Back to Journals » Cancer Management and Research » Volume 11
Efficacy of dose-adjusted EPOCH plus rituximab/R-CHOP regimens and the prognosis analysis in patients with MYC, BCL2/BCL6 gene copy number gain lymphoma and double-hit lymphoma: results from a single institution retrospective clinical study
Authors Ma Q, Chang Y, Li L, Li X, Wang X, Wu J, Fu X, Sun Z, Yu H , Zhang X, Zhou Z, Nan F, Li Z , Liu X, Zhao Q , Li Y, Zhang L, Zhang M, Zhang L
Received 25 October 2018
Accepted for publication 27 December 2018
Published 11 February 2019 Volume 2019:11 Pages 1363—1372
DOI https://doi.org/10.2147/CMAR.S192143
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Qianwen Ma,1,2,* Yu Chang,1,2,* Ling Li,1,2 Xin Li,1,2 Xinhua Wang,1,2 Jingjing Wu,1,2 Xiaorui Fu,1,2 Zhenchang Sun,1,2 Hui Yu,1,2 Xudong Zhang,1,2 Zhiyuan Zhou,1,2 Feifei Nan,1,2 Zhaoming Li,1,2 Xiyang Liu,1,2 Qian Zhao,1,2 Yang Li,1,2 Lan Zhang,2,3 Mingzhi Zhang,1,2 Lei Zhang1,2
1Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, Henan, China; 2Lymphoma Diagnosis and Treatment Center of Henan Province, Zhengzhou 450000, Henan, China; 3Department of Pathology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, Henan, China
*These authors contributed equally to this work
Purpose: To compare the efficacy of rituximab, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R-DA-EPOCH) with traditional rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) regimens in double-hit lymphoma (DHL) and gene copy number gain (CNG) lymphoma and to contrast the prognosis of these two disease types.
Methods: We retrospectively examined 127 cases of newly diagnosed diffuse large B-cell lymphoma (DLBCL), and used fluorescence in situ hybridization (FISH) to detect genetic abnormalities in MYC, BCL2, and BCL6.
Results: In the two schemes, the 2-year progression-free survival (PFS) was higher for R-DA-EPOCH group than for R-CHOP (79.8% vs 57.5%, P=0.002), this advantage was also reflected in 2-year overall survival (OS) (81.6% vs 58.5%, P=0.002). In double CNG patients, R-DA-EPOCH regimen was significantly better than R-CHOP (P=0.007 for PFS, P=0.010 for OS), and R-DA-EPOCH has the same advantage in DHL patients (P=0.001 for PFS, P=0.047 for OS). For the two disease types, the PFS for DHL was inferior to that for double CNG (52.9% vs 72.4%, P=0.008), while the OS was not significantly different (P=0.050). Subgroup analysis showed that the PFS for double CNG with MYC and BCL2 was superior to that for DHL with MYC and BCL2 (P=0.043), this trend is also seen in double CNG and DHL with MYC and BCL6 (P=0.036). However, the OS was not significantly different between the two subgroups. Multivariate analyses showed that in DLBCL patients with genetic abnormality detected by FISH, the treatment and disease types were independent prognostic factors. The adverse reaction rates were similar in R-DA-EPOCH and R-CHOP (P>0.05).
Conclusion: Our retrospective study shows that DHL has a poorer prognosis than double CNG. Based on its improved lifetime and good tolerance, R-DA-EPOCH is a promising regimen for DHL or double CNG, which is expected to become the first-line treatment for high-risk DLBCL types based on more clinical research.
