Back to Journals » Clinical and Experimental Gastroenterology » Volume 13
Endoscopic Ultrasound-Guided Gallbladder Drainage: Current Perspectives
Authors Fugazza A , Colombo M , Repici A , Anderloni A
Received 6 December 2019
Accepted for publication 13 April 2020
Published 15 May 2020 Volume 2020:13 Pages 193—201
DOI https://doi.org/10.2147/CEG.S203626
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Andreas M. Kaiser
Alessandro Fugazza,1 Matteo Colombo,1,2 Alessandro Repici,1,2 Andrea Anderloni1
1Digestive Endoscopy Unit, Department of Gastroenterology, Humanitas Clinical and Research Center-IRCCS, Rozzano (MI), Italy; 2Humanitas University, Department of Biomedical Sciences, Milan, Italy
Correspondence: Andrea Anderloni
Digestive Endoscopy Unit, Division of Gastroenterology, Humanitas Research Hospital, Rozzano, Italy
Tel +39 02 82247308
Email [email protected]
Abstract: According to the recently updated Tokyo Guidelines, laparoscopic cholecystectomy still represents the gold standard for the treatment of acute cholecystitis. However, fragile patients, due to comorbidities or poor clinical conditions, have a high surgical risk. In such cases, percutaneous or endoscopic gallbladder drainage is considered the treatment of choice. In particular, endoscopic ultrasound-guided gallbladder drainage with the placement of specifically designed stents is now considered an alternative option. In addition, the opening of an access door to the lumen of the gallbladder could offer new opportunities for the endoscopic treatment of gallbladder diseases. The purpose of this review is to provide an update on the latest available evidence in the literature regarding the endoscopic ultrasound-guided gallbladder drainage.
Keywords: endoscopic ultrasound-guided gallbladder drainage, lumen-apposing metal stent, acute cholecystitis, percutaneous gallbladder drainage; cholecystostomy
Introduction
Surgical cholecystectomy is the gold standard of treatment for acute cholecystitis (AC) as stated in the recently updated Tokyo Guidelines (Figure 1).1 Laparoscopic cholecystectomy should be the first approach to be taken into account regardless of the degree of severity, in particular, if performed by a skilled surgeon, in a setting that assures intensive care management. However, in high-risk surgical patients, such as in case of fragile patients or patients with advanced malignancy or severe organ failure or poor performance status, the morbidity and mortality rates remain high. Indeed, less invasive treatment is required and percutaneous trans-hepatic gallbladder drainage (PTGBD) is considered the standard nonsurgical option in fragile patients. Nevertheless, nowadays endoscopic gallbladder drainage could be considered a possible alternative approach especially when the procedure is performed by skilled endoscopists in high-volume institutes.2 The two widely accepted endoscopic techniques are the endoscopic transpapillary gallbladder drainage (ETGBD) and the transmural endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) (Figure 2). The latter, since the first case reports published in medical literature in 2007,3 has represented an interesting alternative, made even more attractive by the introduction of the lumen-apposing metal stent (LAMS) specifically designed for interventional endoscopic ultrasonography.4 The purpose of this review is to provide an update on the latest available evidence in the medical literature regarding the EUS-GBD in patients with AC.
