Back to Journals » International Journal of Nanomedicine » Volume 18
Encapsulated Oxovanadium(IV) and Dioxovanadium(V) Complexes into Solid Lipid Nanoparticles Increase Cytotoxicity Against MDA-MB-231 Cell Line
Authors Kostrzewa T , Nowak I , Feliczak-Guzik A , Drzeżdżon J, Jacewicz D, Górska-Ponikowska M, Kuban-Jankowska A
Received 18 January 2023
Accepted for publication 14 April 2023
Published 11 May 2023 Volume 2023:18 Pages 2507—2523
DOI https://doi.org/10.2147/IJN.S403689
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Phong A Tran
Tomasz Kostrzewa,1 Izabela Nowak,2 Agnieszka Feliczak-Guzik,2 Joanna Drzeżdżon,3 Dagmara Jacewicz,3 Magdalena Górska-Ponikowska,1,4,5 Alicja Kuban-Jankowska1
1Department of Medical Chemistry, Faculty of Medicine, Medical University of Gdansk, Gdansk, 80-211, Poland; 2Department of Applied Chemistry, Faculty of Chemistry, Adam Mickiewicz University, Poznań, 61-614, Poland; 3Department of Environmental Technology, Faculty of Chemistry, University of Gdansk, Gdansk, 80-308, Poland; 4IEMEST Istituto Euro-Mediterraneo di Scienza e Tecnologia, Palermo, 90127, Italy; 5Department of Biophysics, Institute of Biomaterials and Biomolecular Systems, University of Stuttgart, Stuttgart, 70174, Germany
Correspondence: Tomasz Kostrzewa; Alicja Kuban-Jankowska, Department of Medical Chemistry, Faculty of Medicine, Medical University of Gdansk, Gdansk, 80-211, Poland, Tel +48 58 349 14 50, Fax +48 58 349 14 56, Email [email protected]; [email protected]
Introduction: Solid lipid nanoparticles (SLN) have been considered lately as promising drug delivery system in treatment of many human diseases including cancers. We previously studied potential drug compounds that were effective inhibitors of PTP1B phosphatase – possible target for breast cancer treatment. Based on our studies, two complexes were selected for encapsulation into the SLNs, the compound 1 ([VO(dipic)(dmbipy)] · 2 H2O) and compound 2 ([VOO(dipic)](2-phepyH) · H2O). Here, we investigate the effect of encapsulation of those compounds on cell cytotoxicity against MDA-MB-231 breast cancer cell line. The study also included the stability evaluation of the obtained nanocarriers with incorporated active substances and characterization of their lipid matrix. Moreover, the cell cytotoxicity studies against the MDA-MB-231 breast cancer cell line in comparison and in combination with vincristine have been performed. Wound healing assay was carried out to observe cell migration rate.
Methods: The properties of the SLNs such as particle size, zeta potential (ZP), and polydispersity index (PDI) were investigated. The morphology of SLNs was observed by scanning electron microscopy (SEM), while the crystallinity of the lipid particles was analyzed by differential scanning calorimetry (DSC) and X-ray diffraction (XRD). The cell cytotoxicity of complexes and their encapsulated forms was carried out against MDA-MB-231 breast cancer cell line using standard MTT protocols. The wound healing assay was performed using live imaging microscopy.
Results: SLNs with a mean size of 160 ± 25 nm, a ZP of − 34.00 ± 0.5, and a polydispersity index of 30 ± 5% were obtained. Encapsulated forms of compounds showed significantly higher cytotoxicity also in co-incubation with vincristine. Moreover, our research shows that the best compound was complex 2 encapsulated into lipid nanoparticles.
Conclusion: We observed that encapsulation of studied complexes into SLNs increases their cell cytotoxicity against MDA-MB-231 cell line and enhanced the effect of vincristine.
Keywords: solid lipid nanoparticles, stability of nanoparticles, oxovanadium(IV) and dioxovanadium(V) complexes, triple-negative MDA-MB-231 breast cancer cell line, live imaging microscopy
Introduction
Solid lipid nanoparticles (SLNs) are currently one of the newest and most effective pharmaceutical delivery systems. Due to their small size and application properties, lipid nanoparticles have become a frequent subject of research on modified drug release.1 SLNs are characterized by a number of beneficial properties, including that their structure is based on lipids, which are well tolerated by the human organism, high stability, as well as the ability to transfer hydro- and lipophilic compounds. However, the greatest advantage of SLNs is safeguarding the stability of the encapsulated compound, increasing the bioavailability of the injected drug and their non-toxicity, which is the result of the biodegradable lipid structure.2 Many scientific studies prove the increase of the cytotoxic potency of compounds after their encapsulation into lipid nanoparticles.3 It is suggested that one of the mechanisms releasing therapeutic compounds from SLNs is liposome disintegration due to low pH in cancer cells.4,5
Protein tyrosine phosphatases (PTPs) are enzymes that control cell adhesion and migration. The mechanism of phosphorylation and dephosphorylation of the tyrosine residues of proteins has fundamental significance in the control of cell physiology, including proliferation, differentiation, migration, and tumorigenesis.6–9 In the last decade, there have been many papers suggesting that protein tyrosine phosphatases may be a molecular target in new therapeutic strategies. Compounds containing vanadium were shown to be one of the most effective inhibitors of PTPs.10
The first researches about the use of PTPs inhibition strategies focused on the treatment of type II diabetes, insulin resistance and obesity. This is due to the fact that the protein tyrosine phosphatase PTP1B is a physiological regulator of glucose homeostasis and plays a particularly important role in the regulation of the insulin receptor.11 As a result of the fact that vanadium compounds have been shown to suppress the activity of protein tyrosine phosphatases and to have an insulin-mimetic action, vanadium-based compounds are now being researched for their potential in the treatment of these metabolic disorders.12,13 The initial stage of the clinical studies included the use of two inorganic salts: vanadyl sulfate and ammonium metavanadate. Unfortunately, poor absorption and significant gastrointestinal discomfort prevented further investigation on these substances in antidiabetic treatment.14,15 The vanadium(IV) complex (bis(ethylmaltolato)oxidovanadium(IV)) has been examined in other clinical studies. The pharmacokinetic characteristics and bioavailability were enhanced by the addition of organic ligand in the coordination sphere. Unfortunately, despite the promising outcomes, the effectiveness of tested compound fluctuated.15 On the other hand, abnormal tyrosine phosphorylation shows a pro-oncogenic effect and can lead to an aggressive malignancy.16–19 Literature data indicate that compounds containing vanadium have been shown to have anti-cancer properties, such as the ability to inhibit cellular tyrosine phosphatases or reactive oxygen species (ROS) generation, via controlling a number of important signaling pathways, including oxidation of cysteine residue in PTPs.10,15