Keywords: R-DA-EPOCH, R-CHOP, DHL, double CNG, efficacy and prognosis
Introduction
Diffuse large B-cell lymphoma (DLBCL) is highly invasive but curable. It accounts for 30%–40% of non-Hodgkin’s lymphoma cases,1–3 which shows significant heterogeneity in terms of age of onset, morphological characteristics, immunohistochemical phenotype, sensitivity to chemotherapy, and survival rate.4 With the advent of rituximab, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen has greatly improved the disease remission rates of patients with DLBCL, curing more than 80%–85% of DLBCL patients.3 However, there are still some patients with high-risk DLBCL who show poor prognosis after receiving standard R-CHOP, with a 5-year survival rate well <50%.3,5 The 2016 WHO classification of the lymphoid hematopoietic system6 clearly defined the concurrent translocation of the MYC and BCL2/BCL6 genes as double-hit lymphoma (DHL),7 and expression of the MYC and BCL2/BCL6 proteins by immunohistochemistry is known as double-expression lymphoma (DEL).8,9 Another molecular factor that affects the prognosis of DLBCL is the double gene copy number gain (double CNG), which is defined as simultaneous increase in MYC and BCL2/BCL6 gene copy number detected by fluorescence in situ hybridization (FISH). Several studies have shown that an increased copy number of the MYC/BCL2 genes can lead to poor prognosis.10–13 Previous studies have focused on comparing the prognosis of DHL with that of DEL at the protein level, while few studies have compared DHL with double CNG at the gene level. In this study, we used FISH to detect abnormalities in MYC and BCL2/BCL6 and compared the prognostic difference of DHL and double CNG.
Due to a high-risk international prognostic index (IPI) score, advanced Ann Arbor stage, and elevated lactate dehydrogenase (LDH) levels, DLBCL patients have rapid disease progression (PD) and short median survival times.14,15 In the era of rituximab, the traditional R-CHOP does not effectively improve the survival of patients with high-risk DLBCL, and the optimal treatment remains to be established. In response to these high-risk patients, several studies have attempted to increase their long-term survival using dose- or density-enhanced immunochemotherapy regimens,15–23 such as R-DA-EPOCH (rituximab, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin), R-Hyper CVAD/MA (rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone/methotrexate, and cytarabine), R-ACVBP (rituximab, doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone), and R-CODOX-M/IVAC (rituximab, cyclophosphamide, vincristine, doxorubicin, methotrexate/ifosfamide, etoposide, and cytarabine). However, these studies did not compare the efficacy of R-DA-EPOCH and R-CHOP regimens in patients with DHL and double CNG. It is also worth noting that the highly invasive nature of these disease types confers a higher recurrence rate. Whether consolidation therapy, such as hematopoietic stem cell transplantation, is needed after complete remission (CR) remains controversial.24–26 We aimed to compare the efficacy and adverse effects of R-DA-EPOCH with R-CHOP in patients with DHL and double CNG.
Methods
Clinical characteristics and regimens
We collected 474 cases of newly diagnosed DLBCL patients who underwent FISH detection from January 2015 to June 2018 in the First Affiliated Hospital of Zhengzhou University, 127 patients of which conformed to the criteria of DHL and double CNG (26 cases of DHL and 101 cases of double CNG). Patients’ pathological section was reread by two senior lymphoma pathologists. Patient baseline clinical characteristics included age, gender, Ann Arbor stage, IPI score, serum LDH level, eastern cooperative oncology group score, extranodal lymph node involvement, Ki-67 level, and the presence or absence of a large mass. The genetic abnormality of MYC and BCL2/BCL6 was detected by FISH. Double CNG was defined as simultaneous increase in MYC and BCL2/BCL6 gene copy number, while DHL was defined as the concurrent translocation of MYC and BCL2/BCL6 genes. The Hans classification27 was used to analyze the cell of origin subtype, all patients were treated with R-CHOP or R-DA-EPOCH regimen. The chemotherapy regimens were as follows:
The R-CHOP regimen consisted of rituximab (375 mg/m2) on day 1, followed by CHOP, which consisted of cyclophosphamide 750 mg/m2 as an intravenous infusion on day 1, vincristine 1.4 mg/m2 (the maximum dose 2 mg) as an intravenous infusion on day 1, doxorubicin 50 mg/m2 as an intravenous infusion on day 1, and prednisone 100 mg/m2 orally on days 1–5.
The R-DA-EPOCH regimen consisted of rituximab (375 mg/m2) on day 1, followed by DA-EPOCH. The starting dose level of the DA-EPOCH consisted of etoposide 50 mg/m2 as a continuous intravenous infusion on days 1–4, vincristine 0.4 mg/m2 as a continuous intravenous infusion on days 1–4, doxorubicin 10 mg/m2 as a continuous intravenous infusion on days 1–4, cyclophosphamide 750 mg/m2 intravenous on day 5, and prednisone 60 mg/m2 orally on days 1–5.
