Back to Journals » International Medical Case Reports Journal » Volume 13
Endogenous Nocardial Endophthalmitis Misdiagnosed as Giant Cell Arteritis
Received 3 September 2020
Accepted for publication 22 October 2020
Published 10 November 2020 Volume 2020:13 Pages 597—601
DOI https://doi.org/10.2147/IMCRJ.S277365
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Steven Gagnon,1– 3 Marc Saab1,2,4– 6
1Département d’ophtalmologie et d’oto-rhino-laryngologie – chirurgie cervico-faciale, Université Laval, Québec, QC, Canada; 2Hôpital Régional de Rimouski, Centre intégré de santé et de services sociaux du Bas-Saint-Laurent, Rimouski, QC, Canada; 3Centre Universitaire d’ophtalmologie (CUO), Hôpital du Saint-Sacrement, Québec, QC, Canada; 4Hôpital Charles-LeMoyne, Centre intégré de santé et de services sociaux de la Montérégie-Centre, Greenfield Park, QC, Canada; 5Service d’ophtalmologie, Université de Sherbrooke, Sherbrooke, QC, Canada; 6Département d’ophtalmologie, Université de Montréal, Montréal, QC, Canada
Correspondence: Steven Gagnon 1083, rue du Chevreau, Lévis, QC G6Z 3C3, Canada
Email [email protected]
Purpose: Endogenous endophthalmitis is uncommon but potentially dangerous. We present a fatal presentation of endogenous Nocardial endophthalmitis in the context of steroid use for treatment of giant cell arteritis.
Case Presentation: An 84-year-old Caucasian female presented to the local emergency room with severe headaches, myalgia and shoulder and calf muscle pain. She was treated for a presumed diagnosis of giant-cell arteritis with corticosteroids and subsequently developed an intense retro-orbital pain in the right eye. Fundus examination revealed a white, vascularized chorioretinal mass at the equator of the eye in the inferotemporal quadrant. Antibiotics were given and a vitrectomy was performed. The culture of the vitreous showed Nocardia nova and a diagnosis of disseminated Nocardiosis was made.
Conclusion and Significance: Although uncommon, it is important that ophthalmologists are aware of Nocardial infections as a differential diagnosis of retinal mass, particularly in immunocompromised patients.
Keywords: Nocardiosis, systemic, Nocardia, eye, intraocular, endophthalmitis, endogenous
Introduction
Nocardia is a known opportunistic gram-positive infection. Nocardia can disseminate to virtually any organ, with the typical portal of entry being the respiratory tract.1 In a 1994 literature review of 1050 cases, 39% of Nocardia infections were pulmonary, 32% of the infections were systemic, 17% were cutaneous or affected the central nervous system alone and 12% were extrapulmonary, namely the eyes or bone.2 Eye presentations of Nocardiosis are uncommon, and endogenous bacterial endophthalmitis is even more rare, highlighting the importance of this report.
Case Report
An 84-year-old Caucasian female presented to the local emergency department with a recent history of severe headaches, myalgia and shoulder and calf muscle pain. She had been recently diagnosed with polymyalgia rheumatic in the context of a three-month history of fatigue, weakness and fluctuating fever and was already on tapered dose of corticosteroids. The patient was otherwise known for atrial fibrillation on anticoagulation, atherosclerotic coronary heart disease, hypertension, and dyslipidemia. Past ocular history was unremarkable except for remote bilateral phacoemulsification cataract surgery.
On presentation at the ER, the patient was afebrile. There was no temporal artery tenderness or jaw pain, and C-reactive protein was mildly elevated at 52 mg/L. Platelets count was 313 x 109, white blood cells count was 19.9 x 109. No erythrocyte sedimentation was done. The tests were otherwise unremarkable.
The patient was prescribed oral prednisone 50 mg once daily and admitted. Twenty-four hours later, solumedrol 1g IV was prescribed once daily for 48 hours for a presumed diagnosis of polymyalgia rheumatica and atypical temporal arteritis as the symptoms worsen and C-reactive protein increased to 197 mg\L. At this point, the patient had no ocular symptoms.
Despite initial improvement, the patient relapsed after three days with worsening of symptoms and a new retro-orbital pain of the right eye. On examination by the ophthalmologist, the patient had 20/20 vision bilaterally, with normal adnexal structures and extraocular movements. Intraocular pressure was within normal limits and the visual field exam showed a superonasal quadranopsia. Anterior segment examination showed no sign of inflammation and fundus examination of the right eye showed a white, vascularized chorioretinal mass in the inferotemporal quadrant. The examination was unremarkable for the left eye. The diagnosis given by the general ophthalmologist was a possible malignant tumor and the patient was referred to a retinal specialist for further evaluation.