Despite over thousand vanadium compound medical patents, still none of them is used as a medicine due to the side effects shown.20 One of the strategy to eliminate the side effects of pharmaceuticals is to improve their selectivity through the use of drug delivery systems. Among the possibilities is the encapsulation of biologically active substances into solid lipid nanoparticles. Researchers focus on solid lipid nanoparticles because they can penetrate physiological barriers, have great skin compatibility, stratum corneum penetration, mechanical and chemical flexibility, biodegradability, and active ingredient protection from external influences.21 Moreover, it is indicated that SLNs can selectively deliver active compounds to cancer cells.22–25 An example of a drug used in the treatment of breast cancer encapsulated in SLNs is doxorubicin and tamoxifen. Encapsulation of these drugs into SLNs increased its accumulation in tumors, which elevated antitumor activity, but at the same time reduced side effects and improved its pharmacokinetic properties.4,26,27
According to the best of our knowledge, this work is one of the first in which vanadium complexes have been incorporated into lipid nanoparticles. In the article of Cacicedo et al, Metvan ([VIVO(Me2phen)2(SO4)] was incorporated into the lipid nanoparticles. The incorporation of this compound into SLN enhanced its cytotoxicity against bone cancer cells. In conclusion, the extension and stabilization of the complex were also discussed.28 Studies on protein tyrosine kinase (PTK) inhibitors have also shown an increase in the cytotoxic impact by increasing the bioavailability of the compounds. PTK are antagonists to tyrosine phosphatase and their dysregulation also promotes oncogenesis.29
In our last study five oxovanadium(IV) and dioxovanadium(V) complexes were identified as possible PTP1B inhibitors with anticancer efficacy against the MCF-7 breast cancer cell line, the triple negative MDA-MB-231 breast cancer cell line, and the human keratinocyte HaCaT cell line.30 We discovered that all of the compounds examined were potent PTP1B inhibitors, which correlates with anticancer activity. In this article, based on our previously obtained result, we present the most potent inhibitors on PTP1B phosphatase, [VO(dipic)(dmbipy)]·2 H2O (compound 1) and [VOO(dipic)](2-phepyH)·H2O (compound 2), which have been incorporated into lipid nanoparticles to assess its effect on the stability and cytotoxicity of these compounds (1.27, 1.40, and 1.52 times stronger for compound 1 and 2.17, 1.96, 1.73 times stronger for compound 2, at concentration 25, 50 and 100 µM of active compounds).
Materials and Methods
Chemicals and Reagents
POLOXAMER 188 Poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) and 2-Phenylpyridine (2-phephyH) was purchased from MERCK, Darmstadt, Germany. SOFTISAN 601 (Glycerides, C12-C18 mono- and di-, Glyceryl Stearate) was purchased from AZELIS, Poznan, Poland. 2,6-pyridinedicarboxylic acid (dipic), 4,4′-dimethoxy-2,2′-bipyridine (dmbipy), Dimethylsulfoxide (DMSO), Fetal bovine serum (FBS), 3-(4-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), para-nitrophenyl phosphate (pNPP) were purchased from Sigma-Aldrich. Dulbecco’s Modified Eagle’s Medium (DMEM) and Phosphate-buffered saline (PBS) were purchased from PAN-biotech, Aidenbach, Germany.
Synthesis of Complexes
The dipicolinate oxovanadium(IV) and dioxovanadium(V) complex compounds with 4,4′-dimethoxy-2,2′-bipyridyl and 2-phenylpyridine (Figure 1) were synthesized in the same way as described in the publications:31 for [VO(dipic)(dmbipy)] · 2 H2O and32 for [VOO(dipic)](2-phepyH) · H2O.
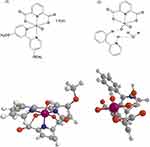 |
Figure 1 Simplified structural formulas of (1) [VO(dipic)(dmbipy)] · 2 H2O and (2) [VOO(dipic)](2-phepyH) · H2O (2D above, 3D below). |
[VO(dipic)(dmbipy)] · 2 H2O was synthesized in powder form and subjected to physico-chemical analysis. The results of elemental analysis of oxovanadium(IV) for [VO(dipic)(dmbipy)] · 2 H2O are 47.46% C, 3.69% H, and 8.77% N, where analysis calculations are the following: 47.10% C, 3.93% H, and 8.68% N. All the results concerning the analysis of the physicochemical properties of this complex are described in the literature.31 However, the second compound complex, ie, [VOO(dipic)](2-phepyH) · H2O was synthesized as crystals and for this reason it was subjected to XRD analysis. The complete crystallographic characterization of the obtained dipicolinate complex of dioxovanadium(V) with 2-phenylpyridine has been described in the publication.32
Synthesis of Solid Lipid Nanoparticles (SLN)
Synthesis of Hollow SLN-Type Lipid Nanoparticles Using HPH
Two phases were prepared: an aqueous phase (Poloxamer®188 (1.25% w/w) and demineralized water (95.75% w/w)) and a lipid phase, containing Softisan®601 (3.00% w/w). Both phases were heated to a temperature of 45°C, mixed with each other, and then subjected to initial high-speed homogenization for 30 seconds. Using an Ultra-Turrax®DI25 homogenizer. Then the obtained microemulsion was subjected to hot high-pressure homogenization with a Panda Plus 2000 homogenizer for 3 minutes (Set pressure 500 bar, temperature 45℃).
Synthesis of SLN-Type Lipid Nanoparticles with Active Substance Using HPH
Two phases were prepared: water, an aqueous phase, consisting of Poloxamer®188 (1.25% w/w) and demineralized water (95.25% w/w), and a lipid phase, containing Softisan®601 (3.00% w/w) and active substance (0.5% w/w). Both phases were heated to a temperature of 45°C, mixed with each other, and then subjected to high-speed preliminary homogenization for 30 seconds using an Ultra-Turrax®DI25 homogenizer. Then the obtained microemulsion was subjected to hot high-pressure homogenization with a Panda Plus 2000 homogenizer for 3 minutes. Set pressure 500 bar, temperature 45℃.
Lyophilization - Part of the Test Samples
The lyophilization process consisted in placing the preparation in a lyophilization chamber. As a result, the obtained lyophilizates (in powder form) were carefully protected against moisture absorption so that they could be stored at room temperature without cooling. Thus, thanks to the freeze-drying process, the risk of damage to the substance has been significantly reduced – compared to other methods of dehydration (at higher temperatures).