For the R-DA-EPOCH regimen, when grade 4 myelosuppression was not reached at the end of the previous cycle of treatment, 20% of the original dose was increased in subsequent cycles until the patient reached grade 4 myelosuppression. The cycle of two regimens was 21 days. All patients were inserted peripheral central line before treatment. Chemotherapy-related adverse reactions were graded according to the WHO criteria for cancer treatment results,28 specifically, granulocyte colony-stimulating factor (G-CSF) was given when patients suffered leukopenia or neutropenia, recombinant human thrombopoietin (TPO) was used for thrombocytopenia. If necessary, the infusion of blood components can be implemented. Symptomatic supportive care was given for non-hematological toxicities. This study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. Informed consent for the collection of medical information was obtained from all patients. All procedures performed in the study were in accordance with the ethical standards of the institutional research committee. The patient consent was written informed consent, and that this study was conducted in accordance with the Declaration of Helsinki.
Observation of efficacy and follow-up
According to the International Working Group’s efficacy evaluation criteria,29,30 the efficacy is divided into CR, CR unconfirmed (CRu), partial remission (PR), disease stabilization (SD), and PD. An 18F-FDG PET-CT scan was used to evaluate the standard uptake value of the lesion before treatment, while a CT scan was used to evaluate the efficacy every 2 cycles. The lesion was evaluated by PET-CT again after 4 cycles, and the final evaluation was performed after 6–8 cycles. Evaluation of adverse reactions was implemented after every cycle. The primary study endpoints were progression-free survival (PFS), which refers to the time from the start of the study to PD or death from any cause, and overall survival (OS), which refers to the time from the start of the study to death from any cause or the end of the study. The secondary study endpoints were the objective response rate (ORR), defined as the proportion of patients with tumor shrinkage reaching a certain level and maintained for a certain period of time, including the proportion of CR, CRu, and PR. During the first 2 years after treatment, patients were followed up every 3 months during the first year and every 6 months during the second year. The follow-up deadline was September 30, 2018.
Statistical analysis
All data were analyzed using SPSS 22.0. The chi-squared test and Fisher’s exact test were used to compare categorical variables. The survival rate of each group was estimated by the Kaplan–Meier method, and the survival rate between two groups was compared by a log-rank test. Prognostic factors were analyzed using COX proportional hazards regression model. All P-values were two-sided with a significance level of 0.05.
Results
Patient characteristics
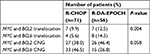
Among the 474 patients who underwent the FISH detection, 127 patients conformed to the criteria of DHL and double CNG, 26 (20.5%) with DHL and 101 (79.5%) with double CNG lymphoma, of which 56 (44.1%) were treated with the R-DA-EPOCH regimen and 71 (55.9%) with R-CHOP. The median age was 55 years (18–85 years), and 74 (58.3%) of the patients had a grade 3/4 Ann Arbor stage. Patients with an IPI score of 3–5 accounted for 31.5%. Of the 127 patients, 4 of whom (3.1%) received transplantation, 1 patient received chimeric antigen receptor T-cell immunotherapy after disease relapse. Patient’s characteristics are listed in Table 1, and the composition of DHL and double CNG is shown in Table 2.
Short-term efficacy
The median number of chemotherapy cycles was 6 (range: 4–8), and all 127 patients enrolled could be evaluated for efficacy, including CR (65 cases, 51.2%), CRu (18 cases, 14.1%), PR (10 cases, 7.9%), SD (15 cases, 11.8%), and PD (19 cases, 15.0%). The ORR was 73.2%. The chi-squared test was used to compare ORR between two groups, the results showed that the ORR in patients treated with R-DA-EPOCH was significantly higher than in R-CHOP group (85.7% vs 63.4%, P=0.005), while the difference in ORR between two disease types was not significant (P=0.131).
