Back to Journals » OncoTargets and Therapy » Volume 12
Elevated apolipoprotein B predicts poor postsurgery prognosis in patients with hepatocellular carcinoma
Authors Yan X, Yao M, Wen X, Zhu Y , Zhao E , Qian X , Chen X, Lu W, Lv Q, Zhang L, Lu F
Received 29 October 2018
Accepted for publication 25 January 2019
Published 12 March 2019 Volume 2019:12 Pages 1957—1964
DOI https://doi.org/10.2147/OTT.S192631
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Xiaotong Yan,1,* Mingjie Yao,2,* Xiajie Wen,2 Yiwei Zhu,3 Erjiang Zhao,4 Xiangjun Qian,2 Xiangmei Chen,2 Weiquan Lu,4 Quanjun Lv,3 Ling Zhang,5 Fengmin Lu1,2
1Department of Epidemiology and Biostatistics, College of Public Health, Zhengzhou University, Zhengzhou 450001, China; 2Department of Microbiology and Infectious Disease Center, School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China; 3Department of Nutrition and Food Hygiene, College of Public Health, Zhengzhou University, Zhengzhou 450001, China; 4Department of Medical Records, Henan Cancer Hospital, Zhengzhou 450008, China; 5Department of Hepatobiliary Surgery, Henan Cancer Hospital, Zhengzhou 450008, China
*These authors contributed equally to this work
Aims: To date, curative resection remains to be the most optimal therapeutic choice of hepatocellular carcinoma (HCC), though the overall survival (OS) remains extremely unsatisfactory. To better manage the HCC patients, we evaluated the prognosis predicting values of apolipoprotein B (ApoB) and low-density lipoprotein cholesterol (LDL-C) on the long-time survival of patients who underwent surgical treatment in this study.
Methods: A subgroup of 164 patients from our previously described follow-up cohort were enrolled in this study, of whom the pre-surgery ApoB and LDL-C measurements were available. They had been followed until January 2017, with a 19.5 months median survival time. The prognosis predicting values of serum ApoB, LDL-C, and other clinical variables were evaluated through Cox univariate and multivariate analyses, meanwhile, Kaplan–Meier analysis was conducted to obtain the OS curves.
Results: Pre-surgery ApoB was an independent prognosis predicting factor with HR as 1.396 (P=0.033), elevated ApoB was associated with worse postsurgery prognosis in HCC patients. Concordantly, Spearman’s correlation analysis revealed that value of pre-surgery ApoB was to some extent correlated with tumor size (r=0.355, P<0.001). In line with this, further univariate and multivariate logistic regression analysis revealed that patients with higher ApoB value were more likely to have larger tumor size (≥5 cm), with the OR value as high as 2.221 (95% CI: 1.288–3.830, P=0.004). Additionally, level of ApoB was found to be highly correlated with the serum level of LDL-C (r=0.686, P<0.001).
Conclusion: ApoB could be a valuable novel prognosis predicting marker for HCC patients who underwent curative liver resection. Moreover, elevated ApoB level could indicate worse outcome in HCC patients, which could be explained by the relationship between ApoB and residual liver function.
Keywords: hepatocellular carcinoma, curative resection, survival, apolipoprotein B
Introduction
Primary liver cancer is the sixth most commonly diagnosed human malignancies and the fourth leading cause of cancer-related death worldwide in 2018, with about 841,000 new cases and 782,000 deaths annually, and hepatocellular carcinoma (HCC) accounts for 75%–80% of primary liver cancer.1 Although new therapeutic regimens have been developed in recent years, to date, surgery remains to be the optimal therapeutic choice for HCC. Unfortunately, the prognosis of HCC remains unsatisfactory because of high recurrence and metastasis postsurgery.2,3 So, to better manage the HCC patients, it has always been important to find proper preoperative clinical and pathological features and markers to predict the prognosis of HCC after surgery.
