development of graphene oxide composite aerogel with proanthocyanidins with hemostatic properties as a delivery system. ACS Appl Mater Interfaces. 2018;10(9):7717–7729. Copyright (2018) American Chemical Society.52 |
Aerogel-Based Biomaterials for Bone Regeneration
Bones constitute the vertebrate skeleton, which not only support and protect the body but also are the place to produce red and white blood cells, and store minerals. Bones are strong and hard, due to the complex internal and external microstructures. They are formed with a composite of ~70% mineral (mostly hydroxyapatite (HA) nanocrystals) and ~30% organics (e.g., collagen and glycoproteins) by dry weight.57,58 Cancellous bone has porous structure (porosity 40–90%) made of a connected network of mineralized organic matrix.59,60 These biomineralized networks in the cancellous bone simultaneously provide large specific surface area, interconnected pores, and excellent mechanical properties. Aerogels-based biomaterials have 3D cross-linked networks that partially mimic the natural microstructure of cancellous bone. On the other hand, synthesized HA-based biomaterials display high biocompatibility, degradability, and bioactivity similar to the inorganic constituent of bones, making them excellent candidates for bone tissue engineering/repair applications.61
The rich diversities of natural structures have been extensively investigated in recent decades, providing inspirations as well as challenges for the fabrication of various biomaterials.62 One-dimensional structure particularly biomimetics the native structure of mineralized collagen fibrils, and may be practically used in the fabrication of HA-based biomaterials. In recent years, a novel solvothermal strategy has been applied to synthesis HA nanowires and microtubes with a great length to diameter ratio, and the length of these HA nanowires and microtubes can be up to several tens of micrometers.63–65 These HA nanowires and microtubes have been further manufactured into various materials with different structures, including ordered structures and aerogel structures.66–69 For example, to mimic the microstructures of cancellous bone, Zhang et al69 applied a new strategy to prepare ultralight HA nanowire aerogels with 3D-interconnected and porous meshwork microstructure (Figure 5). The HA nanowire aerogels presented an ultrahigh porosity (∼99.7%), ultralow density (8.54 mg/cm3), and high elasticity. These HA nanowire aerogels can be used in air filter and continuous oil–water separation. Significantly, these HA nanowire aerogels exhibited a highly porous structure, which is similar to the porous meshwork of the cancellous bones in both nanoscale and macroscale. Therefore, the HA nanowire aerogel is regarded as highly promising for biomedical applications, especially for bone repair or tissue engineering.
Comparing with common HA materials (e.g., HA nanoparticle), one-dimensional HA materials with big aspect ratio (e.g., HA nanowire) usually display good flexibility and can be used for preparing composite scaffolds with collagen or other macromolecules to mimic the structure of natural bone. Sun et al70 reported HA nanowires/collagen-based porous scaffold which was prepared by a freeze drying method and subsequent chemical crosslinking. The HA nanowires/collagen aerogel scaffold had good mechanical properties and can promote the adhesion and spreading of mesenchymal stem cells. Moreover, the in vivo experiments revealed that the HA nanowires/collagen-based porous scaffold significantly enhanced the regeneration of bone defects. Sun et al71 also reported zinc‐containing nanoparticle‐decorated HA nanowires which were prepared by a one‐step solvothermal method. As shown in Figure 6, the zinc‐containing HA nanowires have shown hierarchical rough surface and could be well incorporated into composite porous scaffolds with chitosan by a freeze‐drying method. The composite porous scaffolds with enhanced mechanical properties not only induced the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells (rBMSCs) but also promoted the regeneration of bone defects.
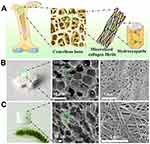 |
Figure 5 (A) Schematic illustration of the microstructure of the cancellous bone. (B) Representative images and SEM micrographs of the cancellous bone from the femur of a mature New Zealand white rabbit. (C) Representative images and SEM micrographs of the HA nanowire aerogel. The HA nanowire aerogel exhibits a highly porous structure, which is similar to the porous meshwork of the cancellous bone in both nanoscale and macroscale. Note: Reprinted with permission from Zhang Y-G, Zhu Y-J, Xiong Z-C, Wu J, Chen F. Bioinspired ultralight inorganic aerogel for highly efficient air filtration and oil-water separation. ACS Appl Mater Interfaces. 2018;10(15):13019–13027. Copyright (2018) American Chemical Society.69 |
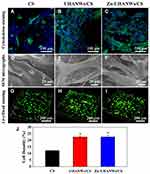 |
Figure 6 Cell adhesion and proliferation on the porous scaffolds. (A–C) Cytoskeleton staining and (D–F) SEM images of rBMSCs on the scaffolds after 3 days of culture: (A) and (D) CS, (B) and (E) UHANWs/CS, and (C) and (F) Zn‐UHANWs/CS; (G–I) live/dead cell staining (green for live cells, and red for dead cells) of rBMSCs on the scaffolds after 7 days of culture: (G) CS, (H) UHANWs/CS, and (I) Zn‐UHANWs/CS; (J) the live cell densities (defined as the green area/total area×100%) on the porous scaffolds of CS, UHANWs/CS, and Zn‐UHANWs/CS. *p<0.05 compared with the CS scaffold group.Note: Reproduced with permission from Sun T-W, Yu W-L, Zhu Y-J, et al. Porous nanocomposite comprising ultralong hydroxyapatite nanowires decorated with zinc-containing nanoparticles and chitosan: synthesis and application in bone defect repair. Chem Eur J. 2018;24(35):8809–882. © 2018 Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim.71 |
Moreover, Zhang et al68 reported porous and ultralight HA microtube-based ceramic aerogels with an ordered structure. Firstly, HA microtubes with a single HA phase, monodisperse and high biocompatibility were successfully synthesized by a one-step solvothermal method. Then, HA microtube-based ceramic aerogels with porous and ordered structures were prepared through a freeze-drying method and subsequent sintering at 1300°C under an air atmosphere. The unique tubular structure has been well preserved in HA microtube ceramic aerogels after sintering. The HA microtube aerogels displayed high porosities (89%-96%), ultralow densities (94.1 to 349.9 mg/cm3), high compressive strengths (up to 1 MPa), interconnected tubular channels, high permeability, and excellent biocompatibility. Meanwhile, the monodispersed HA microtubes could be well blended with chitosan to prepare porous composite scaffolds by a freeze-drying method.72 The HA microtubes greatly improved the mechanical properties of the composite scaffolds, comparing with the pure chitosan scaffolds and chitosan–HA nanorod composite scaffolds. Meanwhile, the hollow structure of HA microtubes was propitious to drug-loading. The HA microtubes-chitosan composite scaffold displayed a good drug-loading capacity, sustained drug release behavior, and antibacterial activity. The ultralong microtube was a new structure of HA-based materials, displaying great potential for biomedical applications. The HA microtube-based ceramic aerogels and composite porous scaffolds have displayed distinguished physical, chemical, and biological properties, compared with other reported HA materials, and might be promising candidates for further applications in bone regenerative medicine.