Long-term efficacy
The median follow-up time was 12 months (range: 1–33 months); until the end of follow-up, 28 patients died (9 [16.1%] patients were treated with R-DA-EPOCH regimen and 19 [26.7%] cases were treated with R-CHOP). We found that in the two regimens, the 2-year survival rate of R-DA-EPOCH group was significantly superior than that of R-CHOP (79.8% vs 57.5%, P=0.002 for PFS; 81.6% vs 58.5%, P=0.002 for OS) (Figure 1). We analyzed the survival rates of different regimens in patients with double CNG and DHL, and the results showed that in patients with double CNG, the PFS and OS for the R-DA-EPOCH regimen were significantly better than those for R-CHOP (82.8% vs 63.2% for PFS, P=0.007; 85.3% vs 65.0% for OS, P=0.010) (Figure 2), similarly, in DHL patients, the PFS and OS for R-DA-EPOCH regimen were higher than those for R-CHOP (73.0% vs 28.1%, P=0.001 for PFS, 68.4% vs 38.2%, P=0.047 for OS) (Figure 3).
For the two disease types, the PFS of DHL group was significantly worse than that of double CNG (52.9% vs 72.4%, P=0.008) (Figure 4), while no significant differences were observed in OS (52.9% vs 75.2%, P=0.050). We performed subgroup analyses on the two disease types, the results showed that the PFS for double CNG with MYC and BCL2 was superior to that for DHL with MYC and BCL2 (MB2) (73.3% vs 48.2%, P=0.043) (Figure 5), this trend is also seen in double CNG and DHL with MYC and BCL6 (MB6) (73.2% vs 51.4%, P=0.036) (Figure 6). However, the OS was not significantly different between the two subgroups (P=0.161 for MB2; P=0.166 for MB6).
  | Figure 4 PFS (A) and OS (B) in double CNG and DHL patients. Abbreviations: CNG, copy number gain; DHL, double-hit lymphoma; OS, overall survival; PFS, progression-free survival. |
Univariate and multivariate analysis
Both univariate and multivariate analyses showed that in DLBCL patients with genetic abnormality detected by FISH, the treatment regimen and disease types were independent prognostic factors while the other factors were not (Table 3). Patients treated with R-DA-EPOCH regimen had a lower risk of death than traditional R-CHOP (PFS: R-DA-EPOCH: HR 0.173; 95% CI: 0.064–0.466; P=0.001; OS: R-DA-EPOCH: HR 0.248; 95% CI: 0.104–0.589; P=0.002), while patients with DHL had a higher risk of death than patients with double CNG (PFS: DHL: HR 4.549; 95% CI: 1.926–10.746; P=0.001; OS: DHL: HR 2.506; 95% CI: 1.120–5.606; P=0.025).
Adverse effects
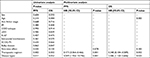
Adverse events (AEs) were evaluated in all 127 patients, the main AE in our cohort was hematologic. The incidence of grade 3/4 leukopenia was 41.7%, grade 3/4 neutropenia accounted for 27.6% of all patients, the incidence of grade 3/4 thrombocytopenia was 8.7%, and grade 3/4 anemia occurred in 14.2% patients. The non-hematological toxicity were mild, 10.2% of patients had grade 3/4 elevated alanine aminotransferase/aspartate transaminase levels; 6.3% had pulmonary infection caused by neutropenia; the incidence of nausea/vomiting was 40.9%, but mainly 1/2 grade AEs; only 7.9% of patients had grade 3/4 of gastrointestinal reactions and 3 (2.4%) had allergic reaction. No serious cardiotoxicity, chemotherapy-related deaths, or secondary tumors were observed. The grade 3/4 myelosuppression in R-DA-EPOCH was heavier than in R-CHOP, but there was no statistical difference (P>0.05), and other AEs were similar (Table 4).
Discussion
Due to the lack of evidence for the optimal treatment of DHL and double CNG in the era of rituximab, physicians can only choose treatments ranging from R-CHOP to R-CHOP-like regimens. The innovation of our research is that we compared the prognosis of DHL and double CNG, and contrast the efficacy of the R-DA-EPOCH and R-CHOP regimens in these two disease types. As far as we know, this is the first report comparing the efficacy of two regimens in this two disease types.