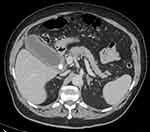 |
Figure 1 Acute cholecystitis (AC) with distended gallbladder, stones, mucosal hyper-enhancement and pericholecystic fluid noted on computed tomography (CT) scan. |
Endoscopic Ultrasound-Guided Gallbladder Drainage (EUS-GBD)
Endoscopic Ultrasound-guided Gallbladder Drainage (EUS-GBD) is now considered a well-established alternative treatment to surgery in case of AC. Firstly described in 2007,3 as mentioned above, the EUS-GBD has gained in popularity progressively. Originally, due to the absence of specifically designed stents for EUS-GBD, a variety of plastic stents (straight, single, and double pigtail) and self-expandable metal stents (SEMS) have been used. One of the first prospective studies was the pilot one published by Song et al5 in 2010: eight patients with AC and unsuitable candidates for cholecystectomy underwent an EUS-guided cholecystoenterostomy with the single-step placement of a 7F double-pigtail plastic stent. Technical and clinical success were achieved in all patients, with three (37.5%) adverse events (AE) reported. During follow-up periods (median 186 days) no recurrence of cholecystitis occurred. With regard to SEMS, one of the first and most important studies was the one carried out by Choi et al6 in 2014. EUS-GBD with SEMS placement was technically and clinically successful in 62/63 patients. During the study, a total of 7 (11.2%) AE were reported, including duodenal perforation, self-limiting pneumoperitoneum, distal stent migration and cholecystitis due to stent occlusion. Approximately 96% of the patients had no recurrence of AC during follow-up. With the aim of overcoming the high rates of AE, modified stents with flared ends and LAMS have been firstly studied and then introduced in the clinical practice (Table 1). In 2016 Anderloni et al7 published a systematic review and pooled analysis with the intention to evaluate the outcomes of the different types of stents. With regard to plastic stents, pooled technical and clinical success rates were 100% with an AE frequency of 18.2% (pneumoperitoneum, bile leakage, and bile peritonitis and stent migration). Pooled technical and clinical success rates obtained using SEMS were 98.6% and 94.4%, respectively, with a pooled AE rate of 12.3% (pneumoperitoneum, duodenal perforation, stent migration and worsening of cholecystitis due to stent occlusion). Analyzing the results of the different types of metal stents (partially covered versus fully covered) the authors have found that the clinical success rate was significantly greater for the former compared to the latter (98% vs 70%). Moreover, AE occurred more commonly in the fully covered group, although the difference did not reach statistical significance. Lastly, the pooled technical and clinical success rates for LAMS were 91.5% and 90.1%, respectively, with lower AE frequency (9.9%). The authors attribute the reduced technical and clinical success of LAMS, compared to other stents, to the learning curve associated with the placement of this new system. In more recent years, Jain et al8 published a systematic review of 189 patients who underwent EUS-GBD by LAMS for AC. Technical and clinical success rate reported was 95.2% and 96.7%, respectively. Furthermore, they observed a small risk of recurrent cholecystitis (5.1%), gastrointestinal bleeding (2.6%) and stent migration (1.1%). In particular, researchers highlighted a reduced stent deployment time when an electrocautery-enhanced LAMS (EC-LAMS) was used, compared to procedures with a non-cautery LAMS (3.1 min vs 7.7 min). Investigators concluded that EUS-GBD using LAMS could be considered a safe and efficacious alternative, but it must be performed by skilled endoscopists in high-volume centers.
 |
Table 1 Available Lumen Apposing Metal and Biflanged Stents |
Technical Aspects
The procedure can be performed with the patient under conscious sedation or general anesthesia, in an endoscopic room with all fluoroscopic equipments. As the first step, a linear array echoendoscope is advanced into the distal gastric antrum or duodenal bulb in order to identify the target. A diagnostic EUS examination should always be performed in order to locate vessels and other structures surrounding the intended needle path. However, the best site to puncture has not been defined yet. Tyberg et al9 have tried to investigate the possible clinical differences between transgastric and transduodenal approaches to EUS-GBD, and they found no differences in terms of clinical failure or AE. Teoh et al10 carried out a comparative analysis of EUS-GBD done with the two possible techniques. Interestingly, the authors reported no significant difference in technical success, clinical success and AE rate based on the