Investigations by an infectious disease specialist led to a concomitant diagnosis of emphysematous cystitis. Piperacillin tazobactam 3.3 g IV q 6 hours was prescribed to the patient and given the partial response to antibiotics, the corticosteroids were gradually eliminated. Piperacillin tazobactam was changed for ampicillin 2 g IV q 6 hours.
One day later, a PET scan was performed with results suggesting an inflammatory or infectious etiology. It showed two pulmonary opacities of unknown etiology and hypermetabolism in the right calf muscle, thought to be a hematoma. Large vessel arteritis was absent.
Three days later at follow-up, the patient’s vision in the right eye was significantly decreased from 20/20 to counting fingers. Panuveitis was present with a stage 4 vitreal haze on fundus exam. A B-scan was performed and revealed that the mass had increased in size and was extending into the vitreous. A diagnosis of endogenous endophthalmitis with a probable fungal etiology was made. A vitrectomy with a chorioretinal biopsy of the lesion was performed the next day. Intravitreal amphotericin B 5 mg/0.1 mL, vancomycin 1 mg/0.1 mL and ceftazidime 2.25 mg/0.1 mL were administered during the procedure. A biopsy of the left calf mass was made by needle biopsy.
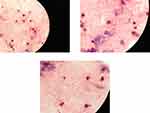
The culture of the vitreous (Figure 1) and biopsy of the left calf mass (Figure 2) showed high quantities of Nocardia nova. Disseminated Nocardiosis was therefore the final diagnosis.
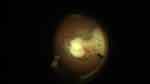 |
Figure 1 White chorioretinal mass. Posterior uveitis with a white chorioretinal mass at the inferotemporal quadrant seen during the vitrectomy and the biopsy. |
Ampicillin was stopped and changed for meropenem 2 g IV q 8h and TMP-SMX 400 mg IV q8h for 4 doses. A dose of 2 mg of intravitreal ceftriaxone was also planned. After the intraocular injection and the parenteral antibiotics, a regression of the intraocular mass, right calf mass and two pulmonary opacities was observed. A considerable improvement of the patient’s general condition was also noted. However, the patient decided to cease treatment given her age and other comorbidities. She was transferred to palliative care and died 18 days later.
Discussion
While eye presentations of Nocardiosis are uncommon, endogenous bacterial endophthalmitis is even more rare. In a retrospective analysis of microbiological profile of culture-proven cases of exogenous and endogenous endophthalmitis by Ramakrishnan and al. between January 1997 and December 2006, no endogenous endophthalmitis was caused by Nocardia. Of the 364 bacterial infectious endophthalmitis in the study, only 24 isolated Nocardia spp. and were all exogenous endophthalmitis cases, mostly after intraocular surgeries or penetrating ocular injuries.3,4
A search for other cases of endogenous intraocular Nocardial infections was conducted on PubMed. We searched for the keywords “nocardiosis”, “Nocardia”, “eye”, “intraocular”, “endophthalmitis” and “endogenous”. Between January 2000 and April 2020, only 26 cases of endogenous intraocular Nocardiosis infection have been reported.5–29 Most of these cases reported white masses or abscesses. One case reported a similar presentation of systemic Nocardiosis mimicking a giant-cell arteritis.29 This highlights the importance of keeping a large differential diagnosis, especially before prescribing corticosteroids.
Aside from the rarity of these cases, the diagnosis of Nocardia infection can be difficult as laboratories need adequate specimens, which often requires an invasive procedure to obtain.30 However, an earlier recognition of Nocardiosis upon eye examination may have saved the patient’s life. Prognosis could also have been different had she not have received large doses of corticosteroids the weeks prior to her ophthalmology consultation. Corticosteroids have inhibitory effects on a wide range of immune responses including profound effects on the cellular functions of leukocytes and endothelial cells, resulting in a reduction in the ability of leukocytes to adhere to the vascular endothelium and exit circulation.31–33 While this may be desired in the management of inflammatory and autoimmune disorders, corticosteroids can be detrimental to patients with active infections and should therefore be used with caution as it may delay diagnoses of infectious causes.