The biomaterials with multifunction such as high biocompatibility, biodegradability, bioactivity, and drug delivery properties are promising in bone repair applications. HA nanowires have displayed potentials for further functionalization. For example, the HA nanowire@magnesium silicate nanosheets (HANW@MS) core-shell porous hierarchical nanocomposites have been prepared by a simple template method.73 Comparing with HA nanowires, the core-shell porous hierarchical nanostructures exhibited remarkably increased specific surface areas and pore volumes, which were suitable for drug loading and sustained release. The porous scaffold was subsequently prepared by incorporating the HANW@MS into the chitosan (CS) matrix. The resultant HANW@MS/CS scaffolds not only promoted cell attachment and growth but also stimulated the osteogenic and angiogenic differentiation of stem cells. Moreover, the HANW@MS/CS porous scaffolds significantly induced the in vivo bone regeneration, owing to the high bioactive performance (Figure 7). Apart from the one-dimensional structure, the self-assembling HA materials have also been fabricated into aerogel-based porous scaffolds. Copper (Cu)-doped mesoporous HA microspheres (Cu-MHMs) have been synthesized by a microwave-assisted hydrothermal method and demonstrated a hierarchical structure and a highly specific surface area.74 The Cu-MHMs were then incorporated into a scaffold with CS macromolecule, which maintained a high porosity and a property to continuously release Cu ions. Importantly, the in vivo experiment suggested that the Cu-MHM/CS porous scaffolds enhanced bone regeneration by simultaneously inducing osteogenesis and angiogenesis of injured tissue, which was favorable for the reconstruction of vascularized tissue-engineered bone.
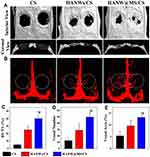 |
Figure 7 Micro-CT assessment of newly formed bone and blood vessels in rat calvarial defect regions after implantation of scaffolds of CS, HANWs/CS, and HANW@MS/CS for 12 weeks. (A) Three-dimensional (3-D) and coronal views of reconstructed calvarial images. (B) Newly formed blood vessels presented by 3-D reconstructed images. Morphometric analysis was completed for the percentage of newly formed bone volume (BV/TV) (C), blood vessel number (D), and blood vessel area (E) in the defects. *p < 0.05 compared to CS scaffold; #p < 0.05 compared to HANWs/CS scaffold. Note: Reprinted with permission from Sun T-W, Yu W-L, Zhu Y-J, et al.Hydroxyapatite nanowire@magnesium silicate core-shell hierarchical nanocomposite: synthesis and application in bone regeneration. ACS Appl Mater Interfaces. 2017;9(19):16435–16447. Copyright (2017) American Chemical Society.73 |
Recently, more and more biomaterials have been developed for bone-related studies apart from the HA-based aerogel biomaterials. Strontium (Sr)-doped amorphous calcium phosphate porous microspheres (SrAPMs) with a higher specific surface area than normal HA materials were synthesized using fructose 1,6-bisphosphate trisodium salt. Then, the biomimetic SrAPMs based porous scaffolds with a morphology of aerogel were further constructed with collagen, which were applied as antibiotic carriers to induce bone regeneration.75 Weng et al76 reported ultralight 3D composite nanofiber aerogels which were composed of electrospun PLGA-collagen-gelatin and Sr–Cu co-doped bioactive glass fibers, with incorporation of heptaglutamate E7 domain-specific BMP‐2 (E7‐BMP‐2) peptides. A sustained release of E7‐BMP‐2 peptide from the degradable aerogels significantly enhanced bone healing and defect closure over 8 weeks, in comparison to blank control. This study for the first time demonstrated the fabrication of 3D nanofiber aerogels from 2D electrospun fibers with therapeutic osteoinductive BMP‐2 mimicking peptides for cranial bone tissue regeneration. Recently, Li et al77 developed a sugarcane aerogel derived borate glass scaffolds, through an in situ bioglass modification strategy (Figure 8). The multilevel structure of this Gramineae plant was maintained in the borate glass scaffolds. As a result, the as-prepared 3D scaffolds highly resemble the element compositions and structures of native bones. Moreover, the results of in vivo implantation indicated that the microstructural orientation of this 3D scaffold significantly promotes the regeneration of bone defect.
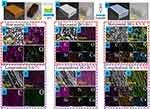 |
Figure 8 (A) Optical image of sugarcane aerogel (SA); (B–D) SEM and EDS of transverse section of the sugarcane aerogel (Horizontal SA/III); (E–G) SEM and EDS of longitudinal section of the sugarcane aerogel (Vertical SA/IV); (H) Optical image of sugarcane derived borate bioglass scaffolds (SBBS); (I–K) SEM and EDS of transverse section of the SBBS (Horizontal BG-B/V); (L–N) SEM and EDS of longitudinal section of the SBBS (Vertical BG-B/VI); (O) Optical image of the cured SBBS; (P–R) SEM and EDS of transverse section of the cured SBBS (Horizontal BG-A/VII); (S–U) SEM and EDS of longitudinal section of the cured SBBS (Vertical BG-A/VII).Note: Reprinted with permission from Li T, Ai F, Shen W, et al. Microstructural orientation and precise regeneration: a proof-of-concept study on the sugar-cane-derived implants with bone mimetic hierarchical structure. ACS Biomater Sci Eng. 2018;4(12):4331–433. Copyright (2018) American Chemical Society.77 |
Aerogel-Based Biomaterials for Drug Delivery
Due to the tuned and controlled structures with high surface areas, aerogel-based biomaterials have been considered as a promising candidate for drug loading and delivery. In order to be applied in different applications, aerogels can be synthesized in various shapes and surface properties. Three steps are generally included for synthesizing aerogel-based carriers for drug delivery, including sol-gel process, aging, and drying.78 Aerogels can increase the bioavailability of poorly soluble drugs, and improve both the stability and release kinetics of drugs due to the high surface area. In the last decades, the research of aerogel-based biomaterials has made significant progress in pharmaceutical sciences. Various inorganic, organic, and composite aerogels have been developed and possess huge potential in different drug delivery applications.79
Polysaccharides from plants have been widely applied in various fields and presented as an alternative to non-renewable synthetic polymers from fossil. Polysaccharide-based aerogels are attractive candidates as drug carriers due to their exceptional biocompatibility, biodegradability, porous structure, large surface area, and drug-loading capacity.80 For example, aerogel microspheres made from starch, pectin, and alginate have been prepared and used to load and release different drugs.81,82 Significantly, these studies point out the possibility of tuning drug loading and release kinetics by choosing suitable polysaccharide-based aerogels.