puncture site. Endoscopists can choose the site they prefer to use for puncture. However, as reported in a recent commentary by Perez-Miranda11 some technical aspects should be taken into account, which may lead to one access being preferred over the other. Duodenum is less mobile than the stomach, resulting in a less technically challenging procedure and with a decreased risk of stent migration or dislodgment. Furthermore, in the duodenal bulb, the risk of stent occlusion by food is lower than in the transgastric approach. On the other hand, the reasons that favour transgastric access are to obtain a simpler target, as gallbladder body represents a larger entry site, and to have less important consequences in case of AE, because stomach has an easier surgical access than duodenal bulb. After selecting the best entry point, a standard 19-gauge needle is used to puncture the gastric antrum or the duodenal bulb to access the gallbladder using real-time ultrasound and color Doppler imaging. Next, a contrast medium is injected to obtain fluoroscopic images of the biliary tree. A standard biliary guidewire is then coiled into the gallbladder lumen. At this point, the newly created tract must then be dilated over the guidewire using a balloon dilator or cystotome. Subsequently, a preloaded stent is then advanced over the guidewire. The distal flange of the stent is first deployed into the gallbladder under ultrasonographic or fluoroscopic guidance. Then, the proximal flange of the stent (plastic stent, SEMS and LAMS) is deployed into the duodenum or the stomach, thereby performing cholecystoenterostomy or cholecystogastrostomy, respectively. The optimal positioning of the stent can be confirmed endoscopically or fluoroscopically and also via aspiration of biliary juice. If an EC-LAMS (Hot-Axios, Boston Scientific, Marlborough, MA, USA) is being used, the puncture site, dilation of the tract and stent deployment can all be performed simultaneously, thereby decreasing the procedure time (Figures 3–6). As mentioned above, this technique requires a proven expertise in the field of advanced interventional EUS. Although this is an established fact, in the current scientific literature there is not a clear definition of who might be considered an endosonographers expert in interventional procedures and especially what is the minimum number of procedures that should be carried out to gain competency in EUS-GBD. Interestingly, Teoh et al12 have tried to find an answer to this question conducting an international multicenter retrospective study on the outcomes of patients with AC treated with EUS-GBD. Indeed, they compared the outcomes of endoscopists who had completed more than 25 EUS-GBD with those who had performed less than 25 procedures. The authors reported a higher percentage of procedures that lasted more than 30 mins by endoscopists with less experience when compared with more experienced colleagues (67% vs 49%, p<0.006). Differences also occurred in terms of unplanned procedural events (13.5% vs 5.8%, p= 0.012) and 30-day AE (19.2% vs 11.5%, p= 0.031). Interestingly no significant differences were noted in terms of technical or clinical success rate. Finally, researchers concluded that endoscopists attempting EUS-GBD should only do so when they are familiar with other EUS interventional techniques, such as drainage of pancreatic fluid collections.
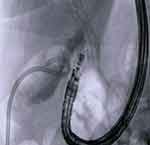
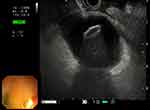 |
Figure 3 Endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) with first flange opening of the electrocautery lumen-apposing metal stent (EC-LAMS). |
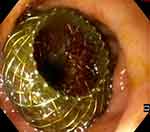 |
Figure 4 Endoscopic image obtained after electrocautery lumen-apposing metal stent (EC-LAMS) deployment in the gastric lumen. |
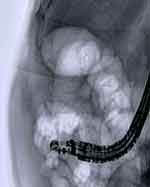 |
Figure 5 Fluoroscopic view of newly positioned electrocautery lumen-apposing metal stent (EC-LAMS) into the gallbladder lumen. |
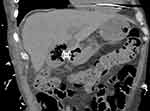 |
Figure 6 Computed tomography (CT) scan after 2 months of follow-up showing a cholecystoduodenostomy using electrocautery lumen-apposing metal stent (EC-LAMS). |
Percutaneous Transhepatic Gallbladder Drainage (PTGBD)
In patients who are not surgical candidates, PTGBD has been traditionally considered the treatment of choice for draining the gallbladder. Indeed, the technical success of PTGBD is high, approximately 95%, and the procedure could be easily carried out by general clinicians. However, clinical success range from 56% to 100%13 and the rate of AE could reach 14%, including bleeding, pneumothorax, biliary peritonitis, and premature catheter removal or dislodgement.14,15 Nevertheless, the external catheter requires continuous care and additionally could be associated with pain, discomfort and cosmetic disfigurement.10 Furthermore, up to 33% of patients could develop recurrent cholecystitis after catheter removal.16
