Back to Journals » Infection and Drug Resistance » Volume 10
Efficacy of intravenous tigecycline in patients with Acinetobacter complex infections: results from 14 Phase III and Phase IV clinical trials
Authors Tucker H, Wible M, Gandhi A, Quintana A
Received 6 June 2017
Accepted for publication 6 September 2017
Published 3 November 2017 Volume 2017:10 Pages 401—417
DOI https://doi.org/10.2147/IDR.S143306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Hal Tucker, Michele Wible, Ashesh Gandhi, Alvaro Quintana
Pfizer Inc, Collegeville, PA, USA
Background: Acinetobacter infections, especially multidrug-resistant (MDR) Acinetobacter infections, are a global health problem. This study aimed to describe clinical outcomes in patients with confirmed Acinetobacter spp. isolates who were treated with tigecycline in randomized clinical trials.
Materials and methods: Data from 14 multinational, randomized (open-label or double-blind), and active-controlled (except one) Phase III and IV studies were analyzed using descriptive statistics.
Results: A total of 174 microbiologically evaluable patients with Acinetobacter spp. infections (including MDR infections) were identified, and 95 received tigecycline to treat community-acquired pneumonia (CAP), diabetic foot infections (DFIs), hospital-acquired pneumonia (HAP), complicated intra-abdominal infections (cIAIs), infections with resistant pathogens (RPs), or complicated skin and skin-structure infections. The rate of cure of tigecycline for most indications was 70%–80%, with the highest (88.2%) in cIAIs. The rate of cure of the comparators was generally higher than tigecycline, but within each indication the 95% CIs for clinical cure for each treatment group overlapped. For most Acinetobacter isolates, the minimum inhibitory concentration of tigecycline was 0.12–2 µg/mL, with seven at 4 µg/mL and one at 8 µg/mL. The cure rate by tigecycline was 50% (95% CI 12.5%–87.5% in CAP) to 88.2% (95% CI 66.2%–97.1% in cIAIs) for all Acinetobacter, and 72.7% (95% CI 54.5%–93.2% in HAP) to 100% (95% CI 25%–100.0% in cIAIs) for MDR Acinetobacter. For the comparators, it was 83.8% (95% CI 62.8%–95.9% in HAP) to 100% (95% CI 75%–100% in cIAIs and 25%–100.0% in RPs) and 88% (95% CI 66%–97% in HAP) to 100% (95% CI 25%–100% in cIAIs and 75%–100% in DFIs), respectively.
Conclusion: These findings suggest that with appropriate monitoring, tigecycline may be a useful consideration for Acinetobacter infections alone or in combination with other anti-infective agents when other therapies are not suitable.
Keywords: tigecycline, Acinetobacter, community-acquired pneumonia, complicated intra-abdominal infections, complicated skin and skin-structure infections
Introduction
The continuing increase in antimicrobial-resistant infections is a global threat to public health.1 Infections caused by Acinetobacter spp. are primarily associated with nosocomial infections in patients with severe illness. Among all Acinetobacter spp., A. baumannii has been identified as the causative pathogen in approximately 80% of reported infection cases.2 A. baumannii, A. baumannii complex, and other Acinetobacter species require careful assessment, due to the variability in the populations in which they present, variability in resistance patterns (including multidrug-resistant [MDR], extensively DR [XDR], and panresistant strains), and the potential for hypervirulence. XDR strains may be susceptible only to polymyxins or tigecycline, and truly panresistant strains complicate treatment further.3
As noted in US prescribing information, tigecycline has in vitro activity against Acinetobacter spp., but efficacy against Acinetobacter spp. has not been established in clinical trials, and thus tigecycline is not approved by the US Food and Drug Administration (FDA) to treat this organism in any indication;4 however, small numbers of Acinetobacter isolates have been identified from the tigecycline clinical program across 14 clinical trials, including approved and unapproved indications. Tigecycline has demonstrated in vitro activity against Acinetobacter isolates in different regions.5–7 From these studies and the findings of a five-center study that tested 103 clinical (including MDR) Acinetobacter spp. strains,8 a provisional susceptibility breakpoint for tigecycline for Acinetobacter spp. has been proposed. Considering that tigecycline demonstrates comparable activity against Acinetobacter spp. and Enterobacteriaceae and the FDA-approved tigecycline breakpoint for Enterobacteriaceae is ≤2 µg/mL,4 ≤2 µg/mL was suggested as a provisional standard by Jones et al, and this breakpoint was used in this paper when reviewing sensitivity data.8 However, there is no FDA- or European Committee on Antimicrobial Susceptibility Testing-approved breakpoint for Acinetobacter.
Many Acinetobacter isolates are resistant to commonly prescribed antibiotics. The Centers for Disease Control reported in 2013 that 63% of the 12,000 health-care-associated Acinetobacter infections occurring in the US each year were MDR,9 and a recent systematic review and meta-analysis by Lemos et al10 reported that resistance to carbapenems has been increasing in the past few years worldwide. For instance, it increased from 9% in 1995 to 40% in 2004 in the US and from 14% in 2003 to 46% in 2008 in Taiwan.