The starch-based aerogels with excellent biocompatibility and biodegradability have been widely used in drug delivery, due to a wide range of sources and the porous structure.83–85 Pawar et al86 reported a starch-based drug delivery material which was developed by converting native starch from hydrogel to aerogel. This starch-based aerogels improved the dissolution and oral bioavailability of a poorly water-soluble antifungal drug of Itraconazole. Marco et al87 reported a maize starch-based aerogel which was suitable for loading vitamin E and vitamin K3 using supercritical CO2 adsorption. The loading rate of two poorly water-soluble vitamins was about 95–98%, and the dissolution rate was also largely improved.
Carrageenan, as an anionic polysaccharide with high availability, thermo-stability, and biocompatibility, offered many advantages in drug delivery. Alnaief et al88 produced aerogel-based microparticles using three different commercial carrageenan. The biodegradable carrageenan aerogel-based microparticles had high specific surface area (ranging between 33 and 174 m2/g), and good pore volumes and sizes. Obaidat et al89 reported a emulsion-gelation technique to prepare carrageenan aerogel-based microparticles, and the drug of Ibuprofen was successfully loaded in these porous microparticles. These aerogel-based microparticles displayed significantly enhanced drug release properties. Salgado et al90 produced barley and yeast β-glucans aerogels by a gelation process in aqueous solution, followed by solvent exchange and drying with supercritical CO2, which were used for acetylsalicylic acid loading and release. Lopez-Iglesias et al49 reported chitosan-based aerogel beads with a fibrous structure, which showed high porosity (>96%) and large surface area (>200 m2/g). This chitosan-based aerogel beads are applied to load vancomycin drug for chronic wounds. Veres et al91 reported aerogel beads (d=3–5 mm) of iron(III)-crosslinked alginate, and loaded with ibuprofen through an adsorptive deposition process from supercritical CO2. The pH of the medium and the size of aerogel particles showed significant effects on drug release, and the incorporation of ascorbic acid also accelerated the drug release. Different methods were developed to prepare nanostructured aerogel particles with controlled release profile and aerodynamic performance of relevance for oral inhalation purposes. Lopez-Iglesias et al92 developed alginate-based aerogel microspheres through thermal inkjet printing followed by supercritical drying. These aerogel microspheres were used for sustained pulmonary drug delivery to manage and treat asthma attacks and chronic obstructive pulmonary diseases.
The natural polymer of cellulose usually has a low density and high porosity, biocompatibility, and biodegradability, which have huge pharmaceutical potentials in drug delivery and other biomedical applications.93 The cellulose aerogels can be prepared by sol–gel process and gel drying, using various sources of cellulose including nanocellulose, bacterial cellulose, regenerated cellulose, and cellulose derivatives. For example, nanocellulose-based materials have an extremely high surface area, hygroscopic, and swelling tendency, leading to a maximum drug-loading performance. Meanwhile, the floating tendency, swelling ability, and the mucoadhesive property of nanocellulose aerogel also induced the controllable drug release property, making it possible to release drugs through skin penetration and implant coating.94 The cellulose-based composite or grafted aerogels have also been investigated as drug carriers. Wang et al95 reported complex aerogels made from chitosan, carboxymethyl cellulose, and graphene oxide, which were synthesized using calcium ion as the crosslinker. The sustained release of 5-fluorouracil from the complex aerogels was complied with a drug release model of Fickian diffusion. Furthermore, this complex aerogel displayed pH-responsive drug release properties, due to the pH sensitivity of chitosan and cellulose, which could be further developed as an effective chemotherapeutic agent in tumor treatment. Zhao et al96 reported aerogels of polyethylenimine-grafted cellulose nanofibrils (CNFs-PEI) as a new drug delivery system (Figure 9). The CNFs-PEI aerogels had a high drug-loading capability (287.39 mg/g) using a water-soluble drug of sodium salicylate (NaSA), and this drug-loading process was well complied to Langmuir isotherm and pseudo-second-order kinetics models. Thereafter, a sustained and controlled drug release behavior of these CNFs-PEI aerogels was observed, which was highly dependent on pH and temperature. Considering the unique pH- and temperature-responsive drug release properties and high biodegradability and biocompatibility, the CNFs-PEI aerogels were promising candidates in controlled drug delivery.