Endoscopic Transpapillary Gallbladder Drainage (ETGBD)
Currently, for the non-surgical treatment of patients with AC, the most accepted endoscopic alternatives are the ETGBD or the EUS-GBD. The transpapillary approach was first reported in 1990 and could be carried out with standard endoscopic retrograde cholangiopancreatography (ERCP) techniques; it ensures physiological drainage of the biliary tract without the need of transmural injury.17 However, cannulation of the cystic duct in patients with AC can be challenging. In addition, this technique carries all the ERCP-related complications such as bleeding, perforation and post-ERCP pancreatitis. One of the largest studies (194 patients) that assessed ETGBD outcomes was published in 2010 by Itoi et al.18 The authors have found a technical success rate and clinical success rate of 81% and 75%, respectively, with an AE rate of 3.6%. In 2011 Lee et al19 conducted a multicenter prospective follow-up study on 29 patients with AC that were unfit for surgery, in order to evaluate the outcomes of this procedure. ETGBD was successful in 23 (79.3%) and the mean procedure time was 22.4 ± 11.5 min. In terms of post-procedural AE, two patients suffered from mild pancreatitis (8.7%) and two from cholestasis (8.7%); during the follow-up period, late AE were recorded in four patients (20%), including distal migration, cholangitis, and recurrent biliary pain. Median stent patency was 760 days. Later in 2013, Maekawa et al20 published a retrospective study of 46 elderly patients with AC treated with endoscopic gallbladder stenting. The procedure was successful in 31 patients (77.5%), without any immediate post-procedural AE. In this study, the mean procedural time was 27.6 ± 15.1 min. Most of the patients (30/31) did not have a recurrence of AC and 29 patients (93.5%) remained asymptomatic until the last follow-up (ranged from 1 month to 5 years).
EUS-GBD versus PTGBD
Most of the data present in literature arise from non-comparative studies; however, some authors have decided to compare the two techniques (Table 2).9,10,21–23 The first comparative study was a randomized controlled trial published in 2012 by Jang et al21 and reported that EUS-GBD is comparable with PTGBD in terms of the technical success (97% vs 97%; p < 0.001), clinical success (100% vs 96%; p < 0.0001) and AE (7% vs 3%; p = 0.492), although only small nasobiliary tubes were placed across the gastrointestinal wall. A recent systematic review showed no statistically significant differences in technical (OR 0.43, 95% CI 0.12 to 1.58; p = 0.21; I2=0%) and clinical success (OR 1.07, 95% CI 0.36 to 3.16; p = 0.90; I2 = 44%) between EUS-GBD and PTGBD. The pooled OR for overall AE was 0.43 (95% CI 0.18 to 1.00; p = 0.05; I2 = 66%), with lower AE of EUS-GBD. Furthermore, EUS-GBD was associated with shorter hospital stays, and fewer re-interventions and readmissions compared with PTGBD. No statistically significant difference in conversion rate to open surgery has been observed between EUS-GBD and PTGBD (9% vs 12%; p = 0.99).24 Moreover, regarding AE, the study of Teoh et al10 showed that overall AE (32.2% vs 74.6%, p<0.001) and severe AE (23.7% vs 74.6%, p<0.001) were significantly less in the EUS-GBD group as compared to the PTGBD group. In addition, Irani et al23 focused on post-procedure pain and they interestingly find that it was significantly lower following EUS-GBD in comparison to PTGBD. Taking in account that PTGBD may reduce the patient’s quality of life due to the presence of external drainage as it lengthens the mean hospital stay often due to the need for reintervention, in addition to an increased incidence of AE, EUS-GBD may be preferable, especially for patients who are less likely to undergo future surgical treatment. An important fact that must be considered, however, is that EUS-GBD is not available in all hospitals. Therefore, in peripheral hospitals, the PTGBD approach can and must still be considered the first alternative. Interestingly, Minaga et al25 have recently assessed the clinical efficacy and safety of EUS-GBD replacement of previously placed PTGBD, in a multicenter retrospective study. The conversion procedure has reached a success rate of 90.5% with no reported early AE. There were only three late AE, and two patients required re-intervention. On the basis of these data, in patients where drainage needs to be maintained PTGBD could be converted to EUS-GBD. The conversion between the two procedures is safe, feasible and effective, particularly in reducing the patient’s discomfort (Figure 7).