The limited data on clinical efficacy of tigecycline against Acinetobacter have mainly come from case reports, case series, or retrospective analyses,11–21 including reports showing that the efficacy of colistin–tigecycline therapy for XDR A. baumannii infections18–20 or A. baumannii ventilator-associated pneumonia (VAP)21 was comparable to other treatment options (eg, high-dose sulbactam, colistin, high-dose prolonged infusion of a carbapenem, and colistin–imipenem–cilastatin). Nevertheless, the safety and efficacy of tigecycline in treating clinical infections caused by A. baumannii have not been established in adequate and well-controlled clinical trials.13 Since Acinetobacter infections are often severe or life-threatening and less common than many other infections, it is challenging to obtain sufficient data correlating clinical outcomes with microbiological isolates. In the absence of a clinical trial aimed specifically at investigating tigecycline use in Acinetobacter infections, this analysis was undertaken to investigate the available data on Acinetobacter infections from the large data set provided by the tigecycline clinical trial program.
This analysis describes the clinical efficacy of tigecycline or comparator antibiotics in the treatment of patients with infections caused by Acinetobacter spp. (including MDR Acinetobacter spp.) in the microbiologically evaluable (ME) population (defined in the “Materials and methods” section) from 14 multinational Phase III and Phase IV clinical trials. In addition to the clinical response by minimum inhibitory concentration (MIC) and microbiological eradication in the ME population, all-cause mortality in patients in the tigecycline- and comparator-treatment groups is also described.
Materials and methods
Data sources
All Phase III and IV clinical trials investigating the efficacy and safety of tigecycline treatment for approved (complicated skin and skin-structure infections [cSSSIs], complicated intra-abdominal infections [cIAIs], and community-acquired pneumonia [CAP; approved in the US]) and unapproved (diabetic foot infections [DFIs] and hospital-acquired pneumonia [HAP], including VAP) indications, and resistant pathogen (RP) studies that were sponsored by Wyeth Research (now a wholly owned subsidiary of Pfizer) were included. Patient groups, trial designs, comparators, and outcomes have been described previously.22–26 All trials were randomized (open-label or double-blind) and active-controlled, except that there was no comparator for one trial investigating the efficacy of tigecycline in difficult-to-treat serious infections caused by resistant Gram-negative organisms.25 All studies were conducted in compliance with the Declaration of Helsinki, with each protocol approved by institutional review board(s) and/or independent ethics committee(s), and all local regulatory requirements were followed. All participants of the studies provided written informed consent. Data from these 14 Phase III and IV studies were used in the current analysis.23–36
Patient population
All patients (all aged 18 years and older) enrolled in these Phase III and IV trials who were infected with Acinetobacter spp. at baseline were identified, including a subset of patients with infections caused by MDR Acinetobacter spp. MDR Acinetobacter was defined as any isolate that was resistant to at least three classes of antibiotics.
The primary population for efficacy was the ME population, with additional supportive analyses performed using the microbiological modified intent-to-treat (m-MITT) population. The MITT population was used as the safety cohort. The ME population were patients who met all prespecified eligibility criteria for the respective protocols with an identifiable primary isolate(s) that was susceptible to tigecycline and the comparator. The MITT population comprised all patients in the ITT population who received at least one dose of study drug. The m-MITT population comprised patients who had evident infections, as defined by individual study protocols and one or more isolates identified at baseline.23–36
Antibiotic therapy
Tigecycline was administered intravenously, initially at 100 mg and followed by 50 mg every 12 hours in all groups except DFIs.23–25,27–36 For patients with DFIs, the dosage was 150 mg once daily.26 Comparators were administered at routine dosages, described in detail in individual studies.23–36 For most (all except two) of the trials, tigecycline was administered as monotherapy. For patients in the Phase III trial investigating the safety and efficacy of tigecycline in treatment for serious infections with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci, if polymicrobial infection with Gram-negative bacteria was suspected, non-study antimicrobial agents or irrigants could be administered as adjunctive therapy.34 For patients with HAP who were enrolled in the Phase III trial comparing tigecycline with imipenem–cilastatin, other therapies were included as per trial protocols to provide appropriate adjunctive therapy for Pseudomonas aeruginosa or methicillin-resistant S. aureus in both cohorts.35
In the three Phase III and IV cSSSI trials, five Phase III and IV cIAI trials, and the two Phase III CAP trials, no additional therapy was added to the tigecycline-treatment arms. Comparator arms contained single or combined therapies, including vancomycin plus aztreonam, imipenem–cilastatin, ceftriaxone sodium plus metronidazole, levofloxacin, vancomycin or linezolid, vancomycin ± ertapenem, and ampicillin–sulbactam or amoxicillin–clavulanate, depending upon the particular study.22–24
End points
Demographic and baseline characteristics were summarized for all patients with Acinetobacter infections in the ME population by infection type and treatment group. Clinical and microbiological responses by treatment group at test of cure were assessed for patients with Acinetobacter infections in the ME and m-MITT populations and a subset of patients with MDR Acinetobacter infections. Cure was defined as resolution or improvement of the infection such that no further antibacterial therapy was required. Clinical response was calculated as rate of cure at test of cure. Reasons for discontinuation of study drugs and study withdrawals were summarized for the MITT population overall and by infection type. All-cause mortality was summarized for the MITT population and also by infection type.