DHL is a subtype of DLBCL with both MYC and BCL2/BCL6 translocations. Studies showed that this type has invasive clinical manifestations and a poor response to R-CHOP, with a mean OS fluctuating between 5 months and 2 years.14,31–34 DHL patients are prone to happen central invasion (13%), while after given the prevention treatment such as preventive intrathecal injection, the central recurrence rate is reduced from 15% to 3%.35,36 In addition, studies have shown that patients with CNG of MYC/BCL2 have poor prognosis.10–13 Yoon et al11 studied 145 patients with DLBCL, 17 (12%) of whom exhibited increased BCL2 copy number and had poor prognosis. Collectively, these studies show that MYC or BCL2 CNG confers inferior survival rates. However, these studies only examined MYC or BCL2 CNG individually, not in combination. Our study analyzed the prognostic difference between DHL and double CNG, and the results showed that DHL had a shorter 2-year PFS than double CNG (P=0.008), while the OS was not significantly different (P=0.050). Subgroup analysis showed that patients with double CNG (MB2) had a higher PFS to patients with DHL (MB2) (P=0.043), similarly, patients with double CNG (MB6) had a superior PFS to patients with DHL (MB6) (P=0.036), while the OS in two subgroups was not significantly different. The aforementioned results suggested that DHL has a poorer prognosis than double CNG. Considering the short observation period and small sample size of DHL patients, some bias may exist in analysis of small subgroups, and further clinical studies are needed to confirm these findings.
At present, there is no international consensus on the standardized treatment for DHL/double CNG. Some investigators have analyzed prognosis by increasing the dose or density of chemotherapy and observed better therapeutic effects.15–23,26 In recent years, two large-scale retrospective clinical analyses17,36 have shown that the application of R-DA-EPOCH regimen significantly increased the PFS compared with other regimens in DHL. Petrich et al17 investigated 311 patients in a multicenter retrospective analysis, and results showed that each enhancement protocol significantly improved the PFS rate (R-DA-EPOCH compared with R-CHOP, P=0.0463; R-Hyper CVAD, P=0.001; R-CODOX-M/IVAC, P=0.036), while no significant difference was observed in OS (P=0.119). Howlett et al36 investigated 394 patients from 11 studies, and this meta-analysis showed that the median PFS of R-CHOP, R-EPOCH, and dose intensity groups (refers to R-Hyper-CVAD, R-M/A, R-CODOX-M/IVAC) were 12.1, 22.2, and 18.9 months, respectively. Furthermore, the PD risk of R-DA-EPOCH was reduced by 34% compared with that of R-CHOP (P=0.032). However, there was no significant difference in the OS between different regimens. Récher37 compared the efficacy of the R-ACVBP and R-CHOP regimens in an open-label randomized trial, and observed a superior 3-year event-free survival (EFS), PFS, and OS in the R-ACVBP group compared with the R-CHOP group after a median follow-up of 44 months (81% vs 67%, P=0.0035 for EFS; 87% vs 73%, P=0.0015 for PFS; 92% vs 84%, P=0.0071 for OS). However, hematological toxicity in R-ACVBP group was significantly increased. H. Sun38 studied 32 patients with DHL in a retrospective analysis, and the results showed that R-CODOX-M/IVAC combined with transplantation increased the PFS and OS to 41% and 53%, respectively. Taken together, these studies have shown that although a stronger chemotherapy regimen can improve the survival of patients with high-risk DLBCL, the toxicity of intensive regimens is increased compared with that of R-CHOP regimen.37 Our study showed that the ORR of the R-DA-EPOCH group was higher than that of R-CHOP group (P=0.005). For 2-year PFS and OS, R-DA-EPOCH also showed significant advantages over R-CHOP (79.8% vs 57.5%, P=0.002 for PFS; 81.6% vs 58.5%, P=0.002 for OS). Separately analyzing the survival of patients with DHL and double CNG, we found that for double CNG patients, the R-DA-EPOCH regimen achieved a higher 2-year PFS and OS than traditional R-CHOP (82.8% vs 63.2%, P=0.007 for PFS; 85.3% vs 65.0%, P=0.010 for OS), similarly, in DHL patients, the PFS and OS for R-DA-EPOCH regimen were higher than those for R-CHOP (73.0% vs 28.1%, P=0.001 for PFS; 68.4% vs 38.2%, P=0.047 for OS). These results are consistent with those of studies by Petrich et al17 and Howlett et al36, which indicate that the R-DA-EPOCH demonstrates therapeutic advantages both in DHL and double CNG.