Since a majority of HCC patients have liver fibrosis or cirrhosis as underlying disease, the residual liver function has been considered as an essential factor in determining the outcomes of HCC patients who underwent curative resection.4 Therefore, factors reflecting the residual liver function should be considered as the preferred candidate predictors for the postsurgery overall survival (OS) of HCC patients. Since organ liver plays a fundamental role in lipid metabolism, and alterations of liver function are correlated with modifications of circulating lipids, so the measurement of blood lipid content could be important to evaluate the progression of liver disease.5,6 Apolipoprotein (Apo), which mainly includes apolipoprotein A1 (ApoA1) and apolipoprotein B (ApoB), is a group of serum lipoproteins synthesized in the liver. It has been reported that lower serum ApoA1 level 1 was associated with poor prognosis among HCC patients.7 However, whether ApoB is related to the prognosis of HCC patients remains unknown.
ApoB is the main Apo of chylomicron and low-density lipoprotein (LDL). The role of ApoB includes carrying lipids, participating in lipoprotein metabolism, and in the recognition of lipoprotein receptors. ApoB mRNA editing is the deamination of a specific cytidine (nt 6666) to uridine in the ApoB transcript. This deamination results in the formation of an ApoB protein (ApoB48) about one-half the size of the full-length genomically encoded ApoB (apoB100). In humans, the liver synthesizes ApoB100, the major protein component of plasma LDL. The small intestine produces ApoB48, a protein necessary for the secretion of chylomicrons. Lipoproteins containing ApoB48 are rapidly cleared from plasma and are not converted to LDL.8,9 ApoB100 is the dominating protein in plasma compared with minute amounts of ApoB48 even in the postprandial state. Therefore, ApoB is the nomenclature most often used unless specific studies are performed focusing on ApoB48.10 Seventy five percent of LDL combines with LDL receptor (LDLR) through its surface ApoB100 and is cleared by the liver and its peripheral tissue; so, the level of low-density lipoprotein cholesterol (LDL-C) in the serum is closely related to ApoB count. It has been shown that high plasma LDL-C level was associated with poor disease-free survival in patients with breast tumor and advanced stage epithelial ovarian cancers.11,12 So we wondered whether the serum levels of ApoB and LDL-C could be used to predict the postsurgery survival among HCC patients.
In this study, we mainly explored whether ApoB and LDL-C were independent prognosis predictors of HCC patients after surgical treatment through the use of Cox univariate and multivariate analyses. In the end, the possible mechanism was discussed.
Methods
Study population
A total of 164 patients who underwent surgical treatment from February 2009 to July 2013 in Henan Cancer Hospital, Zhengzhou, China, were enrolled in this study. The majority of patients were infected with hepatitis B virus (HBV) and they were part of the formal following-up cohort previously reported.4,13,14 In the current study, each enrolled patient met all the following criteria: 1) patients were diagnosed with HCC only, but with no concomitant intrahepatic cholangiocarcinoma, or any other malignancies, to eliminate the confounding effects from disease etiology; 2) patients had ApoB and LDL-C data and the other clinical data possible; 3) patients were successfully followed up until January, 2017; and 4) liver resection was performed on the resectable HCC and none of them received chemotherapy or radiotherapy before the surgery. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Peking University Health Science Center, Beijing, China. Written informed consents were obtained from all the participants. For patients who were children, written informed consents were obtained from their guardians.
Clinical data collection
Data of each patient were collected with the permission of the hospital’s ethnic committee, including gender, age at the surgery, cirrhotic status, presence of portal vein tumor thrombus (PVTT), tumor size, HBV (+/−), Barcelona Clinic Liver Cancer (BCLC) stage (A/B/C), and Child–Pugh stage (A/B). The other laboratory data included total bilirubin (T-bil) value, alanine transaminase value, glutamic oxalacetic transaminase or aspartate transaminase (AST) value, alkaline phosphatase (ALP) value, g-glutamyl transpeptidase (GGT) value, albumin (Alb) value, prothrombin time (PT) value, prealbumin (PA) value, total bile acid (TBA) value, ApoA1 value, LDL-C value, very low-density lipoprotein cholesterol value, high-density lipoprotein cholesterol (HDL-C) value, and ApoB value. Only the data from the last examination before surgery were taken into analysis if multiple laboratory tests were performed. In addition, the Child–Pugh grades A–C were determined by the following five assessments: Alb, T-bil, the PT/international normalized ratio, ascites, and hepatic encephalopathy; the BCLC stages were determined by Child–Pugh classification, performance status, and tumor status, and were classified into classes A–D.