Statistical methods
No formal statistical hypothesis was tested on the Acinetobacter-infected population; results were descriptive only, with no comparisons between treatment groups except for baseline demographics. Baseline categorical variables (eg, sex, prior antibiotic failure, and bacteremia) were compared between treatment groups using Fisher’s exact test or c2 test, as appropriate, and continuous variables (eg, age) were compared using analysis of variance. P<0.05 was considered statistically significant. Efficacy data were not combined across different indications. The number and percentage of patients attaining clinical and microbiological success are presented as 95% CIs for each treatment group calculated using the Clopper–Pearson “exact” method.
Results
Patient population
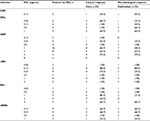
A total of 174 patients in the ME population of the 14 Phase III and IV studies were identified as having Acinetobacter spp. infections and included in this analysis: 95 patients received tigecycline and 79 a comparator drug (Table 1). Acinetobacter spp. were isolated in all infection types, except CAP patients in the comparator group. In general, baseline characteristics were comparable between the tigecycline-treatment group and the comparator group (Table 1), except that patients with cIAIs who were treated with tigecycline were much older (mean age 53.7 years for tigecycline vs 32.7 years for comparator, P=0.003). Results were similar in the m-MITT population (data not shown).
MDR Acinetobacter was found in 66 patients in the ME population, 31 patients in the tigecycline group (eight with DFIs, eleven with HAP, four with cIAIs, seven with RPs, and one with cSSSI) and 35 in the comparator group (seven with DFIs, 25 with HAP, two with cIAIs, and one with cSSSI) (Table 1). In addition, bacteremia occurred in 18 patients: one with the diagnosis of CAP treated with tigecycline, two with DFIs (one treated with tigecycline, one with the comparator), ten with HAP (three treated with tigecycline, seven with the comparator), three with cIAIs (two treated with tigecycline, one with the comparator), and two with RPs (both treated with tigecycline) (Table 1). Additional pathogens isolated from patients with polymicrobial infections are listed in Table S1.
Efficacy
Clinical response
The rate of cure of tigecycline for the approved indications or unapproved infection types ranged from 50% for CAP (n=2) to 88.2% in patients with cIAIs (n=17) (Figure 1, Table S2). Since data were collected from multiple studies in different indications with different comparator-antibiotic regimens, it was determined that a statistical analysis of data pooled across all indications would not be appropriate. Additionally, the significant differences in age in the cIAI trials and low number within each of the other indications limited the ability to interpret comparisons between treatment groups.
For the 66 ME patients with MDR Acinetobacter, the rate of cure was 72.7%–100% for tigecycline and 88%–100% for the comparator group (Figure 1, Table S2). For HAP patients with MDR Acinetobacter, eight of eleven (72.7%, 95% CI 54.5%–93.2%) in the tigecycline group and 22 of 25 (88%, 95% CI 66%–97%) in the comparator group responded to the treatment. For DFI patients with MDR Acinetobacter, seven of eight (87.5%, 95% CI 65.6%–96.9%) in the tigecycline group and seven of seven (100%, 95% CI 75%–100%) in the comparator group responded to the treatment. All seven patients with MDR Acinetobacter in the RP studies were treated with tigecycline, and six were cured (85.7%, 95% CI 21.4%–96.4%). All cIAI patients with MDR Acinetobacter responded to treatment (four of four tigecycline, two of two comparator) (Figure 1, Table S2). Neither of the two CAP patients had MDR Acinetobacter, bacteremia, or prior antibiotic failure, both were treated with tigecycline, and one was cured.
Clinical response to tigecycline was further investigated by MIC (Table 2). Most patients (83) had Acinetobacter isolates (MIC 0.12–2 µg/mL) and the rate of cure varied between 60% and 100%. All patients in the MIC 0.5 µg/mL category (three DFIs, four HAP, one cIAI, five RPs, and two cSSSIs) were cured. For the 14 patients in the MIC 2 µg/mL category, the rate of cure was 62.5% (five of eight) for HAP, 100% (two of two) for cIAIs, and 50% (two of four) for RPs. Seven patients had Acinetobacter isolates with an MIC of 4 µg/mL: three of the four HAP patients and two of the three patients in the RP studies were cured. One HAP patient had Acinetobacter isolates with an MIC of 8 µg/mL; this patient did not respond to tigecycline treatment. Patients with cIAIs had the highest overall rate of cure with Acinetobacter isolates across six MIC categories (0.06–2 µg/mL). The two cIAI patients who did not respond to tigecycline had Acinetobacter isolates with an MIC of 0.12 µg/mL and 0.25 µg/mL (Table 2).