Characteristics of Synthesized Solid Lipid Nanoparticles
The physicochemical characteristics of the obtained lipid nanoparticle dispersions included both the assessment of the basic textural and structural parameters (SEM; DSC and XRD) and the assessment of the stability of the obtained lipid nanoparticle formulations using the dynamic method (DLS) and electrophoretic light scattering (ELS).
Investigation of the Stability of the Obtained Lipid Nanoparticles
The stability of the obtained dispersions of lipid nanoparticles was tested with the use of the Zetasizer Nano ZS device (Malvern Instruments). The stability study included the measurement of three parameters of the physicochemical characteristics – mean particle size (Z-Ave), polydispersity index (PDI), zeta potential (ZP) and consisted of placing 1 mL of the prepared sample into the measuring cell, which was then inserted into the device. The stability of the obtained dispersions of lipid nanoparticles was tested at room temperature after the synthesis and after 7 and 28 days. For each test sample, the procedure was repeated three times, and the arithmetic mean and standard deviation were calculated from the obtained results.
Characteristics of the Lipid Matrix of the Obtained Lipid Nanoparticles
The study of the degree of crystallinity and polymorphs of the lipid matrix of the obtained lipid nanoparticles was carried out based on the results of diffraction analysis obtained with the use of the D8 Advance powder diffractometer with a Johansson monochromator (Bruker). For each tested sample, the procedure was performed once, using the high angle range (2Θ = 6.0–60.0°) and obtaining results in the form of diffractograms.
The study of polymorphic variants of the lipid matrix of the obtained lipid nanoparticles was also carried out based on the results of the DSC thermal analysis obtained with the use of the DSC 8500 differential scanning calorimeter (Perkin Elmer, USA). The measuring principle was to gradually heat the sample from 25°C to 90°C in nitrogen flow (20 mL/min) at a scanning rate of 5°C per minute, keep the sample at 90°C for 1 minute, and then cool it to 25°C with similar parameters. For each tested sample, the procedure was performed once, obtaining results in the form of DSC curves (a graph of the relationship between the measured difference of heat fluxes and temperature) and the determined melting point and reaction enthalpy.
The morphology of lipid nanoparticles was determined using a scanning electron microscope (SEM).
Cell Lines and Culture
MDA-MB-231 breast cancer cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cell lines were grown in Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin as supplements. The cells were grown in a 37°C, 5% CO2 atmosphere.
Cell Viability Assay
An MTT test, which measures cell viability, was carried out in accordance with the standard methodology.30,33–37 Briefly, cells (1.2·106 cells/mL) were treated to serial solutions of tested compounds. In a growth medium containing 1% penicillin/streptomycin, the compounds were dissolved. MTT solution (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) was added at 0.5 mg/mL after 24 hours. Once the purple precipitate was clearly visible under the microscope, 100 µL of DMSO was added to each well. The absorbance at 492 nm was measured using a microplate reader (Biogenet, Jozefow, Poland) and DigiRead Communication Software (Asys Hitech GmbH, Eugendorf, Austria). The percentage of untreated cells was the unit of data expression (control).
Live Imaging Microscopy - Scratch Assay
The scratch test was performed to investigate cell migration in vitro. For this purpose, the CytoSMART LUX2 microscope was used, which enables the visualization of the progress of the gap closing.38,39 The tests were carried out in culture conditions, in an incubator with standard parameters (37°C, 5% CO2 atmosphere). The test was carried out for 24 hours with snapshot every 5 minutes.
Statistical Analysis
The results of triplicate experiments are provided as mean ± SD. Best-fit linear regression was used. Data was entered into GraphPad Prism (GraphPad Software, v.4, La Jolla, CA, USA).30,33–37 Regression analysis was used. This study also used one-way ANOVA combined with Dunnett. The data were expressed as mean ± SD. P < 0.05 indicated significant mean differences.
Results and Discussion
The Dipicolinate Complexes of Oxovanadium(IV) and Dioxovanadium(V) with N, O-Donor Ligands
The dipicolinate complexes of oxovanadium(IV) and dioxovanadium(V) with 4.4′-dimethoxy-2,2′-bipyridyl and 2-phenylpyridine (Figure 1) are known as precatalysts for 2-propen-1-ol, 2-chloro-2-propen-1-ol, and norbornene oligomerizations. These complexes exhibit high catalytic activity upon activation by modified methylaluminoxane MMAO-12. The values of catalytic activity of [VO(dipic)(dmbipy)] · 2 H2O and [VOO(dipic)](2-phepyH) · H2O in the 2-chloro-2-propen-1-ol oligomerization are 522.07 and 281.56 g mmol−1 h−1, respectively.31
In addition, the dipicolinate dioxovanadium(V) complex with 2-phenylpyridine has valuable properties from a biological point of view, namely, it has antioxidant capacity against superoxide anion radical. [VOO(dipic)](2-phepyH) · H2O has stronger antioxidant activity than
Investigation of the Stability of the Obtained Lipid Nanoparticles
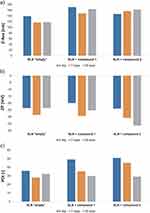
Analyzing the data obtained from the stability assessment of the studied lipid nanoparticle dispersions (non-incorporated (empty) and incorporated with samples of compound 1 and 2), it was observed that the most favorable physicochemical parameters (Figure 2) in terms of mean particle size and polydispersity index were obtained in the case of lipid nanoparticles containing the compound 2 (the lowest PDI, highest absolute value of ZP). The zeta potential determines the potential stability of the studied dispersion of lipid nanoparticles based on the analysis of the movement process of dispersed particles. In theoretical terms, it is defined as the electrical potential existing at the outer boundary of the electric double layer of the particle, contributing to the electrostatic repulsion of particles of the same charge, conditioning the appropriate degree of dispersion. The absolute value of the potential at a level higher than |± 30 mV| defines dispersions of lipid nanoparticles as stable, showing no tendency to aggregation and other changes of unstable nature.40 The obtained results (mean particle size ~ 160 nm, polydispersity index ~ 30%, zeta potential: |±36.00| ± 0.5mV) were considered favorable from the point of view of subsequent medical application.
A positive effect of the incorporation of active substances on physicochemical parameters (↑ ZP) on the long-term stability of lipid nanoparticles containing them was observed.41 Additionally, the polydispersity index (PDI) is an indication of particle quality with respect to the size distribution.
The term “polydispersity” (or “dispersity” as recommended by IUPAC) is used to describe the degree of non-uniformity of a size distribution of particles.42 Controlling and validating this parameter is of key importance for the effective clinical applications of carriers. Thus, the PDI of 0.3 and below is considered to be acceptable and indicates a homogenous population of lipid vesicles43 and other nanocarriers.44
Characteristics of the Lipid Matrix of the Obtained Lipid Nanoparticles
The lipid matrix of the obtained lipid nanoparticles was characterized using three compatible research techniques (SEM, DSC and XRD), obtaining information on the morphology, degree of crystallinity and polymorphic form of the lipid used.