Follow-up
The patients were followed up by telephoning or visiting them or their relatives to consult on their physical conditions. The first follow-up was carried out 1 month after hospital discharge when the patients were advised to return to the hospital for a routine examination. The second follow-up was at the end of the third month after surgery. After that, the patients were followed-up every 3 months for the first year, every 6 months for the second and the third year, and once a year for the fourth and the fifth year. We would ask for the exact date of death and the main cause if a patient died during the follow-up. The last case of patient was enrolled in June 2013, the last instance of follow-up was conducted in January 2017. The patients who were alive at the end of the research period were censored.
Statistical analyses
The Cox univariate and multivariate analysis models were used to identify the potential independent factors related to survival. The OS rate after surgery was tested by the Kaplan–Meier and the differences among the curves were analyzed using the log-rank analysis; the 1-, 3-, and 5-year survival rates were conducted by Life Tables. Correlations between ApoB and basic clinical data were analyzed using the Spearman’s correlation. The associations between the patients’ lipid metabolism biomarkers and tumor size were performed by using univariate and multivariate logistic regression. All of the statistical tests were based on a two-sided probability, with a significance level of 0.05, and were performed in SPSS 21.0 (Xishu Software Company, Shanghai, China).
Results
Patient characteristics
The preoperative clinic pathologic data of 164 HCC patients who had undergone curative resection are shown in Table 1. Among them, 140 (85.4%) of the patients were male and 24 (14.6%) were female; the mean age of the patients was 53 years (range 28–78). Besides, 154 patients (93.9%) had a background of cirrhosis, and HBV infection was the major causative factor of the cohort, with 135 (84.9%) patients were HBV positive. Of all, seven patients were lost during the follow-up visit. Therefore, the OS analysis was performed and the prognosis predicting values of ApoB and other 21 clinical variables were evaluated in the remaining 157 patients who were successfully followed-up.
Identification of the independent risk factors for poor prognosis in HCC patients who underwent surgical treatment
To investigate the risk factors for poor prognosis after surgical treatment, the preoperative ApoB and LDL-C levels, as well as other 19 potential clinical variables, were analyzed by univariate analysis. As shown in Table 2, ApoB as well as tumor size, tumor number, PVTT, BCLC, AST, ALP, GGT, and Alb were identified as the candidate risk factors for poor prognosis. Further multivariate analysis through Cox proportional hazards model identified that elevated ApoB level, advanced BCLC stage, and lower Alb level were the independent risk factors for poor prognosis in patients with HCC after surgical treatment (Table 3). Unlike in patients with breast tumor or epithelial ovarian cancers,11,12 level of LDL-C exhibited no prognosis correlation in HCC patients, at least in this cohort.
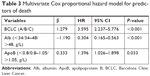  | Table 3 Multivariate Cox proportional hazard model for predictors of death |
Elevated ApoB was associated with worse prognosis in HCC patients
According to the normal reference range of ApoB level (0.8–1.05 g/L), the patients were divided into three groups based on each patient’s preoperative ApoB measurement: group 1 was composed of patients with ApoB level <0.8 g/L, group 2 was composed of patients with ApoB falling in the normal reference range, while group 3 was composed of patients with ApoB level >1.05 g/L. Kaplan–Meier curves for OS rate of patients in three groups are plotted in Figure 1. The OS of the patients in group 3 was significantly poorer than that of the patients in group 1 (P=0.015). The median survival time in three groups was 22.4, 15.5, and 6.03 months, respectively, and the survival time in group 3 is much shorter than in group 1 (P=0.015). In concordance, the 1-, 3-, and 5-year survival rates of patients in group 1 were 66%, 40%, and 29%, respectively, which were much higher than the respective OS rates of 36%, 24%, and 15% in group 3 (P=0.018). However, the 1-, 3-, and 5-year survival rates of patients in group 2 were 57%, 28%, and 21%, which has no statistical difference with group 1 (P=0.249) and group 3 (P=0.125).