Safety
A total of 274 patients in the MITT population with Acinetobacter spp. infection were included in the safety analysis: 154 were treated with tigecycline and 120 with a comparator. Among these patients, eleven (4%) discontinued the study drug: five (3.2%) in the tigecycline group and six (5%) in the comparator group (Table 3). In total, 28 deaths (10.2%) occurred in patients with Acinetobacter spp. in the MITT population: 17 of 154 (11%) in the tigecycline group and eleven of 120 (9.2%) in the comparator group. There were no deaths in the CAP or DFI group. For patients with HAP, the mortality rate was 15.8% (nine of 57) and 17.2% (eleven of 64) for the tigecycline and the comparator groups, respectively. For patients with cIAIs and cSSSIs, no deaths were recorded in the comparator group (zero of 14 and zero of 15), and one death each occurred in the tigecycline groups (one of 21 [4.8%] and one of 16 [6.3%]). For patients in the RP studies, the mortality rate was 15.4% (six of 39) for the tigecycline group, while the only patient in the comparator group survived (Table 3). There were 126 patients in the MITT population with MDR Acinetobacter infection; death occurred in six of 65 (9.2%) of the tigecycline and four of 61 (6.6%) of the comparator group (Table 3).
Discussion
In recent years, infections caused by Acinetobacter and especially MDR Acinetobacter have been increasingly reported worldwide.11–14 In the US, an estimated 7,000 such infections occurred each year, causing 500 deaths.9 However, treatment options, especially for carbapenem-resistant Acinetobacter infections, are limited.37,38
This analysis evaluated the efficacy of tigecycline and comparators against Acinetobacter infection in 174 ME patients from 14 Phase III and IV clinical trials. Clinical response was observed in patients with different types of infections, including CAP, DFIs, HAP, cIAIs, RPs, and cSSSIs, and the rate of cure with tigecycline was at least 50%. In addition, 80% or more of the patients who had had previous antibiotic failure responded to tigecycline therapy, except for patients with HAP. Further, in a subgroup of patients with MDR Acinetobacter infection, the rate of cure for tigecycline was 100% for patients with cIAIs, over 85% for patients with DFIs, and RPs, and 73% for patients with HAP. Since data were collected from multiple studies in different indications with different antibiotic regimens, an analysis of efficacy data pooled across all indications would not be appropriate. Additionally, comparisons between treatment groups were limited, due to baseline differences and/or small numbers, although efficacy rates were generally higher for the comparator-treated patients. For the approved indications (ie, CAP, cIAIs, and cSSSIs), the current analysis found that the MIC for tigecycline against Acinetobacter ranged from 0.06 µg/mL to 2 µg/mL.
For patients included in the current analysis who received tigecycline, HAP was the most common infection type. However, noninferiority of tigecycline to the comparator was not achieved for the treatment of HAP/VAP in a Phase III trial, and thus tigecycline is not approved for the treatment of HAP.35 The failure of this Phase III HAP/VAP trial was largely responsible for the most recent recommendation in the 2016 HAP/VAP guidelines by the Infectious Diseases Society of America and the American Thoracic Society for a “strong recommendation” based on “low-quality evidence” against the use of tigecycline for the treatment of HAP/VAP caused by Acinetobacter spp.39 A subsequent Phase II HAP/VAP study with alternative dosing not included in the present analysis failed to enroll fully, but did show a trend toward improved outcomes vs comparator at higher tigecycline doses.40
Approximately 20% of the patients included in the current analysis had DFIs, 40% of whom were treated with tigecycline. However, tigecycline did not show noninferiority to ertapenem ± vancomycin in this Phase III trial in DFI patients with and without osteomyelitis,26 and thus tigecycline efficacy for this indication remains unproven.
Tigecycline has been shown to be efficacious and safe in the treatment of cSSSIs, cIAIs, and CAP.27–30,32,33 However, across all tigecycline Phase III and IV clinical trials (cSSSIs, cIAIs, CAP, HAP/VAP, and DFIs), meta-analyses demonstrated an all-cause mortality imbalance favoring comparators.22,41 This has resulted in label language that states tigecycline should be used only when other antibiotics are not suitable (US prescribing information and summary of product characteristics by the European Medicines Agency), and thus one may expect tigecycline use is more likely for patients who have few if any other choices due to MDR and XDR pathogens.