Scanning Electron Microscope
The images obtained because of the SEM analysis (Figure 3) made it possible to assess the shape and morphology of the investigated dispersions of lipid nanoparticles. The analysis of the images confirmed the spherical-continuous shape of the obtained lipid nanoparticles and their size in the range of 2 μm (up to 200 nm), predisposing them to be used as components of medical products. The images show that the high-pressure homogenization method produces a polydisperse sample with a wide size distribution.45 Moreover, some of the particles formed large “clusters” (agglomerates). This is a characteristic phenomenon due to the “sticky” nature of the lipid, especially after long-term storage. This effect has already been observed many times in other studies on the synthesis of SLNs incorporated with active substances of different origins [as above].
 |
Figure 3 SEM images of SLNs (A) “empty” (B) containing compound 1, and (C) containing compound 2. |
Differential Scanning Calorimetry
On the DSC curves (Figure 4) of the carrier systems containing compound 1 and compound 2, a characteristic exothermic peak (for cooling) was observed, at higher temperatures than for pure lipid, and an increase in enthalpy (heat of reaction) confirming the effective incorporation of active compounds into the nanoparticles, not disturbing the expected structure (β’ form) of the lipid matrix. The detailed results of the parameters determined during the DSC analysis are presented in the table above (Table 1). DSC analysis of lipid nanoparticles concerned in particular the study of thermal properties of lipids (included in the tested SLN), ie, the analysis of thermal phenomena potentially occurring in lipids (eg, crystallization, melting, thermal oxidation.46 On this basis, it was possible to identify polymorphism (polymorphism) frequently present in lipids.47 The incorporation of compound 1 and compound 2 into the lipid matrix influenced the course of thermal processes.
 |
Table 1 Melting Point and Enthalpy Values for the Materials Obtained |
 |
Figure 4 Comparison of DSC thermograms of the solid lipid nanoparticles containing oxovanadium(IV) and dioxovanadium(V) complexes. |
Powder X-Ray Diffraction
Typical diffraction reflections at angles 2Θ=19.1° and 23.4° were observed on the diffractograms of the studied lipid nanoparticle dispersions, confirming the β’ polymorph, characteristic for lipids based on triacylglycerols in the colloidal state (Figure 5). In comparison with the diffractogram of the solid lipid, a reduced intensity of diffraction of the identified signals was noted, which was, in turn: (i) “empty” lipid nanoparticles – 4784 a.u. and 3502 a.u.; (ii) lipid nanoparticles incorporated compound 1–2934 a.u. and 1980 a.u.; (iii) lipid nanoparticles incorporated compound 2–2436 a.u. and 1876 a.u., respectively, for 2Θ = 19.1° and 23.4°. As a result, it was again concluded that both the reference sample and SLNs incorporated with active substances have a slightly ordered structure and are in the β’ form.48
 |
Figure 5 Powder X-Ray Diffraction for lipid (red line), “empty” SLN (gray line), SLN incorporated compound 1 (blue line) and SLN incorporated compound 2 (green line). |
The Cytotoxicity Against MDA-MB-231 Breast Cancer Cell Lines
Cytotoxicity against MDA-MB-231 breast cancer cell line was performed for VO(dipic)(dmbipy)] · 2 H2O (compound 1) and [VOO(dipic)](2-phepyH) · H2O (compound 2) and also for their encapsulated form, in concentration of 25, 50 and 100 µM of tested compounds. The concentration of the active compound in the lipid nanoparticle was determined on the basis of weight percentage (10,52% by Weight).
According to the obtained results (Figure 6), complex 1 ([VO(dipic)(dmbipy)· 2 H2O]) at concentrations of 25–100 µM decreased cell viability by 28.40 ± 8.62%, 40.19 ± 3.02% and 51.23 ± 9.64%, respectively, while its encapsulated form ([VO(dipic)(dmbipy)] SLN) at the same concentration of active substance reduced cell viability by 43.54 ± 4.32%, 57.25 ± 3.56% and 67.90 ± 4.63%, respectively. With increasing complex content, the cytotoxicity ratio (defined as the ratio of cell viability after treatment with non-encapsulated compound to lipid nanoparticles containing active substance) was 1.27, 1.40, and 1.52, respectively.
In the case of complex 2 ([VOO(dipic)(2-phephy) · H2O]), we noticed a decrease in cell viability of 25.84 ± 4.45%, 32.01 ± 6.32%, and 47.16 ± 4.45%, respectively, whereas the encapsulated form ([VOO(dipic)(2-phephy)] SLN) decreased cell viability by 67.82 ± 4.65%, 68.51 ± 2.01%, and 71.64 ± 2.42%, respectively. The ratio of non-encapsulated to encapsulated cytotoxicity for compound 2 was 2.17, 1.96, 1.73, respectively.
The obtained results indicate that the encapsulated forms of the active compound showed a stronger cytotoxic effect on MDA-MB-231 cells, with the most noticeable effect being compound 2. As an effective inhibitor of several phosphate metabolizing enzymes, orthovanadate(V), an orthophosphate analogue, has been widely employed to study the processes of various enzymes.49 Vanadium is capable of existing in a wide variety of oxidation states, in addition to the oxyanions and oxycations generated by dissolved in a solution. The chemistry of vanadium is significantly more complicated than that of many other metals, because of its many oxidation states, accessible availability for hydrolysis, and potential for polymerization. There are two forms of vanadium that are able to dissolve in natural waters: V(IV) and V(V). The nutritional qualities and toxicity properties of these forms are distinct from one another. The binding of vanadium to proteins in biological samples is extremely sensitive to changes in their local environment. The redox properties, permeability and bioavailability are crucial to study the effect of vanadium-based compounds.50,51 Chemically attaching lipids to pharmaceuticals in the process known as lipid-drug conjugation (LDC) is an efficient method for increasing the liposolubility of pharmaceuticals. Encapsulation of compounds undergoing redox reactions into SLNs improves its properties.52,53 Similar properties have been investigated for encapsulated epigallocatechin gallate.54,55 In our earlier research, we found that PTP1B inhibition may be one of the molecular mechanisms by which green tea polyphenols exhibit their cytotoxic effects on breast cancer cells MCF-7.36 Comparing the cytotoxicity of epigallocatechin gallate against MCF-7 cells, encapsulation of this compound resulted in an approximately 2-fold decrease in the IC50 value.36,55
In our research encapsulated form of complexes, in the same concentrations of active compounds, shows stronger cytotoxicity effects. In our opinion, based on our research, the lipid nanoparticles protected the stability of the encapsulated compounds, selectively delivered them inside the cell, where their structure was biodegraded, and as a result of these, the complexes could show their biological properties.