Correlations between ApoB and other basic clinical variables
In order to explore the relationship between ApoB and other clinical variables, the bivariate correlations were conducted. As shown in Table 4, the preoperative ApoB was observed to be associated with tumor size and LDL-C, the correlation coefficient between ApoB and LDL-C was noticeably high (r=0.686, P<0.001). It was worthwhile to notice that HCC patients with higher ApoB level were also more likely to have higher ALP, α-fetoprotein (AFP), GGT, but had nothing to do with the Child–Pugh score (Table 4).
Elevated ApoB was a predictive factor for tumor size ≥5 cm at diagnosis
We noticed from Figure 2 that the significant increase in tumor sizes was in parallel with the elevated ApoB level in the serum. In other words, patients in group 3 had tumor larger in size when compared with that in group 1 (P<0.001) and group 2 (P=0.006). To further validate such associations between tumor size and the quantification of patients’ lipid metabolism biomarkers, univariate and multivariate logistic regression analysis was performed. The result showed that gender, ApoB, and ApoA1 are independent predictor factors for tumor size ≥5 cm at diagnosis. The patients who had higher ApoB level were more likely to have the tumor size ≥5 cm, with the OR value as high as 2.221 (95% CI: 1.288–3.830, P=0.004). Conversely, the patients who had higher ApoA1 count were less likely to have the tumor size ≥5 cm, with the OR value of 0.430 (95% CI: 0.219–0.844, P=0.014). (Table 5).
Discussion
The outcome of a patient after receiving certain treatment is of importance when a therapeutic strategy has to be recommended. In addition to tumor-related factors, including tumor size, number of tumors, vascular invasion, and AFP,14–17 as well as p53 mutation reported by us and others,18 the poor outcome of HCC could also be attributed to the factor, which influenced the basic function of residual liver after curative surgical treatment. Therefore, for the proper management of HCC patients, a practicable and effective prognostic factor with higher accuracy is extremely needed.
Liver plays a fundamental role in lipid metabolism and is thought to be the major assembled center for the production of most endogenous lipids, Apos, and lipoproteins. Recently, abnormal lipid metabolism has been validated to be a vital metabolic reprogramming process in cancer cells,19 and the majority of researches about the relationship between LDL-C and cancer are concentrated in breast cancer. One article suggested that LDL-C levels at diagnosis emerged as a prognostic factor in breast cancer patients and patients with high levels of LDL-C at diagnosis had reduced DFS, which was favored by the strong association of LDL-C level and tumor size before treatment.11 Another published data supported the notion that cancer cells were able to uptake cholesterol from the bloodstream, for example, plasma LDL-C could be used by cancer cells. Also, preoperative ApoB/ApoA1 ratio has been found to be a novel prognostic factor for gastric cancer.20 As it has been reported in the literature that low level of ApoA1 is associated with poor prognosis in HCC patients,7 our article mainly tested whether ApoB, LDL-C, and HDL-C were independent prognosis predictors of HCC patients.
In our results, we identified that tumor size, tumor number, PVTT, BCLC stage, AST, ALP, GGT, Alb, PA, ApoA1, and ApoB were the risk factors for poor prognosis in patients with HCC after surgical treatment through Cox univariate analysis (Table 2). In order to select the independent predictors for postsurgery prognosis of HCC patients, further Cox multivariate analysis was conducted, and the result showed that BCLC stages, Alb, and ApoB were significant predictors (Table 3). LDL-C level at diagnosis has been suggested as a prognostic factor in breast cancer patients.11 However, it exhibited no prognostic correlation in HCC patients, at least in this cohort. As ApoB was related to LDL-C, with correlation coefficient between them as high as 0.686 (P<0.001), the relationship between LDL-C and cancer has been reported in several articles12,21 and some metabolic-related index, such as LDL-C was found to be closely related to cancer cells.22–24 So, we could speculate that tumors might grow rapidly in the high ApoB group, which is consistent with the correlation between ApoB and tumor size in our result (r=0.355, P<0.001). To validate the associations between the patients’ lipid metabolism biomarkers and tumor size, further analysis was performed using univariate and multivariate logistic regression. The result showed that gender, ApoB, and ApoA1 are independent predictor factors for tumor size ≥5 cm at diagnosis. The patients who had higher ApoB level were more likely to have the tumor size ≥5 cm, with the OR value as high as 2.221 (95% CI: 1.288–3.830, P=0.004). Conversely, the patients who had higher ApoA1 count were less likely to have the tumor size ≥5 cm, with the OR value of 0.430 (95% CI: 0.219–0.844, P=0.014). It has been proved that ApoA1 can inhibit tumor cell proliferation, induce apoptosis, and impair their extracellular matrix degradation.7 So, we could speculate that ApoB might be related to the tumor growth.