Clinicians should be aware of the possibility of resistance and/or rapid development of resistance to tigecycline. In a report of two seriously ill patients with bloodstream infections with A. baumannii, tigecycline therapy failed.42 MICs for the A. baumannii isolates were >2 µg/mL (at the time therapy was deemed a failure), but initial MICs at the beginning of therapy were not reported. One died, and another was switched to another therapy and survived. In both cases, tigecycline dosing was as per presently approved dosing for treatment of cIAIs and cSSSIs.
In another case report by Reid et al,43 a 53-year-old woman who had undergone a kidney and liver transplant 5 months previously had a complicated course with urinary tract infection, subsequent pneumonia, paraspinal abscess, and spinal osteomyelitis. During a protracted treatment course that included two separate courses of tigecycline, an initial A. baumannii tigecycline MIC of 1.5 mg/L eventually increased to 24 mg/L. Therapy was changed, and the patient survived until discharge. The authors concluded that patients being treated for A. baumannii infection with tigecycline could develop resistance after brief exposure to the drug. It should be noted that tigecycline does not have an approved indication for urinary tract infections or osteomyelitis.
In 2008, Karageorgopoulos et al44 reviewed the evidence available at the time with regard to tigecycline treatment for MDR (including carbapenem-resistant) Acinetobacter infections. They identified 22 microbiological studies reporting 2,384 Acinetobacter isolates (1,906 were A. baumannii). Assuming a breakpoint of susceptibility ≤2 mg/L for tigecycline, in nine of 18 studies reporting data on MDR Acinetobacter, 90% of these Acinetobacter isolates were susceptible to tigecycline, in eight of 18 tigecycline had inadequate activity against MDR Acinetobacter, and in one study results were variable based on the testing method. Seven of 15 studies reported data on carbapenem-resistant Acinetobacter, demonstrating 90% susceptibility. Another study revealed 89% of imipenem-resistant isolates were susceptible to tigecycline, and there was inadequate activity in the remaining six studies. Of note, there were ten reported colistin-resistant Acinetobacter spp., and nine were susceptible to tigecycline. An additional 17 (same clone) Acinetobacter isolates that were intermediate to colistin were all susceptible to tigecycline.
Also in Karageorgopoulos et al,44 eight studies assessed the clinical effectiveness of tigecycline vs MDR Acinetobacter spp. or Acinetobacter spp. with clinically significant resistance. A total of 42 patients had MDR Acinetobacter infections treated with tigecycline: 31 had respiratory tract infections (mainly VAP), four of these had bacteremia, four additional patients had primary or secondary bacteremia, and most were critically ill. Two-thirds of the patients also received therapy concurrently with other agents potentially active against Acinetobacter spp. A total of 32 of the 42 patients had a “favorable” clinical course, but intermediately susceptible strains were noted to have a worse prognosis, and VAP recurred in three patients. Finally, in three of the 42 patients, A. baumannii strains resistant to tigecycline emerged after tigecycline therapy had begun, and two of three resulted in clinical failure. The authors concluded that tigecycline had “considerable, though not consistent, antimicrobial activity against MDR (including carbapenem-resistant) Acinetobacter spp.”
Gordon and Wareham45 reviewed clinical and microbiological outcomes when tigecycline was used to treat MDR A. baumannii. This retrospective study of 34 patients revealed that 23 (68%) patients who received tigecycline for MDR A. baumannii or polymicrobial infections that included A. baumannii had a positive clinical outcome, and ten had microbiological clearance. Fourteen (41%) patients died, with nine deaths attributable to sepsis. Three of these deaths had documented Gram-negative bacteremia on tigecycline therapy, and one developed resistance. The authors noted the poor correlation between microbiological clearance and clinical outcome, especially in those patients with respiratory tract infections. They also stated that the study results did not support empirical tigecycline monotherapy for Gram-negative bloodstream infections. Noted limitations included the retrospective design, small numbers, and the fact that more than half of the patients received concomitant antibiotic treatment with tigecycline.