Cell Migration
The scratch assay studies cell migration in vitro. Creating a scratch in a cell monolayer, capturing pictures at different time intervals to monitor gap closure, and quantifying gap closure and/or cell migration rate are the first steps of a wound healing test. In our studies, using real-time microscopy, we observed the growth of cells treated with the compound at a concentration of 10 µM, both non-encapsulated form and encapsulated form of complexes. Snapshots were taken by the microscope every 5 minutes for 24 hours. Based on artificial intelligence algorithms, the surface of the uncovered scratch was calculated. The percentage of overgrowth was calculated as the ratio of the scratch area to the initial area at the representative images.
In the study of complexes not encapsulated into lipid nanoparticles (Figure 7), compound 1 showed no significant differences from the control. However, cells treated with compound 2 stopped growing at 27% free space after 24 hours. In the study of encapsulated forms of compounds into lipid nanoparticles (Figure 8), we also tested empty nanoparticles as a control. Compound 2 deserves attention again. Encapsulation of this compound and testing the activity at a concentration of 10 µM of active substance caused stop growing cells after 24 hours and reaching a confluence of 35%, whereas compound 1 showed 57% of confluence.
 |
Figure 7 Representative images for the wound healing assay against MDA-MD-231 breast cancer cell line for compound 1 and 2 at concentration 10 µM. |
 |
Figure 8 Representative images obtained from wound healing assay against MDA-MD-231 breast cancer cell line for encapsulated form of compound 1 and 2 at concentration of active substances 10 µM. |
Protein tyrosine phosphatases, also known as PTPs, are the enzymes that are responsible for regulating the tyrosine phosphorylation process. This mechanism is what controls cell adhesion and migration. Aberrant tyrosine phosphorylation may cause cancer with abnormal growth and metastasis.16,17,56,57 PTP1B, an ubiquitously expressed nonreceptor PTPs localizes in the endoplasmic reticulum, may be a therapeutic target for the prevention and treatment of breast cancer.58,59 This enzyme increases growth of HER2-positive and triple-negative breast cancer cells, because it promotes the proliferation, and suppresses the apoptosis.60 The movement of individual cells, cell sheets, and cell clusters from one place to another is known as the cell migration.61 PTP1B is responsible for cell adhesion and migration of cancer.62 These processes are related to prognosis, tumor growth rate and the potential for metastasis. The wound healing assay can be used to extrapolate results from in vitro to in vivo conditions.63,64 The effect of reducing the migration and proliferation of cancer cells can be used in supportive therapy and inhibition of PTP1B appears to be an effective adjuvant treatment option.
Recent studies indicate that dietary vanadium may have cancer-preventative properties.10,49,51,65 Our research demonstrates that the encapsulation of compounds into lipid nanoparticles may affect the speed of cancer cell growth (Figure 9). Compounds which were encapsulated into SLNs were selected as inhibitors of PTP1B (based on our previous research; compound 1 IC50 185.4 ± 9.8 nM; Ki 74.2 ± 4.1, compound 2 IC50 167.2 ± 8.0 nM; Ki 66.7 ± 3.3 nM).30 Interestingly, the cell growth was stopped in both the encapsulated and non-encapsulated forms of this compound. However, the effect was almost twice as strong for the compounds encapsulated into lipid nanoparticles. What needs to be emphasized, also empty SLNs show an anti-migration effect, which is the same as for compound 1. Additionally, Compound 2 showed a large discrepancy in activity in the first 6 hours of action. This may be related to the unstable oxidation form of the vanadium(V) complex. Probably, the encapsulation of this complex resulted in maintaining the stability of the compound. In light of this, we propose that lipid nanoparticles could serve as an effective delivery system for vanadium compounds to pass through the membrane, where they can interact with molecular targets.
The Influence of the Tested Compounds on the Cytotoxicity of Vincristine
Vincristine is a drug that is used to treat breast cancer in advanced stages. Unfortunately, it has many side effects.66 To reduce the toxicity of the utilized chemotherapeutic drugs, attempts are thus undertaken to lower the dosages. Because of this, treatments with a synergistic effect are frequently employed, allowing the dosages of the pharmaceuticals to be used to be decreased. For this reason, we present the results for the co-incubation of tested vanadium complexes with a selected concentration of vincristine, which showed a low cytotoxic effect. In our research, we examined the effect of co-incubation of complex compounds in concentration of 10 µM of active substance (forms non-encapsulated and encapsulated into lipid nanoparticles) with vincristine at concentration of 250 nM (Figure 10).
Cell viability after 250nM vincristine treatment was 76.57 ± 5.86% and for compound 1 and 2 in concentration of 10 µM was 82.31 ± 5.44% and 92.69 ± 5.18%, respectively. Encapsulation of these compounds into SLNs resulted in an increase in cytotoxicity by 17.8 ± 2.04% for compound 1 and 31.53 ± 7.18% for compound 2. Vincristine co-incubation with complexes increased the cytotoxic potency. For compound 1 cell viability was 62.16 ± 2.71% and for compound 2 53.46 ± 4.35%. For encapsulated forms, cell viability was respectively: 45.03 ± 3.02% and 42.66 ± 2.63%. Encapsulation of compounds into lipid nanoparticles resulted in an increase in cytotoxic potency by 17.11% for compound 1 and 10.81% for compound 2.
In our opinion, this study confirms that compounds that have inhibitory properties against PTP1B can be considered as potential strategies to complement standard treatment. The obtained results are promising, but further preclinical and early phase studies are needed. In addition, it is necessary to conduct research in relation to normotypic cells. However, based on many studies, appropriately selected or modified lipid nanoparticles show cytocompatibility and safety against healthy cells.27,44 Based on our research, co-incubation of substances with lipid nanoparticle-encapsulated substances increased their cytotoxicity against breast cancer cell line. We believe that this kind of therapeutic approach can be taken in designing new therapeutic protocols that also use treatment-supporting substances.