 |
Figure 9 Schematic illustration of (A) the preparation process of the polyethylenimine (PEI) grafted cellulose nanofibrils (CNFs) aerogels and (B) the reaction mechanism of the grafting process. Note: Reprinted with permission from Zhao J, Lu C, He X, Zhang X, Zhang W, Zhang X. Polyethylenimine-grafted cellulose nanofibril aerogels as versatile vehicles for drug delivery. ACS Appl Mater Interfaces. 2015;7(4):2607–2615. Copyright (2015) American Chemical Society.96 |
Apart from the aforementioned macromolecule-based aerogels, the inorganic aerogels have also been studied as drug carriers. Silicon-based aerogels are the most popular inorganic aerogels, and numerous researches have proved that silicon aerogels have outstanding performances in delivering both hydrophobic and hydrophilic drugs.97–100 The physical characteristics (e.g., pore diameter, surface area, and micropore volume) significantly affect the density and the drug-loading capacity of silica aerogels.101 Therefore, further studies on the design and structural control of silica aerogels are warranted for developing suitable physical characteristics for drug delivery. Zhang et al102 developed a surfactant-free method to prepare silica aerogel microspheres with hierarchically porous structure, which had a huge packing density ranging from 62 to 230 mg/cm3 and BET surface area ranging from 800 to 960 m2/g. This low density and high BET surface area might benefit their drug loading and release. Rajanna et al103 described a method to produce hollow silica aerogel microspheres (HSAMs) and granular silica aerogel microparticles (GSAMs) for drug delivery, using an inexpensive and biocompatible silica source of rice husk ash (RHA). This method involved a two-step sol–gel process, solvent exchange and subsequently drying with supercritical CO2. The HSAMs showed high loading capacity of ibuprofen (0.47 g per g of HSAM). Furthermore, a fast drug release profile of ibuprofen from this HSAMs system was observed, indicating that HSAMs were good carriers for drug delivery. Afrashi et al104 compared the drug loading and release properties of silica-aerogel, poly (vinyl alcohol) nanofibers and films. The result of drug (fluconazole) release experiment indicated that the silica aerogel had faster drug release performance than other samples, due to high surface area and porosity. About 80% of the loaded drugs on silica aerogels were released within 60 min, while the same amount of the pure drug of fluconazole was released in 225 min. The high drug-loading capacity and fast release kinetics confirmed that silica-aerogel is suitable for drug delivery in certain applications.105
Composite aerogels bring the advantages of inorganic and organic materials together, resulting in promising carriers with controlled properties for drug delivery. For example, silica-gelatin composite aerogels, as new drug delivery devices, were not only nontoxic but also affect the cells behavior.106 Veres et al107 reported a silica-gelatin composite aerogel (3 wt.% gelatin content), and a strong hydration of the silica-gelatin skeleton could be facilitated with rapid desorption and dissolution of loaded drugs from aerogel. Follmann et al108 reported organic–inorganic composite aerogel with one-dimensionally aligned pores, which were achieved by dispersing mesoporous silica nanoparticles in a polymer solution and freeze-drying. These aerogels were demonstrated as sustained and prolonged release systems for a hydrophobic drug, with high performance on encapsulation efficiency (>75%) and sustained drug release (over 50 days or more). These new composite aerogels could be highly appealing biomaterials for drug delivery.
Conclusion and Outlook
Aerogels, being ultra-light materials, have cross-linked 3D structures which are constructed with polymers, inorganic materials, and composites. Aerogels display high porosity with excellent surface area and partially resemble the physical characteristics of natural ECM. Therefore, aerogel-based biomaterials may interact with adjacent cells, and then modulate diverse cellular functions such as attachment, migration, and differentiation. The porous 3D structure in aerogel-based biomaterials may enable cells attachment and growth in both planar and perpendicular directions, which avoid restricting polarity of cells. Meanwhile, the biocompatibility and biodegradation of aerogel-based biomaterials can be well regulated through the selection of substances. With porous structure and high permeability, aerogel-based biomaterials have attracted growing interest in the biomedical field. This review highlights the recent advances of aerogel-based biomaterials and their biomedical applications including wound healing, bone regeneration, and drug delivery, expecting to help researchers understand the current status and tendency of aerogel-based biomaterials in biomedical application.
Aerogel-based biomaterials with high porosity are propitious to absorbed large amounts of aqueous fluids, and to provide adequate moisture balance and pH for promoting wound healing. The loading of drugs in the aerogel-based biomaterials can provide a fast local administration of the growth factors and antibiotics at the wound site to promote tissue regeneration and prevent infections. Through the selection of substances and the additives of functional components or drugs, aerogels could realize a series of functions to shorten wound healing time and inhibit the pathological inflammation, such as protection, absorption, coagulation, resistance, duration, and antimicrobial activity. Meanwhile, aerogel-based biomaterials made from HA and its composite can simultaneously mimic the structure and chemical properties of bone, and have promising prospects in the applications of bone regeneration. Synthesized HA and its composite-based aerogel-based biomaterials display high biocompatibility, degradability, and bioactivity. These aerogels simulate the biomineralized networks in the cancellous bone, and provide large specific surface area, interconnected pores for cell growth, which is regarded as highly promising for bone repair or tissue engineering. Furthermore, aerogel-based biomaterials are also displaying high performance in drug delivery. With the tuned and controlled porous structures, aerogel-based biomaterials improve the wettability and reduced crystallinity of drugs, and raise the bioavailability of poorly soluble drug by improving both stability and release kinetics of drugs. With these superiorities, aerogel-based biomaterials will be promising candidates for drugs/proteins loading and delivery.
Although some significant progress have been made in the preparation and application of aerogel-based biomaterials, there are still some problems that need to be solved urgently. At present, most of the studies in aerogel-related fields still focus on the preparation strategy and performance investigation, including the regulation on the structures or properties. There is relatively little research conducted on the aerogel-related molecular dynamics theory or computational materials science. Meanwhile, the components and functions of aerogel-based biomaterials should meet the strict requirements in biomedical application. It is an important strategy for designing and preparing the functional aerogel-based composite biomaterials by selecting different components and exploring their synergistic effects. Aerogels have high porosity, which usually leads to insufficient mechanical properties. For example, aerogels-based biomaterials are often brittle and have low strength. The strategy for preparing aerogel-based biomaterials with controllable elasticity and strength is very meaningful. Although there are many problems that need to be studied, as a new functional material system, aerogel-based biomaterials have already shown promising application prospects in the field of biomedicine.
Acknowledgments
The financial support from the National Natural Science Foundation of China (31771081, 81700969), the Science and Technology Commission of Shanghai Municipality (18ZR1445100, 19441901900, 19ZR1439700), the National Health and Medical Research Council (NHMRC) Early Career Fellowship (1105035) is gratefully acknowledged. Longpo Zheng and Shaodi Zhang are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Brightman AO, Rajwa BP, Sturgis JE, McCallister ME, Robinson JP, Voytik-Harbin SL. Time-lapse confocal reflection microscopy of collagen fibrillogenesis and extracellular matrix assembly in vitro. Biopolymers. 2000;54(3):222–234. doi:10.1002/(ISSN)1097-0282
2. Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–1219. doi:10.1126/science.1176009
3. Dalby MJ, Garcia AJ, Salmeron-Sanchez M. Receptor control in mesenchymal stem cell engineering. Nat Rev Mater. 2018;3(3):17091. doi:10.1038/natrevmats.2017.91
4. Hussey GS, Dziki JL, Badylak SF. Extracellular matrix-based materials for regenerative medicine. Nat Rev Mater. 2018;3(7):159–173.