In a review of recent literature, a report on 79 patients in intensive care units with A. baumannii VAP noted that a colistin–tigecycline regimen (in which the tigecycline dose was doubled during the first 48 hours to 100 mg twice a day, and then continued at 50 mg twice a day) vs a colistin–imipenem regimen demonstrated no difference between the two groups in 28-day survival.21 However, these clinical data in this small retrospective study are still inadequate to draw any definitive conclusions with regard to dosing and treatment of Acinetobacter infections with tigecycline. In a recent prospective observational study comparing a colistin–tigecycline regimen vs a colistin–carbapenem regimen for the treatment of XDR A. baumannii bacteremia, after adjustment for demographic characteristics and comorbidities, it was found that there was excess 14-day mortality only in the colistin–tigecycline subgroup that had an MIC >2 mg/L.20
A systematic review by Poulikakos et al of 1,040 patients with infections caused by DR Acinetobacter spp. in 12 studies found that in three studies, combination therapy (tigecycline was administered in some combinations) was superior to monotherapy. Based on the available data, the authors acknowledged that combination therapy may be preferred for the treatment of DR Acinetobacter infections in severely ill patients.46
Kim et al47 reported on 70 patients (40 on colistin-based therapy and 30 who received tigecycline-based therapy) who were treated for MDR or XDR A. baumannii pneumonia. This retrospective study of intensive care-unit patients from 2009 to 2010 revealed no significant differences in baseline characteristics and no differences with regard to clinical outcomes, except for significantly higher nephrotoxicity in the colistin group. It is notable that patients received the approved usual tigecycline dosing (loading dose of 100 mg, followed by 50 mg every 12 hours), but the authors noted in the discussion that higher doses were studied in a small VAP clinical trial reported by Ramirez et al.40 Both clinical and microbiological outcomes were numerically higher in the Kim et al study, and mortality numerically lower in patient groups that received combination therapy vs monotherapy. Monotherapy was independently associated with increased clinical failure as per multivariate analysis, and thus the authors concluded combination therapy may be more useful for MDR and XDR A. baumannii pneumonia. They also concluded clinical outcomes were comparable for tigecycline and colistin-based therapy for MDR and XDR A. baumannii pneumonia.
The case reports, studies, and reviews described herein20,42,44–47 reflect the need for treatment options for MDR and XDR A. baumannii. Resistance to myriad anti-infectives, including carbapenems, and the lack of approved and effective options has resulted in off-label use of tigecycline, which has variable in vitro activity and a presumptive unapproved breakpoint for A. baumannii, but no clinical indication for this organism, and no clinical indication to treat patients with HAP/VAP and bloodstream infections. The literature appropriately cautions about tigecycline use as monotherapy in these off-label indications, and as noted, tigecycline did have a failed clinical trial in HAP/VAP, has low serum levels due to its large volume of distribution, and there are case reports of the development of resistance while on therapy. The approved dosing for tigecycline for cIAIs, cSSSIs, and CAP may not be the appropriate dosing for other indications, and although we and others note the use of higher doses in a small clinical trial,40 there is no labeled safety information on higher dosing. Further, as suggested in some studies and reviews, it is possible that tigecycline may be most effective when used with concomitant therapy for certain indications.
The current descriptive analysis has some limitations. Firstly, this analysis included data from various studies with different dosing regimens, comparators, and patient populations, which made pooling efficacy data inappropriate, and thus the findings were descriptive only. Secondly, numbers in some indications were small and the patient population was diverse, limiting the ability to do treatment-group comparisons and making interpretation of findings difficult. Additionally, tigecycline was administered with other antibiotics in some indications (eg, HAP/VAP),35 and thus it is difficult to assess the effect of tigecycline in isolation. Further, in some infections, such as cIAIs, where the presence of multiple pathogens is the rule rather than the exception, clinical cure in the presence of Acinetobacter and even eradication of Acinetobacter does not always mean that Acinetobacter was the primary causative pathogen.
Conclusion
The clinical trial data discussed in this paper could be hypothesis- generating for a larger and well-controlled systematic trial. However, it is unclear that such a study will be carried out any time in the near future. Therefore, this paper serves to fill a gap with randomized controlled clinical trial data that are presently available. Despite the noted limitations, we believe these data could be useful to clinicians who treat critically ill patients with Acinetobacter infections. We have described clinical outcomes and MICs, as well as dosing information from clinical trials, along with a literature review from real-world clinical data and microbiology. While tigecycline may be considered by physicians14–21 as an option for the treatment of patients with MDR Acinetobacter infections alone or in combination with other anti-infective agents when other available antibiotics are not suitable, additional study would be required to properly assess efficacy and determine the correct dosing regimen.11–14
Acknowledgments
The Phase III and IV trials were sponsored by Wyeth Research (now a wholly owned subsidiary of Pfizer). The current study was funded by Pfizer. The authors would like to thank Jeffrey Goodrich at Pfizer for programming support. Medical writing support was provided by Shuang Li, PhD at Engage Scientific (Horsham, UK) and was funded by Pfizer.
Disclosure
HT, MW, and AQ are employees of Pfizer. AG was an employee of Pfizer at the time the study was conducted. This study used data from clinical trials that have been published previously. Information related to trial registration can be found in the original publications cited as references. The authors report no other conflicts of interest in this work.