Conclusions
Despite observable advances in cancer treatment, contemporary medicine continues to struggle with the issue of completely successful chemotherapy. Consequently, there is a pressing need for a greater application of existing knowledge in the field of the development and synthesis of new compounds as well as the method of their delivery to the body, which will be characterized by appropriate efficacy, selectivity, and specificity of action on cancer cells. Research in bioinorganic chemistry has recently focused on designing and synthesizing new compounds with the aim of treating human diseases. Compounds based on metals or capable of binding metal ions, such as vanadium-based compounds, are particularly promising. Vanadium complexes are one of the compounds that inhibit protein tyrosine phosphatase. The chemical form (oxidation state, ligand type, and geometric arrangement) of vanadium-based complexes affects their physicochemical and biological properties. The fact that inorganic PTP inhibitors are typically non-specific and can have an effect on a diverse selection of human enzymes is the greatest disadvantage associated with these compounds. In our latest work, we presented five oxovanadium(IV) and dioxovanadium(V) complexes as potential PTP1B inhibitors with anticancer activity against MCF-7 breast cancer cells, triple negative MDA-MB-231 breast cancer cells, and human keratinocyte HaCaT cells. All compounds inhibited PTP1B, corresponding with anticancer action. Compounds presented in this paper, compound 1 ([VO(dipic)(dmbipy)] · 2 H2O) and compound 2 ([VOO(dipic)](2-phepyH) · H2O), caused the highest inhibitory effect, with IC50 185.4 ± 9.8 and 167.2 ± 8.0 nM, respectively. To investigate the effect of encapsulation on the cytotoxicity activity against MDA-MB-231 cell line, we chose these substances, which had the greatest activity against PTP1B, to encapsulate into solid lipid nanoparticles. The stability evaluation data showed that lipid nanoparticle dispersions containing compound 2 had the best physicochemical parameters. As a result of the conducted experiments, we noticed that the encapsulation of these compounds increased also their cytotoxic activity against the MDA-MB-231 cell line. Encapsulated forms of compounds showed significantly higher cytotoxicity (1.27, 1.40, and 1.52 times stronger for compound 1 and 2.17, 1.96, 1.73 times stronger for compound 2, at concentration 25, 50 and 100 µM of active compounds), also in co-incubation with vincristine. In addition, these compounds showed a better anti-migration effect. In the future, it is necessary to conduct other studies to compare the toxicity to normotypic cells. Thanks to this, it will be possible to determine the safety of the lipid nanoparticles used.
Abbreviations
2-phephyH, 2-Phenylpyridine; dipic, 2,6-pyridinedicarboxylic acid; DMEM, Dulbecco’s Modified Eagle’s Medium; DMSO, Dimethylsulfoxide; dmbipy, 4,4′-Dimethoxy-2,2′-bipyridine; FBS, Fetal bovine serum (FBS); DSC, Differential Scanning Calorimetry; MTT, 3-(4-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; pNPP, para-nitrophenyl phosphate; PBS, Phosphate-buffered saline; PDI, polydispersity index; PTP, Protein Tyrosine Phosphatase; PTP1B, Protein Tyrosine Phosphatase PTP1B; SEM, Scanning electron microscope; SLN, Solid Lipid Nanoparticles; Z-Ave, Comparison of average particle size; ZP, zeta potential; XRD, X-Ray Diffraction.
Institutional Review Board Statement
The Institutional review board statement and ethical approval was not required for these studies.
Acknowledgments
M.G.-P. acknowledges to ST46 project (Medical University of Gdansk, Poland).
Funding
These studies were supported by Grant No. 664/259/63/73-3319 from Medical University of Gdańsk Grant (“Excellence Initiative—Research University”).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Carbone C, Arena E, Pepe V, et al. Nanoencapsulation strategies for the delivery of novel bifunctional antioxidant/σ1 selective ligands. Colloids Surf B Biointerfaces. 2017;155:238–247. doi:10.1016/J.COLSURFB.2017.04.016
2. Souto EB, Müller RH. Lipid nanoparticles: effect on bioavailability and pharmacokinetic changes. Handb Exp Pharmacol. 2010;197(197):115–141. doi:10.1007/978-3-642-00477-3_4
3. García-Pinel B, Porras-Alcalá C, Ortega-Rodríguez A, et al. Lipid-based nanoparticles: application and recent advances in cancer treatment. Nanomaterials. 2019;9:4. doi:10.3390/NANO9040638
4. Fontana G, Maniscalco L, Schillaci D, Cavallaro G, Giammona G. Solid lipid nanoparticles containing tamoxifen characterization and in vitro antitumoral activity. Drug Deliv. 2005;12(6):385–392. doi:10.1080/10717540590968855
5. Scioli Montoto S, Muraca G, Ruiz ME. Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Front Mol Biosci. 2020;7:319. doi:10.3389/FMOLB.2020.587997/BIBTEX
6. den Hertog J, Groen A, van der Wijk T. Redox regulation of protein-tyrosine phosphatases. Arch Biochem Biophys. 2005;434(1SPEC):11–15. doi:10.1016/j.abb.2004.05.024
7. Elson A. Stepping out of the shadows: oncogenic and tumor-promoting protein tyrosine phosphatases. Int J Biochem Cell Biol. 2018;96:135–147. doi:10.1016/J.BIOCEL.2017.09.013
8. Chen PJ, Zhang YT. Protein tyrosine phosphatase 1B (PTP1B): insights into its new implications in tumorigenesis. Curr Cancer Drug Targets. 2022;22(3):181–194. doi:10.2174/1568009622666220128113400
9. Julien SG, Dubé N, Read M, et al. Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nat Genet. 2007;39(3):338–346. doi:10.1038/ng1963
10. Evangelou AM. Vanadium in cancer treatment. Crit Rev Oncol Hematol. 2002;42(3):249–265. doi:10.1016/S1040-8428(01)00221-9
11. Galic S, Hauser C, Kahn BB, et al. Coordinated regulation of insulin signaling by the protein tyrosine phosphatases PTP1B and TCPTP. Mol Cell Biol. 2005;25(2):819. doi:10.1128/MCB.25.2.819-829.2005
12. Tsiani E, Fantus IG. Vanadium compounds: biological actions and potential as pharmacological agents. Trends Endocrinol Metabol. 1997;8(2):51–58. doi:10.1016/S1043-2760(96)00262-7