5. Robb KP, Shridhar A, Flynn LE. Decellularized matrices as cell-instructive scaffolds to guide tissue-specific regeneration. ACS Biomater Sci Eng. 2018;4(11):3627–3643. doi:10.1021/acsbiomaterials.7b00619
6. Kistler S. Coherent expanded aerogels and jellies. Nature. 1931;127(3211):741. doi:10.1038/127741a0
7. McNaught AD, Wilkinson A. International Union of Pure and Applied Chemistry. Compendium of Chemical Terminology: IUPAC Recommendations.
8. Aliev AE, Oh J, Kozlov ME, et al. Giant-stroke, superelastic carbon nanotube aerogel muscles. Science. 2009;323(5921):1575–1578. doi:10.1126/science.1168312
9. Du A, Zhou B, Zhang Z, Shen J. A special material or a new state of matter: a review and reconsideration of the aerogel. Materials. 2013;6(3):941–968. doi:10.3390/ma6030941
10. Du A, Wang HQ, Zhou B, et al. Multifunctional silica nanotube aerogels inspired by polar bear hair for light management and thermal insulation. Chem Mater. 2018;30(19):6849–6857. doi:10.1021/acs.chemmater.8b02926
11. Wan WC, Zhang RY, Ma MZ, Zhou Y. Monolithic aerogel photocatalysts: a review. J Mater Chem A. 2018;6(3):754–775. doi:10.1039/C7TA09227J
12. Mi HY, Jing X, Cai ZY, Liu YJ, Turng LS, Gong SQ. Highly porous composite aerogel based triboelectric nanogenerators for high performance energy generation and versatile self-powered sensing. Nanoscale. 2018;10(48):23131–23140. doi:10.1039/C8NR05872E
13. Wei N, Ruan LM, Zeng W, et al. Compressible supercapacitor with residual stress effect for sensitive elastic-electrochemical stress sensor. ACS Appl Mater Interfaces. 2018;10(44):38057–38065. doi:10.1021/acsami.8b12745
14. Li LY, Lu FX, Wang C, et al. Flexible double-cross-linked cellulose-based hydrogel and aerogel membrane for supercapacitor separator. J Mater Chem A. 2018;6(47):24468–24478. doi:10.1039/C8TA07751G
15. Shabangoli Y, Rahmanifar MS, El-Kady MF, Noori A, Mousavi MF, Kaner RB. Thionine functionalized 3D graphene aerogel: combining simplicity and efficiency in fabrication of a metal-free redox supercapacitor. Adv Energy Mater. 2018;8(34):1802869. doi:10.1002/aenm.201802869
16. Yuan DS, Zhang T, Guo Q, Qiu FX, Yang DY, Ou ZP. Recyclable biomass carbon@SiO2@MnO2 aerogel with hierarchical structures for fast and selective oil-water separation. Chem Eng J. 2018;351:622–630. doi:10.1016/j.cej.2018.06.132
17. Maleki H, Duraes L, Garcia-Gonzalez CA, Del Gaudio P, Portugal A, Mahmoudi M. Synthesis and biomedical applications of aerogels: possibilities and challenges. Adv Colloid Interfac. 2016;236:1–27. doi:10.1016/j.cis.2016.05.011
18. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Bio. 2014;15(12):786–801. doi:10.1038/nrm3904
19. Amani H, Arzaghi H, Bayandori M, et al. Controlling cell behavior through the design of biomaterial surfaces: a focus on surface modification techniques. Adv Colloid Interfac. 2019;6(13):1900572.
20. Kuttor A, Szaloki M, Rente T, et al. Preparation and application of highly porous aerogel-based bioactive materials in dentistry. Front Mater Sci. 2014;8(1):46–52. doi:10.1007/s11706-014-0231-2
21. Vrettos K, Karouta N, Loginos P, Donthula S, Gournis D, Georgakilas V. The role of diamines in the formation of graphene aerogels. Front Mater. 2018;5:20. doi:10.3389/fmats.2018.00020
22. Amani H, Mostafavi E, Arzaghi H, et al. Three-dimensional graphene foams: synthesis, properties, biocompatibility, biodegradability, and applications in tissue engineering. ACS Biomater Sci Eng. 2019;5(1):193–214. doi:10.1021/acsbiomaterials.8b00658
23. Barrios E, Fox D, Sip YYL, et al. Nanomaterials in advanced, high-performance aerogel composites: a review. Polymers. 2019;11(4):726. doi:10.3390/polym11040726
24. Sani NS, Malek NANN, Jemon K, Kadir MRA, Hamdan H. Preparation and characterization of hydroxyapatite incorporated silica aerogel and its effect on normal human dermal fibroblast cells. J Sol-Gel Sci Techn. 2019;90(2):422–433. doi:10.1007/s10971-019-04946-z
25. Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. 2017;49:1–15. doi:10.1016/j.actbio.2016.11.068
26. Stergar J, Maver U. Review of aerogel-based materials in biomedical applications. J Sol-Gel Sci Techn. 2016;77(3):738–752. doi:10.1007/s10971-016-3968-5
27. Horsley V, Watt F. Repeal and replace: adipocyte regeneration in wound repair. Cell Stem Cell. 2017;20(4):424–426. doi:10.1016/j.stem.2017.03.015
28. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–321. doi:10.1038/nature07039
29. Bando T, Yokoyama H, Nakamura H. Wound repair, remodeling, and regeneration PREFACE. Dev Growth Differ. 2018;60(6):303–305. doi:10.1111/dgd.2018.60.issue-6
30. Rosique RG, Rosique MJ, Farina Junior JA. Curbing inflammation in skin wound healing: a review. Int J Inflam. 2015;2015:316235. doi:10.1155/2015/316235