We noticed that Lee et al25 reported that ApoB might play a role in regulating multiple genes involved in HCC development, and ablation of APOB in tumor tissues was significantly associated with poor clinical outcome in HCC patients and increased proliferation of HCC cells. Differently, Lin et al26 showed that ApoB was not an independent risk factor for HCC development in their cohort. As we know, besides malignancy of the tumor tissue, the residual liver function could also affect the HCC patient’s outcome. ApoB is synthesized and degraded in the liver, the content of ApoB in serum is related not only to the speed of synthesis but also to the state of degradation. ApoB is a major constitutive protein of LDL and VLDL. Triglycerides, ApoB100, and cholesterol are assembled into VLDL after liver synthesis and then secreted into the blood circulation. The lipoprotein esterase attached to the vessel wall breaks down the triglycerides of VLDL into free fatty acids and glycerol, providing energy to peripheral tissues. In this process, VLDL is transformed to intermediate-density lipoprotein and LDL, LDL combines with LDLR through ApoB100 on its surface and ApoB100 is broken down into amino acids in the liver for the next cycle. We speculated that the elevated ApoB level in the serum reflected the reduced expression of LDLR, and the poor postoperative prognosis might be associated with the poor residual liver function.
To the best of our knowledge, this is the first report to discover the clinical significance of serum ApoB levels for predicting the postoperative prognosis of HCC patients. Of course, there are some limitations in our study. For instance, as the sample size is not very large and the data are collected from a single hospital, the representation of our result is limited. Besides, the majority of patients in our cohort have the background of HBV infection, so the results are more suitable for HBV-related HCC patients. As a result, the aforementioned result obtained through this observation study need to be confirmed in the future with multicenter cohort studies and the underlying mechanism relevant to ApoB’s effect on the overall postoperative survival of HCC patients also need to be studied. Since the measurement of ApoB is becoming common in clinical practice and the lipoprotein metabolism could be an important therapeutic target in HCC patients, we expect that more data will be generated and eventually clear the potential use of ApoB in the management of HCC patients with tumors >5 cm.
Conclusion
ApoB could be a valuable novel prognosis predicting marker for HCC patients who underwent curative liver resection. And elevated ApoB level in serum could indicate worse outcome in HCC patients, which could be explained by the relationship between ApoB and residual liver function.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No. 2017YFC0908100), the National S & T Major Project for Infectious Diseases (Nos 2017ZX10201201, 2017ZX10202202, 2017ZX10302201, and 2017ZX10202203), the project from the Beijing Municipal Science & Technology Commission (No. Z161100000116047), and the project funded by the China Postdoctoral Science Foundation (No. 2017M620544 and 2018T110014).