 |
Table 2 Comparison Between EUS-GBD and PTGBD |
EUS-GBD versus ETGBD
Currently, only a few studies on this topic are available in medical literature comparing the two techniques in terms of technical, clinical, and long-term results. Itoi et al18 in 2010 performed a systematic review to evaluate the potential role of each gallbladder drainage technique, reporting for ETGBD a relatively high pooled success rate of 96% (95% CI, 91.1–98.7%), accompanied, however, by a pooled response rate of only 88% (95% CI, 81.2–93.2%). Subsequent studies have instead shown relatively low technical success rates ranging from 76% to 79%, and this could be explained by the intrinsic difficulty in accessing the cystic duct.19,26 Oh et al27 have recently conducted a retrospective study on 172 patients, 76 who underwent EUS-GBD drainage and 96 who were treated transpapillary, comparing the different outcomes of the techniques under examination. The Authors reported a technical success rate (99.3% vs 86.6%, P < 0.01) and clinical success rate (99.3% vs 86%, p < 0.01) significantly higher in the EUS-GBD group than in the ETGBD group. In addition, taking into account the procedure-related AE rate (7.1% vs 19.3%, p = 0.02), the EUS-GBD showed a significantly better safety profile. In the EUS-GBD group, the AE were three cases of pneumoperitoneum, one duodenal perforation, and two patients that experienced recurrent biliary pain while in the ETGBD group eight patients experienced post-ERCP pancreatitis and one recurrent biliary pain. Also, with regard to long-term outcomes, assessed as cholecystitis or cholangitis recurrence rate, EUS-GBD showed better results (3.2% vs 12.4%) when compared to ETGBD drainage. The authors, therefore, concluded that in patients with AC who are not candidates for surgery, EUS-GBD represents a more appropriate treatment compared to the transpapillary approach. ETGBD can still be considered in patients with choledocholithiasis, as it allows concomitant stone removal and physiologic drainage without the creation of a fistula.
Long-Term Outcomes of EUS-GBD
Recently, Yuste et al28 have performed a retrospective evaluation of a case series including subjects who underwent EUS-GBD using LAMS, in order to evaluate the safety and clinical outcomes after at least 1 year of follow up. In this study, no LAMS-related AE were identified after the first 12 months of follow up, and only 4.5% of patients required re-admission for the gallstone-related disease. Interestingly, these results are also confirmed by a larger study published by Choi et al6 where the rates of readmission for the gallstone-related disease were 3.6%; no AE were identified beyond the first year. Previously, Walter et al29 published a multicenter retrospective study in which 15 patients had a LAMS left in situ with a mean follow up of 364 days: 4 procedure-related AE (13%) were reported, although the time to AE was not specified. Taken together, these data propose EUS-GBD as a valuable safe and efficient procedure also in the long-term follow-up, reducing the risk of further biliary events for fragile patients who do not undergo cholecystectomy, carrying low rates of AE.