13. Shaik A, Kondaparthy V, Aveli R, Vijjulatha M, Sree Kanth S, Das Manwal D. Interaction of vanadium metal complexes with protein tyrosine phosphatase-1B enzyme along with identification of active site of enzyme by molecular modeling. Inorg Chem Commun. 2021;126:108499. doi:10.1016/J.INOCHE.2021.108499
14. Koren S, Fantus IG. Inhibition of the protein tyrosine phosphatase PTP1B: potential therapy for obesity, insulin resistance and type-2 diabetes mellitus. Best Pract Res Clin Endocrinol Metab. 2007;21(4):621–640. doi:10.1016/J.BEEM.2007.08.004
15. Irving E, Stoker AW. Vanadium Compounds as PTP Inhibitors. Molecules. 2017;22:12. doi:10.3390/MOLECULES22122269
16. Hendriks WJAJ, Elson A, Harroch S, Stoker AW. Protein tyrosine phosphatases: functional inferences from mouse models and human diseases. FEBS J. 2008;275(5):816–830. doi:10.1111/j.1742-4658.2008.06249.x
17. Östman A, Hellberg C, Böhmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6(4):307–320. doi:10.1038/nrc1837
18. Liu X, Chen Q, Hu XG, et al. PTP1B promotes aggressiveness of breast cancer cells by regulating PTEN but not EMT. Tumour Biol. 2016;37(10):13479–13487. doi:10.1007/S13277-016-5245-1
19. Zheng LY, Zhou DX, Lu J, Zhang WJ, Zou DJ. Down-regulated expression of the protein-tyrosine phosphatase 1B (PTP1B) is associated with aggressive clinicopathologic features and poor prognosis in hepatocellular carcinoma. Biochem Biophys Res Commun. 2012;420(3):680–684. doi:10.1016/J.BBRC.2012.03.066
20. Treviño S, Díaz A, Sánchez-Lara E, Sanchez-Gaytan BL, Perez-Aguilar JM, González-Vergara E. Vanadium in biological action: chemical, pharmacological aspects, and metabolic implications in diabetes mellitus. Biol Trace Elem Res. 2018;188(1):68–98. doi:10.1007/S12011-018-1540-6
21. Musielak E, Feliczak-Guzik A, Nowak I. Synthesis and potential applications of lipid nanoparticles in medicine. Materials. 2022;15:682. doi:10.3390/MA15020682
22. Nassimi M, Schleh C, Lauenstein HD, et al. Low cytotoxicity of solid lipid nanoparticles in in vitro and ex vivo lung models. Inhal Toxicol. 2009;21(SUPPL.1):104–109. doi:10.1080/08958370903005769
23. Serpe L, Catalano MG, Cavalli R, et al. Cytotoxicity of anticancer drugs incorporated in solid lipid nanoparticles on HT-29 colorectal cancer cell line. Eur J Pharm Biopharm. 2004;58(3):673–680. doi:10.1016/J.EJPB.2004.03.026
24. Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59(6):491–504. doi:10.1016/J.ADDR.2007.04.008
25. Bayón-Cordero L, Alkorta I, Arana L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials. 2019;9(3):474. doi:10.3390/NANO9030474
26. Oliveira MS, Aryasomayajula B, Pattni B, Mussi SV, Ferreira LAM, Torchilin VP. Solid lipid nanoparticles co-loaded with doxorubicin and α-tocopherol succinate are effective against drug-resistant cancer cells in monolayer and 3-D spheroid cancer cell models. Int J Pharm. 2016;512(1):292–300. doi:10.1016/J.IJPHARM.2016.08.049
27. Al-Jubori AA, Sulaiman GM, Tawfeeq AT, Mohammed HA, Khan RA, Mohammed SAA. Layer-by-layer nanoparticles of tamoxifen and resveratrol for dual drug delivery system and potential triple-negative breast cancer treatment. Pharmaceutics. 2021;13(7):1098. doi:10.3390/PHARMACEUTICS13071098/S1
28. Cacicedo ML, Ruiz MC, Scioli-Montoto S, et al. Lipid nanoparticles – metvan: revealing a novel way to deliver a vanadium compound to bone cancer cells. New J Chem. 2019;43(45):17726–17734. doi:10.1039/C9NJ01634A
29. Satapathy S, Patro CS. Solid lipid nanoparticles for efficient oral delivery of tyrosine kinase inhibitors: a nano targeted cancer drug delivery. Adv Pharm Bull. 2022;12(2):298–308. doi:10.34172/apb.2022.041
30. Kostrzewa T, Jończyk J, Drzeżdżon J, et al. Synthesis, in vitro, and computational studies of ptp1b phosphatase inhibitors based on oxovanadium(IV) and dioxovanadium(V) complexes. Int J Mol Sci. 2022;23:13. doi:10.3390/IJMS23137034
31. Drzeżdżon J, Pawlak M, Gawdzik B, et al. Dipicolinate complexes of oxovanadium(IV) and dioxovanadium(V) with 2-phenylpyridine and 4,4′-dimethoxy-2,2′-bipyridyl as new precatalysts for olefin oligomerization. Materials. 2022;15(4):1379. doi:10.3390/MA15041379/S1
32. Drzeżdżon J, Pawlak M, Matyka N, Sikorski A, Gawdzik B, Jacewicz D. Relationship between antioxidant activity and ligand basicity in the dipicolinate series of oxovanadium(Iv) and dioxovanadium(v) complexes. Int J Mol Sci. 2021;22(18):9886. doi:10.3390/IJMS22189886/S1
33. Kostrzewa T, Przychodzen P, Gorska-Ponikowska M, Kuban-Jankowska A. Curcumin and cinnamaldehyde as PTP1B inhibitors with antidiabetic and anticancer potential. Anticancer Res. 2019;39(2):745–749. doi:10.21873/ANTICANRES.13171
34. Kostrzewa T, Sahu KK, Gorska-Ponikowska M, Tuszynski JA, Kuban-Jankowska A. Synthesis of small peptide compounds, molecular docking, and inhibitory activity evaluation against phosphatases PTP1B and SHP2. Drug Des Devel Ther. 2018;12:4139–4147. doi:10.2147/DDDT.S186614
35. Kuban-Jankowska A, Gorska-Ponikowska M, Sahu KK, Kostrzewa T, Wozniak M, Tuszynski J. Docosahexaenoic acid inhibits PTP1B phosphatase and the viability of MCF-7 breast cancer cells. Nutrients. 2019;11:11. doi:10.3390/nu11112554
36. Kuban-Jankowska A, Kostrzewa T, Musial C, et al. Green tea catechins induce inhibition of PTP1B phosphatase in breast cancer cells with potent anti-cancer properties: in vitro assay, molecular docking, and dynamics studies. Antioxidants. 2020;9(12):1208. doi:10.3390/ANTIOX9121208
37. Kostrzewa T, Wołosewicz K, Jamrozik M, et al. Curcumin and its new derivatives: correlation between cyto-toxicity against breast cancer cell lines, degradation of ptp1b phosphatase and ros generation. Int J Mol Sci. 2021;22(19):10368. doi:10.3390/IJMS221910368/S1
38. Okada T, Chino Y, Yokoyama K, et al. Design and synthesis of novel pipernonaline derivatives as anti-austerity agents against human pancreatic cancer PANC-1 cells. Bioorg Med Chem. 2022;71:116963. doi:10.1016/J.BMC.2022.116963
39. ’Thijssen – van Loosdregt I, Regnard G, Frankfort M, Koedood N. Collective cell migration assays; 2021.