31. Mercado AM, Quan N, Padgett DA, Sheridan JF, Marucha PT. Restraint stress alters the expression of interleukin-1 and keratinocyte growth factor at the wound site: an in situ hybridization study. J Neuroimmunol. 2002;129(1–2):74–83. doi:10.1016/S0165-5728(02)00174-1
32. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–746. doi:10.1056/NEJM199909023411006
33. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi:10.1177/0022034509359125
34. Ashtikar M, Wacker MG. Nanopharmaceuticals for wound healing - lost in translation? Adv Drug Deliv Rev. 2018;129:194–218. doi:10.1016/j.addr.2018.03.005
35. Rodriguez-Cabello JC, de Torre IG, Ibanez-Fonseca A, Alonso M. Bioactive scaffolds based on elastin-like materials for wound healing. Adv Drug Deliv Rev. 2018;129:118–133. doi:10.1016/j.addr.2018.03.003
36. Sheikholeslam M, Wright MEE, Jeschke MG, Amini-Nik S. Biomaterials for skin substitutes. Adv Healthc Mater. 2018;7(5):1700897. doi:10.1002/adhm.201700897
37. Concha M, Vidal A, Giacaman A, et al. Aerogels made of chitosan and chondroitin sulfate at high degree of neutralization: biological properties toward wound healing. J Biomed Mater Res B. 2018;106(6):2464–2471. doi:10.1002/jbm.b.v106.6
38. Jorfi M, Foster EJ. Recent advances in nanocellulose for biomedical applications. J Appl Polym Sci. 2015;132(14):41719. doi:10.1002/app.41719
39. Zhang HY, Lyu SY, Zhou XM, et al. Super light 3D hierarchical nanocellulose aerogel foam with superior oil adsorption. J Colloid Interf Sci. 2019;536:245–251. doi:10.1016/j.jcis.2018.10.038
40. Nemoto J, Saito T, Isogai A. Simple freeze-drying procedure for producing nanocellulose aerogel-containing, high-performance air filters. ACS Appl Mater Interfaces. 2015;7(35):19809–19815. doi:10.1021/acsami.5b05841
41. Nordli HR, Chinga-Carrasco G, Rokstad AM, Pukstad B. Producing ultrapure wood cellulose nanofibrils and evaluating the cytotoxicity using human skin cells. Carbohydr Polym. 2016;150:65–73. doi:10.1016/j.carbpol.2016.04.094
42. MB G, MV S. Endotoxin: the uninvited guest. Biomaterials. 2005;26(34):6811–6817. doi:10.1016/j.biomaterials.2005.04.063
43. Parenteau-Bareil RGR, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3:1863–1887.
44. Dharunya G, Duraipandy N, Lakra R, Korapatti PS, Jayavel R, Kiran MS. Curcumin cross-linked collagen aerogels with controlled anti-proteolytic and pro-angiogenic efficacy. Biomed Mater. 2016;11(4):045011. doi:10.1088/1748-6041/11/4/045011
45. Govindarajan D, Durabandy N, Sriyatsan KV, et al. Fabrication of hybrid collagen aerogels reinforced with wheat grass bioactives as instructive scaffolds for collagen turnover and angiogenesis for wound healing applications. ACS Appl Mater Interfaces. 2017;9(20):16940–16951. doi:10.1021/acsami.7b05842
46. Durairaj V, Hoda M, Shakya G, Babu SP, Rajagopalan R. Phytochemical screening and analysis of antioxidant properties of aqueous extract of wheatgrass. Asian Pac J Trop Med. 2014;7S1:S398–404. doi:10.1016/S1995-7645(14)60265-0
47. Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29(3):322–337. doi:10.1016/j.biotechadv.2011.01.005
48. Radwan-Praglowska J, Piatkowski M, Janus L, Bogdal D, Matysek D, Cablik V. Microwave-assisted synthesis and characterization of antioxidant chitosan-based aerogels for biomedical applications. Int J Polym Anal Ch. 2018;23(8):721–729. doi:10.1080/1023666X.2018.1504471
49. Lopez-Iglesias C, Barros J, Ardao I, et al. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr Polym. 2018;204:223–231. doi:10.1016/j.carbpol.2018.10.012
50. Quan KC, Li GF, Tao L, Xie Q, Yuan QP, Wang X. Diaminopropionic acid reinforced graphene sponge and its use for hemostasis. ACS Appl Mater Interfaces. 2016;8(12):7666–7673. doi:10.1021/acsami.5b12715
51. Quan KC, Li GF, Luan D, Yuan QP, Tao L, Wang X. Black hemostatic sponge based on facile prepared cross-linked graphene. Colloid Surface B. 2015;132:27–33. doi:10.1016/j.colsurfb.2015.04.067
52. Mellado C, Figueroa T, Baez R, et al. Development of graphene oxide composite aerogel with proanthocyanidins with hemostatic properties as a delivery system. ACS Appl Mater Interfaces. 2018;10(9):7717–7729. doi:10.1021/acsami.7b16084
53. Berthet M, Gauthier Y, Lacroix C, Verrier B, Monge C. Nanoparticle-based dressing: the future of wound treatment? Trends Biotechnol. 2017;35(8):770–784. doi:10.1016/j.tibtech.2017.05.005
54. Rose S, Prevoteau A, Elziere P, Hourdet D, Marcellan A, Leibler L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature. 2014;505(7483):382–385. doi:10.1038/nature12806
55. Meddahi-Pellé ALA, Marcellan A, Louedec L, Letourneur D, Leibler L. hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew Chem Int Ed Engl. 2014;34(12):6369–6373. doi:10.1002/anie.201401043
56. Meng-Meng Lu JB, Dan S, Jing Q, Ming L. antibacterial and biodegradable tissue nano-adhesives for rapid wound closure. Inter J Nanomed. 2018;13:5849–5863.
57. Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev. 2008;108(11):4754–4783. doi:10.1021/cr8004422
58. Wegst UGK, Bai H, Saiz E, Tomsia AP, Ritchie RO. Bioinspired structural materials. Nat Mater. 2015;14(1):23–36. doi:10.1038/nmat4089
59. Tertuliano OA, Greer JR. The nanocomposite nature of bone drives its strength and damage resistance. Nat Mater. 2016;15(11):1195–1202. doi:10.1038/nmat4719
60. Chen PY, McKittrick J, Meyers MA. Biological materials: functional adaptations and bioinspired designs. Prog Mater Sci. 2012;57(8):1492–1704. doi:10.1016/j.pmatsci.2012.03.001
61. Li YF, Li Q, Zhu SS, et al. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials. 2010;31(34):9006–9014. doi:10.1016/j.biomaterials.2010.07.112
62. Ortiz C, Boyce MC. Materials science - bioinspired structural materials. Science. 2008;319(5866):1053–1054. doi:10.1126/science.1154295
63. Lu B-Q, Zhu Y-J, Chen F. Highly flexible and nonflammable inorganic hydroxyapatite paper. Chem Eur J. 2014;20(5):1242–1246. doi:10.1002/chem.v20.5
64. Li H, Wu D, Wu J, Dong L-Y, Zhu Y-J, Hu X. Flexible, high-wettability and fire-resistant separators based on hydroxyapatite nanowires for advanced lithium-ion batteries. Adv Mater. 2017;29(44):1703548. doi:10.1002/adma.201703548
65. Zhang Y-G, Zhu Y-J, Chen F, Sun T-W, Jiang -Y-Y. Ultralong hydroxyapatite microtubes: solvothermal synthesis and application in drug loading and sustained drug release. CrystEngComm. 2017;19(14):1965–1973. doi:10.1039/C6CE02394K
66. Chen F, Zhu Y-J. Large-scale automated production of highly ordered ultralong hydroxyapatite nanowires and construction of various fire-resistant flexible ordered architectures. ACS Nano. 2016;10(12):11483–11495. doi:10.1021/acsnano.6b07239
67. Sun T-W, Zhu Y-J, Chen F. Highly flexible multifunctional biopaper comprising chitosan reinforced by ultralong hydroxyapatite nanowires. Chem Eur J. 2017;23(16):3850–3862. doi:10.1002/chem.201605165
68. Zhang Y-G, Zhu Y-J, Chen F, Sun T-W. Biocompatible, ultralight, strong hydroxyapatite networks based on hydroxyapatite microtubes with excellent permeability and ultralow thermal conductivity. ACS Appl Mater Interfaces. 2017;9(9):7918–7928. doi:10.1021/acsami.6b13328
69. Zhang Y-G, Zhu Y-J, Xiong Z-C, Wu J, Chen F. Bioinspired ultralight inorganic aerogel for highly efficient air filtration and oil-water separation. ACS Appl Mater Interfaces. 2018;10(15):13019–13027. doi:10.1021/acsami.8b02081
70. Sun T-W, Zhu Y-J, Chen F. Hydroxyapatite nanowire/collagen elastic porous nanocomposite and its enhanced performance in bone defect repair. RSC Adv. 2018;8(46):26133–26144.
71. Sun T-W, Yu W-L, Zhu Y-J, et al. Porous nanocomposite comprising ultralong hydroxyapatite nanowires decorated with zinc-containing nanoparticles and chitosan: synthesis and application in bone defect repair. Chem Eur J. 2018;24(35):8809–8821. doi:10.1002/chem.v24.35
72. Zhang Y-G, Zhu Y-J, Chen F, Sun T-W. A novel composite scaffold comprising ultralong hydroxyapatite microtubes and chitosan: preparation and application in drug delivery. J Mater Chem B. 2017;5(21):3898–3906. doi:10.1039/C6TB02576E
73. Sun T-W, Yu W-L, Zhu Y-J, et al. Hydroxyapatite nanowire@magnesium silicate core-shell hierarchical nanocomposite: synthesis and application in bone regeneration. ACS Appl Mater Interfaces. 2017;9(19):16435–16447. doi:10.1021/acsami.7b03532
74. Yu W, Sun T-W, Ding Z, et al. Copper-doped mesoporous hydroxyapatite microspheres synthesized by a microwave-hydrothermal method using creatine phosphate as an organic phosphorus source: application in drug delivery and enhanced bone regeneration. J Mater Chem B. 2017;5(5):1039–1052. doi:10.1039/C6TB02747D
75. Yu W, Sun T-W, Qi C, et al. Strontium-doped amorphous calcium phosphate porous microspheres synthesized through a microwave-hydrothermal method using fructose 1,6-bisphosphate as an organic phosphorus source: application in drug delivery and enhanced bone regeneration. ACS Appl Mater Interfaces. 2017;9(4):3306–3317. doi:10.1021/acsami.6b12325
76. Weng L, Boda SK, Wang H, Teusink MJ, Shuler FD, Novel XJ. 3D hybrid nanofiber aerogels coupled with BMP-2 peptides for cranial bone regeneration. Adv Healthc Mater. 2018;7(10):1701415. doi:10.1002/adhm.201701415
77. Li T, Ai F, Shen W, et al. Microstructural orientation and precise regeneration: a proof-of-concept study on the sugar-cane-derived implants with bone mimetic hierarchical structure. ACS Biomater Sci Eng. 2018;4(12):4331–4337. doi:10.1021/acsbiomaterials.8b01052
78. Aegerter MA, Koebel MM. 2011. Advances in Sol–Gel Derived Materials and Technologies. New York: Springer, Aerogel handbook.
79. Ulker Z, Erkey C. An emerging platform for drug delivery: aerogel based systems. J Control Release. 2014;177:51–63. doi:10.1016/j.jconrel.2013.12.033
80. Galante YM, Merlini L, Silvetti T, et al. Enzyme oxidation of plant galactomannans yielding biomaterials with novel properties and applications, including as delivery systems. Appl Microbiol Biot. 2018;102(11):4687–4702. doi:10.1007/s00253-018-9028-z
81. Garcia-Gonzalez CA, Jin M, Gerth J, Alvarez-Lorenzo C, Smirnova I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr Polym. 2015;117:797–806. doi:10.1016/j.carbpol.2014.10.045
82. Goncalves VSS, Gurikov P, Poejo J, et al. Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur J Pharm Biopharm. 2016;107:160–170. doi:10.1016/j.ejpb.2016.07.003
83. De Marco I, Iannone R, Miranda S, Riemma S. An environmental study on starch aerogel for drug delivery applications: effect of plant scale-up. Int J Life Cycle Assess. 2018;23(6):1228–1239. doi:10.1007/s11367-017-1351-6
84. De Marco I, Riemma S, Iannone R. Life cycle assessment of supercritical impregnation: starch aerogel + α-tocopherol tablets. J Supercrit Fluids. 2019;143:305–312. doi:10.1016/j.supflu.2018.09.003
85. Ubeyitogullari A, Brahma S, Rose DJ, Ciftci ON. In vitro digestibility of nanoporous wheat starch aerogels. J Agric Food Chem. 2018;66(36):9490–9497. doi:10.1021/acs.jafc.8b03231
86. Pawar J, Ali MT, Fule R, et al. Biodegradable porous starch spheres as a novel carrier for enhancement of dissolution rate and oral bioavailability of itraconazole. Curr Drug Deliv. 2017;14(7):944–954. doi:10.2174/1567201813666160920154209
87. De Marco I, Reverchon E. Starch aerogel loaded with poorly water-soluble vitamins through supercritical CO2 adsorption. Chem EngRes Des. 2017;119:221–230.