References
World Health Organization. Antimicrobial resistance. 2016. Available from: http://www.who.int/mediacentre/factsheets/fs194/en. Accessed April 12, 2017. | ||
Centers for Disease Control and Prevention. Acinetobacter in healthcare settings. 2010. Available from: https://www.cdc.gov/HAI/organisms/acinetobacter.html. Accessed April 12, 2017. | ||
Göttig S, Gruber TM, Higgins PG, Wachsmuth M, Seifert H, Kempf VA. Detection of pan drug-resistant Acinetobacter baumannii in Germany. J Antimicrob Chemother. 2014;69:2578–2579. | ||
US Food and Drug Administration. Tygacil [prescribing information]. 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021821s039lbl.pdf. Accessed April 12, 2017. | ||
Bouchillon SK, Hoban DJ, Johnson BM, Johnson JL, Hsiung A, Dowzicky MJ. In vitro activity of tigecycline against 3989 Gram-negative and Gram-positive clinical isolates from the United States Tigecycline Evaluation and Surveillance Trial (test program; 2004). Diagn Microbiol Infect Dis. 2005;52:173–179. | ||
Seifert H, Stefanik D, Wisplinghoff H. Comparative in vitro activities of tigecycline and 11 other antimicrobial agents against 215 epidemiologically defined multidrug-resistant Acinetobacter baumannii isolates. J Antimicrob Chemother. 2006;58:1099–1100. | ||
Reinert RR, Low DE, Rossi F, Zhang X, Wattal C, Dowzicky MJ. Antimicrobial susceptibility among organisms from the Asia/Pacific Rim, Europe and Latin and North America collected as part of TEST and the in vitro activity of tigecycline. J Antimicrob Chemother. 2007;60:1018–1029. | ||
Jones RN, Ferraro MJ, Reller LB, Schreckenberger PC, Swenson JM, Sader HS. Multicenter studies of tigecycline disk diffusion susceptibility results for Acinetobacter spp. J Clin Microbiol. 2007;45:227–230. | ||
Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. Atlanta: CDC; 2013. Available from: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 2, 2017. | ||
Lemos EV, de la Hoz FP, Einarson TR, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 2014;20:416–423. | ||
Curcio D, Fernandez F, Vergara J, Vazquez W, Luna CM. Late onset ventilator-associated pneumonia due to multidrug-resistant Acinetobacter spp.: experience with tigecycline. J Chemother. 2009;21:58–62. | ||
Tekçe AY, Erbay A, Çabadak H, Yağcı S, Karabiber N, Şen S. Pan-resistant Acinetobacter baumannii mediastinitis treated successfully with tigecycline: a case report. Surg Infect (Larchmt). 2011;12:141–143. | ||
De Luca M, Angelino G, Calò Carducci FI, et al. Multidrug-resistant Acinetobacter baumannii infection in children. BMJ Case Rep. 2011;pii:bcr0220113807. | ||
Moon SY, Peck KR, Chang HH, et al. Clinical experience of tigecycline treatment in infections caused by extensively drug-resistant Acinetobacter spp. Microb Drug Resist. 2012;18:562–566. | ||
Lee YT, Tsao SM, Hsueh PR. Clinical outcomes of tigecycline alone or in combination with other antimicrobial agents for the treatment of patients with healthcare-associated multidrug-resistant Acinetobacter baumannii infections. Eur J Clin Microbiol Infect Dis. 2013;32:1211–1220. | ||
Kanık-Yüksek S, Tezer H, Ozkaya-Parlakay A, et al. Multidrug-resistant Acinetobacter baumannii bacteremia treated with tigecycline in two pediatric burn patients. Pediatr Infect Dis J. 2015;34:677. | ||
Liou BH, Lee YT, Kuo SC, Liu PY, Fung CP. Efficacy of tigecycline for secondary Acinetobacter bacteremia and factors associated with treatment failure. Antimicrob Agents Chemother. 2015;59:3637–3640. | ||
Kwon SH, Ahn HL, Han OY, La HO. Efficacy and safety profile comparison of colistin and tigecycline on the extensively drug resistant Acinetobacter baumannii. Biol Pharm Bull. 2014;37:340–346. | ||
Khawcharoenporn T, Pruetpongpun N, Tiamsak P, Rutchanawech S, Mundy LM, Apisarnthanarak A. Colistin-based treatment for extensively drug-resistant Acinetobacter baumannii pneumonia. Int J Antimicrob Agents. 2014;43:378–382. | ||
Cheng A, Chuang YC, Sun HY, et al. Excess mortality associated with colistin-tigecycline compared with colistin-carbapenem combination therapy for extensively drug-resistant Acinetobacter baumannii bacteremia: a multicenter prospective observational study. Crit Care Med. 2015;43:1194–1204. | ||
Chaari A, Pham T, Mnif B, et al. Colistin-tigecycline versus colistin-imipenem-cilastatin combinations for the treatment of Acinetobacter baumannii ventilator-acquired pneumonia: a prognosis study. Intensive Care Med. 2015;41:2018–2019. | ||
Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis. 2012;54:1699–1709. | ||
Qvist N, Warren B, Leister-Tebbe H, et al. Efficacy of tigecycline versus ceftriaxone plus metronidazole for the treatment of complicated intra-abdominal infections: results from a randomized, controlled trial. Surg Infect (Larchmt). 2012;13:102–109. | ||