40. Doktorovova S, Souto EB. Nanostructured lipid carrier-based hydrogel formulations for drug delivery: a comprehensive review. Expert Opin Drug Deliv. 2009;6(2):165–176. doi:10.1517/17425240802712590
41. Rodenak-Kladniew B, Islan GA, de Bravo MG, Durán N, Castro GR. Design, characterization and in vitro evaluation of linalool-loaded solid lipid nanoparticles as potent tool in cancer therapy. Colloids Surf B Biointerfaces. 2017;154:123–132. doi:10.1016/J.COLSURFB.2017.03.021
42. Danaei M, Dehghankhold M, Ataei S, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57. doi:10.3390/PHARMACEUTICS10020057
43. Shekunov BY, Chattopadhyay P, Tong HHY, Chow AHL. Particle size analysis in pharmaceutics: principles, methods and applications. Pharm Res. 2007;24(2):203–227. doi:10.1007/S11095-006-9146-7
44. Abbas ZS, Sulaiman GM, Jabir MS, et al. Galangin/β-cyclodextrin inclusion complex as a drug-delivery system for improved solubility and biocompatibility in breast cancer treatment. Molecules. 2022;27(14):4521. doi:10.3390/MOLECULES27144521
45. Mazuryk J, Deptuła T, Polchi A, et al. Rapamycin-loaded solid lipid nanoparticles: morphology and impact of the drug loading on the phase transition between lipid polymorphs. Colloids Surf a Physicochem Eng Asp. 2016;502:54–65. doi:10.1016/J.COLSURFA.2016.05.017
46. Kardas M, Grochowska NE. Differential Scanning Calorimetry as a thermoanalytical method used in pharmacy and food analysis. Bromat Chem Toksykol. 2009;2:224–230.
47. Tan CP, Che MY. Differential scanning calorimetric analysis of edible oils: comparison of thermal properties and chemical composition. J Am Oil Chem Soc. 2000;77:143–155. doi:10.1007/s11746-000-0024-6
48. Hou DZ, Xie CS, Huang KJ, Zhu CH. The production and characteristics of solid lipid nanoparticles (SLNs). Biomaterials. 2003;24(10):1781–1785. doi:10.1016/S0142-9612(02)00578-1
49. Chasteen ND, Grady JK, Holloway CE. Characterization of the binding, kinetics, and redox stability of vanadium(IV) and vanadium(V) protein complexes in serum. Inorg Chem. 1986;25(16):2754–2760. doi:10.1021/IC00236A021/ASSET/IC00236A021.FP.PNG_V03
50. Pyrzyńska K, Wierzbicki T. Determination of vanadium species in environmental samples. Talanta. 2004;64(4):823–829. doi:10.1016/J.TALANTA.2004.05.007
51. Crans DC, Mahroof-Tahir M, Keramidas AD. Vanadium chemistry and biochemistry of relevance for use of vanadium compounds as antidiabetic agents. Vanadium Comp. 1995;17–24. doi:10.1007/978-1-4613-1251-2_2
52. Du Y, Ling L, Ismail M, et al. Redox sensitive lipid-camptothecin conjugate encapsulated solid lipid nanoparticles for oral delivery. Int J Pharm. 2018;549(1–2):352–362. doi:10.1016/J.IJPHARM.2018.08.010
53. Mohammed HA, Sulaiman GM, Anwar SS, et al. Quercetin against MCF7 and CAL51 breast cancer cell lines: apoptosis, gene expression and cytotoxicity of nano-quercetin. Nanomedicine. 2021;16(22):1937–1961. doi:10.2217/NNM-2021-0070
54. Fangueiro JF, Andreani T, Fernandes L, et al. Physicochemical characterization of epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf B Biointerfaces. 2014;123:452–460. doi:10.1016/J.COLSURFB.2014.09.042
55. Silva AM, Martins-Gomes C, Fangueiro JF, Andreani T, Souto EB. Comparison of antiproliferative effect of epigallocatechin gallate when loaded into cationic solid lipid nanoparticles against different cell lines. Pharm Dev Technol. 2019;24(10):1243–1249. doi:10.1080/10837450.2019.1658774
56. Alonso A, Sasin J, Bottini N, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117(6):699–711. doi:10.1016/J.CELL.2004.05.018
57. Navis AC, van den Eijnden M, Schepens JTG, Hooft Van Huijsduijnen R, Wesseling P, Hendriks WJAJ. Protein tyrosine phosphatases in glioma biology. Acta Neuropathol. 2010;119(2):157–175. doi:10.1007/s00401-009-0614-0
58. Diluvio G, Del Gaudio F, Giuli MV, et al. NOTCH3 inactivation increases triple negative breast cancer sensitivity to gefitinib by promoting EGFR tyrosine dephosphorylation and its intracellular arrest. Oncogenesis. 2018;7:5. doi:10.1038/S41389-018-0051-9
59. Frangioni JV, Beahm PH, Shifrin V, Jost CA, Neel BG. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992;68(3):545–560. doi:10.1016/0092-8674(92)90190-N
60. Yu M, Liu Z, Liu Y, et al. PTP1B markedly promotes breast cancer progression and is regulated by miR-193a-3p. FEBS J. 2019;286(6):1136–1153. doi:10.1111/FEBS.14724
61. Hulkower KI, Herber RL. Cell migration and invasion assays as tools for drug discovery. Pharmaceutics. 2011;3(1):107–124. doi:10.3390/PHARMACEUTICS3010107
62. Hilmarsdottir B, Briem E, Halldorsson S, et al. Inhibition of PTP1B disrupts cell–cell adhesion and induces anoikis in breast epithelial cells. Cell Death Dis. 2017;8(5):e2769–e2769. doi:10.1038/cddis.2017.177
63. Moutasim KA, Nystrom ML, Thomas GJ. Cell migration and invasion assays. Methods Protoc. 2011;333–343. doi:10.1007/978-1-61779-080-5_27/COVER
64. Pijuan J, Barceló C, Moreno DF, et al. In vitro cell migration, invasion, and adhesion assays: from cell imaging to data analysis. Front Cell Dev Biol. 2019;7:107. doi:10.3389/FCELL.2019.00107/BIBTEX
65. Ferretti VA, León IE. An overview of vanadium and cell signaling in potential cancer treatments. Inorganics. 2022;10(4):47. doi:10.3390/INORGANICS10040047
66. Vincristine. Available from: https://www.breastcancer.org/drugs/vincristine.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.