Conclusions
We present a case of endogenous Nocardia endophthalmitis, a rare but fatal condition. Although uncommon, it is important that ophthalmologists are aware of such entities and keep a large differential diagnosis upon seeing a retinal mass. Accordingly, Nocardia infection should always be considered in immunocompromised patients.
Consent for Publication
Our institution’s ethic committee does not require a consent for such articles as the patient is deceased and the case report does not contain any personal information. They did not need the family’s approval considering that the information was denominalised.
Acknowledgment
Special thanks to Dr Philippe Dolcé for the pictures of the cultures of the left calf centesis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding or grant support.
Disclosure
The authors declare that they have no competing interests.
References
1. Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22(6):
2. Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7(2):213–264. doi:10.1128/CMR.7.2.213
3. Ramakrishnan R, Bharathi MJ, Shivkumar C, et al. Microbiological profile of culture-proven cases of exogenous and endogenous endophthalmitis: a 10-year retrospective study. Eye. 2009;23(4):945–956. doi:10.1038/eye.2008.197
4. Hudson JD, Danis RP, Chaluvadi U, Allen SD. Posttraumatic exogenous nocardia endophthalmitis. Am J Ophthalmol. 2003;135(6):915–917. doi:10.1016/S0002-9394(02)02294-8
5. Wang S, Jiang B, Li Y, Shang Y, Liu Z, Zhang Y. A case report of disseminated nocardiosis with ocular involvement in a myasthenia gravis patient and literature review. BMC Neurol. 2019;19(1). doi:10.1186/s12883-019-1482-4
6. Horvath CE, Brinkmann CK, Ozdoba C, Leib SL, Wolf S, Wolf-Schnurrbusch UEK. Chorioretinal nocardiosis. Retin Cases Brief Rep. 2009;3(3):263–265. doi:10.1097/ICB.0b013e31817376eb
7. Bozbeyoglu S, Yilmaz G, Akova YA, Arslan H, Aydin P, Haberal M. Choroidal abscess due to nocardial infection in a renal allograft recipient. Retina. 2004;24(1):164–166. doi:10.1097/00006982-200402000-00027
8. Dutta Majumder P, Mukherjee M, Therese L, Gopal L, Biswas J. Diagnostic challenge with nocardia subretinal abscess: a case report from tuberculosis-endemic region. Ocul Immunol Inflamm. 2019;27(5):762–765. doi:10.1080/09273948.2018.1462391
9. Pelayes DE, Colombero D, Gioino JM, Zarate JO, Piantoni G. [Endogenous Nocardia asteroides endophthalmitis in a patient with systemic lupus erythematosus]. Medicina. 2004;64(2):146–148. Spanish.
10. Lee B, Drayna P, Maltry AC, Mason CM, Montezuma SR, Koozekanani D. Endogenous nocardia endophthalmitis presenting as a mass lesion in a patient with metatstatic nonsmall cell carcinoma of the lung. Retin Cases Brief Rep. 2019;13(2):145–149. doi:10.1097/ICB.0000000000000545
11. Kawakami H, Sawada A, Mochizuki K, Takahashi K, Muto T, Ohkusu K. Endogenous Nocardia farcinica endophthalmitis. Jpn J Ophthalmol. 2010;54(2):164–166. doi:10.1007/s10384-009-0782-4
12. Ravage ZB, Singerman LJ. Endogenous Nocardial chorioretinitis in an immunocompetent patient. Retin Cases Brief Rep. 2009;3(1):27–30. doi:10.1097/ICB.0b013e31814fae6f
13. Trehan H, Kaushik J, Jain VK, Parihar JKS, Avasthi A. Endogenous nocardial endophthalmitis in an immunosuppressed patient: a serious warning of an underlying life threatening and blinding disorder. J Ophthalmic Vis Res. 2017;12(1):113–116. doi:10.4103/2008-322X.200172
14. Kim JE, Landon RE, Connor TB, Kivlin JD. Endogenous ocular nocardiosis. J AAPOS. 2004;8(2):194–195. doi:10.1016/j.jaapos.2003.09.006
15. Eschle-Meniconi ME, Guex-Crosier Y, Wolfensberger TJ. Endogenous ocular nocardiosis—an interventional case report with a review of the literature. Surv Ophthalmol. 2011;56(5):383–415. doi:10.1016/j.survophthal.2011.03.003
16. de Silva T, Evans C, Mudhar HS, Rennie I, Green ST. Isolated endogenous endophthalmitis secondary to Nocardia spp in an immunocompetent adult. J Clin Pathol. 2006;59(11):1226.