Malignant Distal Biliary Obstruction
In consideration of the excellent results obtained in fragile patients with AC, new possible indications for the EUS-GBD are currently being explored; one of the most interesting is the biliary decompression in malignant distal biliary obstruction when current endoscopic methods fail or are not feasible. ERCP is the treatment of choice for treating malignant obstructive jaundice. However, ERCP can fail due to the presence of surgically altered anatomy, duodenal obstruction, indwelling enteral stents, or periampullary tumor infiltration. In these cases, EUS-guided bile duct drainage (EUS-BD) represents an alternative biliary drainage method and its use is increasingly taken into consideration.30,31 Nevertheless, in some cases, neither of the two opportunities are feasible. As shown by Imai et al32 EUS-GBD may be a suitable rescue alternative to treat malignant distal biliary obstruction when both ERCP and EUS-BD failed. Indeed, the Authors reported that the two echo-endoscopic procedures achieve similar results in terms of functional success (84.2% for EUS-BD and 91.7% for EUS-GBD) and AE rates (19.8% for EUS-BD and 16.7% for EUS-GBD). In conclusion, EUS-GBD may represent a possible alternative in case of failed of conventional approaches when cystic duct patency has been confirmed.
Gallbladder Interventions
Traditionally, the gallbladder is known to remain a difficult organ to assess using the endoscope. With the advent of the LAMS, however, this statement no longer appears to be so well founded. In fact, thanks to the creation of a large caliber anastomosis, the endoscopist has the opportunity to access the lumen of the gallbladder and to perform advanced endoscopic evaluation and interventions. The first case of endoscopic gallbladder polypectomy, even though performed through a surgical cholecysto-gastrostomy, was reported by Chen et al in 2011,33 confirming the feasibility of the procedure. Subsequently, Ge et al34 described a case series of EUS-GBD access using a LAMS followed by gallstones removal and gallbladder polypectomy in two cases. As reported in a retrospective study by Chan et al35 routine peroral cholecystoscopy after large-diameter LAMS placement was successfully completed in the 93.1% and has allowed complete gallstone clearance in 88% of patients, using irrigation, suction, basket and holmium laser lithotripsy. Moreover, in the same study, a variety of image-enhanced modalities for mucosal evaluation of the gallbladder, such as magnifying endoscopy and confocal laser endomicroscopy, were performed. Thanks to the adoption of these techniques, it has been possible to identify not only features suggestive of inflammation, which was subsequently confirmed on endoscopic biopsy, but also the presence of polypoid lesions and in one case of gallbladder adenocarcinoma. Even more recently, Tian et al36 reported another case of successful gallbladder polypectomy of three polyps through a LAMS, avoiding surgical removal of a well-functioning gallbladder. All the Authors conclude that per-oral cholecystoscopy is a feasible and safe procedure for advanced endoscopic evaluation and interventions, representing an unexplored land in the treatment of gallbladder pathologies.
Acknowledgments
The Authors thank Dr Annalisa Cappello for the graphic representation of gallbladder drainage procedures.
Disclosure
Dr Andrea Anderloni reports personal fees from Boston Scientific, personal fees from Olympus, personal fees from Medtronic, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Okamoto K, Suzuki K, Takada T, et al. Tokyo Guidelines 2018: flowchart for the management of acute cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25(1):55–72. doi:10.1002/jhbp.516