88. Alnaief M, Obaidat R, Mashaqbeh H. Effect of processing parameters on preparation of carrageenan aerogel microparticles. Carbohydr Polym. 2018;180:264–275. doi:10.1016/j.carbpol.2017.10.038
89. Obaidat RM, Alnaief M, Mashaqbeh H. Investigation of carrageenan aerogel microparticles as a potential drug carrier. AAPS PharmSciTech. 2018;19(5):2226–2236. doi:10.1208/s12249-018-1021-4
90. Salgado M, Santos F, Rodriguez-Rojo S, Reis RL, Duarte ARC, Jose Cocero M. Development of barley and yeast beta-glucan aerogels for drug delivery by supercritical fluids. J CO2 Util. 2017;22:262–269. doi:10.1016/j.jcou.2017.10.006
91. Veres P, Sebok D, Dekany I, et al. A redox strategy to tailor the release properties of Fe(III)-alginate aerogels for oral drug delivery. Carbohydr Polym. 2018;188:159–167. doi:10.1016/j.carbpol.2018.01.098
92. Lopez-Iglesias C, Casielles AM, Altay A, Bettini R, Alvarez-Lorenzo C, Garcia-Gonzalez CA. From the printer to the lungs: inkjet-printed aerogel particles for pulmonary delivery. Chem Eng J. 2019;357:559–566. doi:10.1016/j.cej.2018.09.159
93. Long L-Y, Weng Y-X, Wang Y-Z. Cellulose aerogels: synthesis, applications, and prospects. Polymers. 2018;10(6):623. doi:10.3390/polym10060623
94. Bhandari J, Mishra H, Mishra PK, Wimmer R, Ahmad FJ, Talegaonkar S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Inter J Nanomed. 2017;12:2021–2023.
95. Wang R, Shou D, Lv O, Kong Y, Deng L, Shen J. pH-Controlled drug delivery with hybrid aerogel of chitosan, carboxymethyl cellulose and graphene oxide as the carrier. Int J Biol Macromol. 2017;103:248–253. doi:10.1016/j.ijbiomac.2017.05.064
96. Zhao J, Lu C, He X, Zhang X, Zhang W, Zhang X. Polyethylenimine-grafted cellulose nanofibril aerogels as versatile vehicles for drug delivery. ACS Appl Mater Interfaces. 2015;7(4):2607–2615. doi:10.1021/am507601m
97. Smirnova I, Suttiruengwong S, Seiler M, Arlt W. Dissolution rate enhancement by adsorption of poorly soluble drugs on hydrophilic silica aerogels. Pharm Dev Technol. 2004;4:443–452.
98. Smirnova I, et al. Comparison of different methods for enhancing the dissolution rate of poorly soluble drugs: case of griseofulvin. Eng Life Sci. 2005;5(3):277–280. doi:10.1002/elsc.200500081
99. Guenther U, SMIRNOVA I, NEUBERT R. Hydrophilic silica aerogels as dermal drug delivery systems — dithranol as a model drug. Eur J Pharm Biopharm. 2008;69(3):935–942. doi:10.1016/j.ejpb.2008.02.003
100. Smirnova I, S S, Arlt W. Feasibility study of hydrophilic and hydrophobic silica aerogels as drug delivery systems. J Non-Cryst Solids. 2004;350:54–60. doi:10.1016/j.jnoncrysol.2004.06.031
101. Mohammadian M, Kashi TSJ, Erfan M, Soorbaghi FP. Synthesis and characterization of silica aerogel as a promising drug carrier system. J Drug Deliv Sci Technol. 2018;44:205–212. doi:10.1016/j.jddst.2017.12.017
102. Zhang Y, Wang J, Zhang X. Surfactant-free synthesis of silica aerogel microspheres with hierarchically porous structure. J Colloid Interf Sci. 2018;515:1–9. doi:10.1016/j.jcis.2018.01.010
103. Rajanna SK, Vinjamur M, Mukhopadhyay M. Mechanism for formation of hollow and granular silica aerogel microspheres from rice husk ash for drug delivery. J Non Cryst Solids. 2015;429:226–231. doi:10.1016/j.jnoncrysol.2015.09.015
104. Afrashi M, Semnani D, Talebi Z, Dehghan P, Maherolnaghsh M. Comparing the drug loading and release of silica aerogel and PVA nano fibers. J Non Cryst Solids. 2019;503:186–193. doi:10.1016/j.jnoncrysol.2018.09.045
105. Rajanna SK, Kumar D, Vinjamur M, Mukhopadhyay M. Silica aerogel microparticles from rice husk ash for drug delivery. Ind Eng Chem Res. 2015;54(3):949–956. doi:10.1021/ie503867p
106. Veres P, Kiraly G, Nagy G, Lazar I, Fabian I, Kalmar J. Biocompatible silica-gelatin hybrid aerogels covalently labeled with fluorescein. J Non Cryst Solids. 2017;473:17–25. doi:10.1016/j.jnoncrysol.2017.07.016
107. Veres P, Keri M, Banyai I, et al. Mechanism of drug release from silica-gelatin aerogel-Relationship between matrix structure and release kinetics. Colloid Surface B. 2017;152:229–237. doi:10.1016/j.colsurfb.2017.01.019
108. Follmann HDM, Oliveira ON
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