17. Chen LY, Kesen MR, Ghafourian A, Nguyen QD, Eberhart CG, Do DV. Isolated endogenous Nocardia endophthalmitis after immunosuppression. J Ophthalmic Inflamm Infect. 2012;2(3):141–143. doi:10.1007/s12348-011-0057-3
18. Milman T, Trubnik V, Shah M, McCormick SA, Finger PT. Isolated Nocardia exalbida endogenous endophthalmitis. Ocul Immunol Inflamm. 2011;19(4):237–239. doi:10.3109/09273948.2011.563898
19. Navarrete-Navarrete N, Escobar Sevilla J, Toribio García M, Urbano F, Sabio JM, Jiménez-Alonso J. A man with unilateral endophthalmitis: a case of disseminated nocardiosis. Case Rep Infect Dis. 2015;2015:607421.
20. Eisenberg MA, Wilker SC. Nocardia asteroides subretinal abscess in patient with acute myelogenous leukemia after allogeneic stem cell transplant. Retin Cases Brief Rep. 2014;8(2):113–115. doi:10.1097/ICB.0000000000000017
21. Yap EY, Fam HB, Leong KP, Buettner H. Nocardia choroidal abscess in a patient with systemic lupus erythematosus. Aust N Z J Ophthalmol. 1998;26(4):337–338. doi:10.1111/j.1442-9071.1998.tb01340.x
22. Schriever S, Mistry-Burchardi N, Grabein B, et al. Nocardia farcinica: schwere Chorioiditis mit lebensbedrohlicher Generalisierung unter systemischer Immunsuppression. Klin Monbl Augenheilkd. 2002;219(3):164–167. doi:10.1055/s-2002-26724
23. Scott M, Mehta S, Rahman HT, Grossniklaus HE, Yeh S. Nocardia veterana endogenous endophthalmitis in a cardiac transplant patient. J Ophthalmic Inflamm Infect. 2013;3(1):44. doi:10.1186/1869-5760-3-44
24. Giuliari GP, Sadaka A, Eagle R, Gonzalez VH. Ocular nocardiosis in a renal transplant patient. Retin Cases Brief Rep. 2012;6(3):245–248. doi:10.1097/ICB.0b013e31822477c4
25. Angermann R, Stattin M, Zehetner C. Ocular nocardiosis: a case report. Ocul Immunol Inflamm. 2018;27(7):1114–1116. doi:10.1080/09273948.2018.1506041
26. Lally DR, Sharma DK, Shields CL, Malloy BC, Garg SJ. Pulmonary nocardiosis initially manifesting as endogenous endophthalmitis. Can J Ophthalmol. 2014;49(2):e59–e62. doi:10.1016/j.jcjo.2014.02.003
27. Lakosha H, Pavlin CJ, Lipton J. Subretinal abscess due to nocardia farcinica infection. Retina. 2000;20(3):247–269. doi:10.1097/00006982-200003000-00008
28. Dodds EM, Echandi LV, Puente SI, Kaufman S. Subretinal abscess due to nocardia farcinicaresistant to trimethoprim- sulfamethoxazole in a patient with systemic lupus erythematosus. Ocul Immunol Inflamm. 2006;14(4):249–251. doi:10.1080/09273940600760514
29. Héron E, Augustin P, Cervera P, et al. Systemic nocardiosis mimicking an ocular relapse of giant-cell arteritis. Rheumatology. 2006;45(5):641–643. doi:10.1093/rheumatology/kel064
30. Georghiou PR, Blacklock ZM. Infection with Nocardia species in Queensland: a review of 102 clinical isolates. Med J Aust. 1992;156(10):692–697. doi:10.5694/j.1326-5377.1992.tb121509.x
31. Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976;84(3):304–315. doi:10.7326/0003-4819-84-3-304
32. Fauci AS, Murakami T, Brandon DD, Loriaux DL, Lipsett MB. Mechanisms of corticosteroid action on lymphocyte subpopulations. VI. Lack of correlation between glucocorticosteroid receptors and the differential effects of glucocorticosteroids on T-cell subpopulations. Cell Immunol. 1980;49(1):43–50. doi:10.1016/0008-8749(80)90054-4
33. Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med. 1993;119(12):1198–1208. doi:10.7326/0003-4819-119-12-199312150-00007
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.