Matthews P, Alpert M, Rahav G, et al. A randomized trial of tigecycline versus ampicillin-sulbactam or amoxicillin-clavulanate for the treatment of complicated skin and skin structure infections. BMC Infect Dis. 2012;12:297. | ||
Vasilev K, Reshedko G, Orasan R, et al. A phase 3, open-label, non-comparative study of tigecycline in the treatment of patients with selected serious infections due to resistant Gram-negative organisms including Enterobacter species, Acinetobacter baumannii and Klebsiella pneumoniae. J Antimicrob Chemother. 2008;62:i29–i40. | ||
Lauf L, Ozsvar Z, Mitha I, et al. Phase 3 study comparing tigecycline and ertapenem in patients with diabetic foot infections with and without osteomyelitis. Diagn Microbiol Infect Dis. 2014;78:469–480. | ||
Sacchidanand S, Penn RL, Embil JM, et al. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: results from a phase 3, randomized, double-blind trial. Int J Infect Dis. 2005;9:251–261. | ||
Breedt J, Teras J, Gardovskis J, et al. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob Agents Chemother. 2005;49:4658–4666. | ||
Oliva ME, Rekha A, Yellin A, et al. A multicenter trial of the efficacy and safety of tigecycline versus imipenem/cilastatin in patients with complicated intra-abdominal infections [study ID numbers: 3074A1–301-WW; ClinicalTrials.gov identifier: NCT00081744]. BMC Infect Dis. 2005;5:88. | ||
Fomin P, Beuran M, Gradauskas A, et al. Tigecycline is efficacious in the treatment of complicated intra-abdominal infections. Int J Surg. 2005;3:35–47. | ||
Chen Z, Wu J, Zhang Y, et al. Efficacy and safety of tigecycline monotherapy vs. imipenem/cilastatin in Chinese patients with complicated intra-abdominal infections: a randomized controlled trial. BMC Infect Dis. 2010;10:217. | ||
Bergallo C, Jasovich A, Teglia O, et al. Safety and efficacy of intravenous tigecycline in treatment of community-acquired pneumonia: results from a double-blind randomized phase 3 comparison study with levofloxacin. Diagn Microbiol Infect Dis. 2009;63:52–61. | ||
Tanaseanu C, Milutinovic S, Calistru PI, et al. Efficacy and safety of tigecycline versus levofloxacin for community-acquired pneumonia. BMC Pulm Med. 2009;9:44. | ||
Florescu I, Beuran M, Dimov R, et al. Efficacy and safety of tigecycline compared with vancomycin or linezolid for treatment of serious infections with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: a phase 3, multicentre, double-blind, randomized study. J Antimicrob Chemother. 2008;62:i17–i28. | ||
Freire AT, Melnyk V, Kim MJ, et al. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis. 2010;68:140–151. | ||
Towfigh S, Pasternak J, Poirier A, Leister H, Babinchak T. A multicentre, open-label, randomized comparative study of tigecycline versus ceftriaxone sodium plus metronidazole for the treatment of hospitalized subjects with complicated intra-abdominal infections. Clin Microbiol Infect. 2010;16:1274–1281. | ||
Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis. 2010;51:79–84. | ||
Doi Y, Murray GL, Peleg AY. Acinetobacter baumannii: evolution of antimicrobial resistance-treatment options. Semin Respir Crit Care Med. 2015;36:85–98. | ||
Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. | ||
Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother. 2013;57:1756–1762. | ||
Verde PE, Curcio D. Imbalanced mortality evidence for tigecycline: 2011, the year of the meta-analysis. Clin Infect Dis. 2012;55:471–472. | ||
Peleg AY, Potoski BA, Rea R, et al. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J Antimicrob Chemother. 2007;59:128–131. | ||
Reid GE, Grim SA, Aldeza CA, Janda WM, Clark NM. Rapid development of Acinetobacter baumannii resistance to tigecycline. Pharmacotherapy. 2007;27:1198–1201. | ||
Karageorgopoulos DE, Kelesidis T, Kelesidis I, Falagas ME. Tigecycline for the treatment of multidrug-resistant (including carbapenem-resistant) Acinetobacter infections: a review of the scientific evidence. J Antimicrob Chemother. 2008;62:45–55. | ||
Gordon NC, Wareham DW. A review of clinical and microbiological outcomes following treatment of infections involving multidrug-resistant Acinetobacter baumannii with tigecycline. J Antimicrob Chemother. 2009;63:775–780. | ||
Poulikakos P, Tansarli GS, Falagas ME. Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant Acinetobacter infections: a systematic review. Eur J Clin Microbiol Infect Dis. 2014;33:1675–1685. | ||
Kim WY, Moon JY, Huh JW, et al. Comparable efficacy of tigecycline versus colistin therapy for multidrug-resistant and extensively drug-resistant Acinetobacter baumannii pneumonia in critically ill patients. PLoS One. 2016;11:e0150642. |
Supplementary material
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.