2. Mori Y, Itoi T, Baron TH, et al. Tokyo Guidelines 2018: management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):87–95. doi:10.1002/jhbp.504
3. Kwan V, Eisendrath P, Antaki F, et al. EUS-guided cholecystenterostomy: a new technique (with videos). Gastrointest Endosc. 2007;66(3):582–586. doi:10.1016/j.gie.2007.02.065
4. Mussetto A, Fugazza A, Fuccio L, et al. Current uses and outcomes of lumen-apposing metal stents. Ann Gastroenterol. 2018;31(5):535–540. doi:10.20524/aog.2018.0287
5. Song TJ, Park DH, Eum JB, et al. EUS-guided cholecystoenterostomy with single-step placement of a 7F double-pigtail plastic stent in patients who are unsuitable for cholecystectomy: a pilot study (with video). Gastrointest Endosc. 2010;71(3):634–640. doi:10.1016/j.gie.2009.11.024
6. Choi JH, Lee SS, Choi JH, et al. Long-term outcomes after endoscopic ultrasonography-guided gallbladder drainage for acute cholecystitis. Endoscopy. 2014;46(8):656–661. doi:10.1055/s-0034-1365720
7. Anderloni A, Buda A, Vieceli F, et al. Endoscopic ultrasound-guided transmural stenting for gallbladder drainage in high-risk patients with acute cholecystitis: a systematic review and pooled analysis. Surg Endosc. 2016;30(12):5200–5208. doi:10.1007/s00464-016-4894-x
8. Jain D, Bhandari BS, Agrawal N, et al. Endoscopic ultrasound-guided gallbladder drainage using a lumen-apposing metal stent for acute cholecystitis: a systematic review. Clin Endosc. 2018;51(5):450–462. doi:10.5946/ce.2018.024
9. Tyberg A, Saumoy M, Sequeiros EV, et al. EUS-guided versus percutaneous gallbladder drainage: isn’t it time to convert? J Clin Gastroenterol. 2018;52(1):79–84. doi:10.1097/MCG.0000000000000786
10. Teoh AYB, Serna C, Penas I, et al. Endoscopic ultrasound-guided gallbladder drainage reduces adverse events compared with percutaneous cholecystostomy in patients who are unfit for cholecystectomy. Endoscopy. 2017;49(2):130–138. doi:10.1055/s-0042-119036
11. Perez-Miranda M. Technical considerations in EUS-guided gallbladder drainage. Endosc Ultrasound. 2018;7(2):79–82. doi:10.4103/eus.eus_5_18
12. Teoh AY, Perez-Miranda M, Kunda R, et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019;7(8):E964–E73. doi:10.1055/a-0915-2098
13. Winbladh A, Gullstrand P, Svanvik J, et al. Systematic review of cholecystostomy as a treatment option in acute cholecystitis. HPB (Oxford). 2009;11(3):183–193. doi:10.1111/j.1477-2574.2009.00052.x
14. McGahan JP, Lindfors KK. Percutaneous cholecystostomy: an alternative to surgical cholecystostomy for acute cholecystitis? Radiology. 1989;173(2):481–485. doi:10.1148/radiology.173.2.2678261
15. Itoi T, Sofuni A, Itokawa F, et al. Endoscopic transpapillary gallbladder drainage in patients with acute cholecystitis in whom percutaneous transhepatic approach is contraindicated or anatomically impossible (with video). Gastrointest Endosc. 2008;68(3):455–460. doi:10.1016/j.gie.2008.02.052
16. Sugiyama M, Tokuhara M, Atomi Y. Is percutaneous cholecystostomy the optimal treatment for acute cholecystitis in the very elderly? World J Surg. 1998;22(5):459–463. doi:10.1007/s002689900416
17. Feretis CB, Manouras AJ, Apostolidis NS, et al. Endoscopic transpapillary drainage of gallbladder empyema. Gastrointest Endosc. 1990;36(5):523–525. doi:10.1016/S0016-5107(90)71134-0
18. Itoi T, Coelho-Prabhu N, Baron TH. Endoscopic gallbladder drainage for management of acute cholecystitis. Gastrointest Endosc. 2010;71(6):1038–1045. doi:10.1016/j.gie.2010.01.026
19. Lee TH, Park DH, Lee SS, et al. Outcomes of endoscopic transpapillary gallbladder stenting for symptomatic gallbladder diseases: a multicenter prospective follow-up study. Endoscopy. 2011;43(8):702–708. doi:10.1055/s-0030-1256226