Disclosure
The authors report no conflicts of interest in this work.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. | ||
Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. | ||
Ji J, Shi J, Budhu A, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361(15):1437–1447. | ||
Wen X, Yao M, Lu Y, et al. Integration of prealbumin into Child–Pugh classification improves prognosis predicting accuracy in HCC patients considering curative surgery. J Clin Transl Hepatol. 2018;6(4):377–384. | ||
Gibbons GF, Wiggins D, Brown AM, Hebbachi AM. Synthesis and function of hepatic very-low-density lipoprotein. Biochm Soc Trans. 2004;32(1):59–64. | ||
Olofsson SO, Borèn J. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J Intern Med. 2005;258(5):395–410. | ||
Ma XL, Gao XH, Gong ZJ, et al. Apolipoprotein A1: a novel serum biomarker for predicting the prognosis of hepatocellular carcinoma after curative resection. Oncotarget. 2016;7(43):70654–70668. | ||
Okuyama S, Marusawa H, Matsumoto T, et al. Excessive activity of apolipoprotein B mRNA editing enzyme catalytic polypeptide 2 (APOBEC2) contributes to liver and lung tumorigenesis. Int J Cancer. 2012;130(6):1294–1301. | ||
Yamanaka S, Balestra ME, Ferrell LD, et al. Apolipoprotein B mRNA-editing protein induces hepatocellular carcinoma and dysplasia in transgenic animals. Proc Natl Acad Sci U S A. 1995;92(18):8483–8487. | ||
Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy-a review of the evidence. J Intern Med. 2006;259(5):493–519. | ||
Rodrigues Dos Santos C, Fonseca I, Dias S, Mendes de Almeida JC. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Cancer. 2014;14:132. | ||
Li AJ, Elmore RG, Chen IY, Karlan BY. Serum low-density lipoprotein levels correlate with survival in advanced stage epithelial ovarian cancers. Gynecol Oncol. 2010;116(1):78–81. | ||
Xie X, Yao M, Chen X, et al. Reduced red blood cell count predicts poor survival after surgery in patients with primary liver cancer. Medicine (Baltimore). 2015;94(8):e577. | ||
Yao M, Zhao J, Lu F. Alpha-fetoprotein still is a valuable diagnostic and prognosis predicting biomarker in hepatitis B virus infection-related hepatocellular carcinoma. Oncotarget. 2016;7(4):3702–3708. | ||
Hu XG, Mao W, Park YK, Xu WG, Kim BW, Wang HJ. Blood neutrophil-to-lymphocyte ratio predicts tumor recurrence in patients with hepatocellular carcinoma within Milan criteria after hepatectomy. Yonsei Med J. 2016;57(5):1115–1123. | ||
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(6):1430–1430. | ||
Reichl P, Mikulits W. Accuracy of novel diagnostic biomarkers for hepatocellular carcinoma: an update for clinicians (review). Oncol Rep. 2016;36(2):613–625. | ||
Wen X, Lu F, Liu S. Prognostic value of p53 mutation for poor outcome of Asian primary liver cancer patients: evidence from a cohort study and meta-analysis of 988 patients. Onco Targets Ther. 2016;9:7425–7433. | ||
Liu Q, Luo Q, Halim A, Song G. Targeting lipid metabolism of cancer cells: a promising therapeutic strategy for cancer. Cancer Lett. 2017;401:39–45. | ||
Ma MZ, Yuan SQ, Chen YM, Zhou ZW. Preoperative apolipoprotein B/apolipoprotein A1 ratio: a novel prognostic factor for gastric cancer. Onco Targets Ther. 2018;11:2169–2176. | ||
Pires LA, Hegg R, Freitas FR, et al. Effect of neoadjuvant chemotherapy on low-density lipoprotein (LDL) receptor and LDL receptor-related protein 1 (LRP-1) receptor in locally advanced breast cancer. Braz J Med Biol Res. 2012;45(6):557–564. | ||
Clendening JW, Pandyra A, Boutros PC, et al. Dysregulation of the mevalonate pathway promotes transformation. Proc Natl Acad Sci U S A. 2010;107(34):15051–15056. | ||
Deberardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20. | ||
Ginestier C, Monville F, Wicinski J, et al. Mevalonate metabolism regulates basal breast cancer stem cells and is a potential therapeutic target. Stem Cells. 2012;30(7):1327–1337. | ||
Lee G, Jeong YS, Kim DW, et al. Clinical significance of APOB inactivation in hepatocellular carcinoma. Exp Mol Med. 2018;50(11):147. | ||
Lin YJ, Lee MH, Yang HI, et al. Predictability of liver-related seromarkers for the risk of hepatocellular carcinoma in chronic hepatitis B patients. PLoS One. 2013;8(4):e61448. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