20. Maekawa S, Nomura R, Murase T, et al. Endoscopic gallbladder stenting for acute cholecystitis: a retrospective study of 46 elderly patients aged 65 years or older. BMC Gastroenterol. 2013;13:65. doi:10.1186/1471-230X-13-65
21. Jang JW, Lee SS, Song TJ, et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012;142(4):805–811. doi:10.1053/j.gastro.2011.12.051
22. Kedia P, Sharaiha RZ, Kumta NA, et al. Endoscopic gallbladder drainage compared with percutaneous drainage. Gastrointest Endosc. 2015;82(6):1031–1036. doi:10.1016/j.gie.2015.03.1912
23. Irani S, Ngamruengphong S, Teoh A, et al. Similar efficacies of endoscopic ultrasound gallbladder drainage with a lumen-apposing metal stent versus percutaneous transhepatic gallbladder drainage for acute cholecystitis. Clin Gastroenterol Hepatol. 2017;15(5):738–745. doi:10.1016/j.cgh.2016.12.021
24. Luk SW, Irani S, Krishnamoorthi R, et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019;51(8):722–732. doi:10.1055/a-0929-6603
25. Minaga K, Yamashita Y, Ogura T, et al. Clinical efficacy and safety of endoscopic ultrasound-guided gallbladder drainage replacement of percutaneous drainage: a multicenter retrospective study. Dig Endosc. 2019;31(2):180–187. doi:10.1111/den.13242
26. McCarthy ST, Tujios S, Fontana RJ, et al. Endoscopic transpapillary gallbladder stent placement is safe and effective in high-risk patients without cirrhosis. Dig Dis Sci. 2015;60(8):2516–2522. doi:10.1007/s10620-014-3371-4
27. Oh D, Song TJ, Cho DH, et al. EUS-guided cholecystostomy versus endoscopic transpapillary cholecystostomy for acute cholecystitis in high-risk surgical patients. Gastrointest Endosc. 2019;89(2):289–298. doi:10.1016/j.gie.2018.08.052
28. Yuste RT, Garcia-Alonso FJ, Sanchez-Ocana R, et al. Safety and clinical outcomes of endoscopic ultrasound-guided gallbladder drainage with lumen-apposing metal stents in patients with dwell time over one year. Ann Gastroenterol. 2019;32(5):514–521. doi:10.20524/aog.2019.0395
29. Walter D, Teoh AY, Itoi T, et al. EUS-guided gall bladder drainage with a lumen-apposing metal stent: a prospective long-term evaluation. Gut. 2016;65(1):6–8. doi:10.1136/gutjnl-2015-309925
30. Anderloni A, Fugazza A, Troncone E, et al. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest Endosc. 2019;89(1):69–76. doi:10.1016/j.gie.2018.08.047
31. Anderloni A, Troncone E, Fugazza A, et al. Lumen-apposing metal stents for malignant biliary obstruction: is this the ultimate horizon of our experience? World J Gastroenterol. 2019;25(29):3857–3869. doi:10.3748/wjg.v25.i29.3857
32. Imai H, Kitano M, Omoto S, et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016;84(1):147–151. doi:10.1016/j.gie.2015.12.024
33. Chen YY, Su PY, Shen WC. Gallbladder polyp treated with endoscopic polypectomy through a cholecystogastrostomy. Endoscopy. 2011;43(Suppl 2 UCTN):E88–E89. doi:10.1055/s-0030-1255983
34. Ge N, Sun S, Sun S, et al. Endoscopic ultrasound-assisted transmural cholecystoduodenostomy or cholecystogastrostomy as a bridge for per-oral cholecystoscopy therapy using double-flanged fully covered metal stent. BMC Gastroenterol. 2016;16:9. doi:10.1186/s12876-016-0420-9
35. Chan SM, Teoh AYB, Yip HC, et al. Feasibility of per-oral cholecystoscopy and advanced gallbladder interventions after EUS-guided gallbladder stenting (with video). Gastrointest Endosc. 2017;85(6):1225–1232. doi:10.1016/j.gie.2016.10.014
36. Tian L, Yang Y, Xiao D, et al. Resection of gallbladder polyps following endoscopic ultrasound-guided cholecystoduodenostomy using a lumen-apposing metal stent. Endoscopy. 2018;50(10):E307–E308. doi:10.1055/a-0631-7970
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.