Since the administration of the R-DA-EPOCH regimen is continuous intravenous infusion, the adverse reactions increase simultaneously. The results showed that the grade of 3/4 myelosuppression in R-DA-EPOCH was heavier than in R-CHOP (Table 4), while there was no statistical difference (P>0.05). In addition, these AEs can be returned to normal within a short period of time after given symptomatic treatment, no chemotherapy-related deaths or secondary tumors occurred, indicating that R-DA-EPOCH regimen is relatively safe.
Conclusion
In summary, our retrospective study showed that DHL has a poorer prognosis than double CNG. Compared with traditional R-CHOP, R-DA-EPOCH is a more effective and well-tolerated regimen for both DHL and double CNG, and is expected to become the first-line treatment for these two disease types. However, due to the potential limitations of single center, small sample, retrospective analysis, further prospective, multicenter, and large-scale studies are needed to establish the status of R-DA-EPOCH regimen and the first-line therapy for these high-risk DLBCL patients.
Acknowledgments
The authors wish to thank Wencai Li, Guannan Wang, and Wugan Zhao for participating in pathological diagnosis and manuscript revision. Qianwen Ma and Yu Chang are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Smith A, Roman E, Howell D, et al. The Haematological Malignancy Research Network (HMRN): a new information strategy for population based epidemiology and health service research. Br J Haematol. 2010;148(5):739–753. | ||
Martelli M, Ferreri AJ, Agostinelli C, di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87(2):146–171. | ||
Smith A, Howell D, Crouch S, et al. Cohort profile: the Haematological Malignancy Research Network (HMRN): a UK population-based patient cohort. Int J Epidemiol. 2018;47(3):700–700g. | ||
Le Gouill S, Talmant P, Touzeau C, et al. The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica. 2007;92(10):1335–1342. | ||
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J. Lymphoma classification – from controversy to consensus: the R.E.A.L. and WHO Classification of lymphoid neoplasms. Ann Oncol. 2000;11(Suppl 1):S3–S10. | ||
Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. | ||
Lin P, Medeiros LJ. High-grade B-cell lymphoma/leukemia associated with t(14;18) and 8q24/MYC rearrangement: a neoplasm of germinal center immunophenotype with poor prognosis. Haematologica. 2007;92(10):1297–1301. | ||
Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from the International DLBCL Rituximab-CHOP Consortium program. Blood. 2013;121(20):4021–4031. | ||
Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–3459. | ||
Dunleavy K, Roschewski M, Abramson JS. Preliminary report of a multicenter prospective phase II study of DA-EPOCH-R in MYC-rearranged aggressive B-cell lymphoma. Blood. 2014;124:395. | ||
Yoon SO, Jeon YK, Paik JH, et al. MYC translocation and an increased copy number predict poor prognosis in adult diffuse large B-cell lymphoma (DLBCL), especially in germinal centre-like B cell (GCB) type. Histopathology. 2008;53(2):205–217 | ||
Kusumoto S, Kobayashi Y, Sekiguchi N, et al. Diffuse large B-cell lymphoma with extra Bcl-2 gene signals detected by FISH analysis is associated with a “non-germinal center phenotype”. Am J Surg Pathol. 2005;29(8):1067–1073. | ||
Monni O, Joensuu H, Franssila K, Klefstrom J, Alitalo K, Knuutila S. Bcl2 overexpression associated with chromosomal amplification in diffuse large B-cell lymphoma. Blood. 1997;90(3):1168–1174. | ||
Sarkozy C, Traverse-Glehen A, Coiffier B. Double-hit and double-protein-expression lymphomas: aggressive and refractory lymphomas. Lancet Oncol. 2015;16(15):e555–e567. | ||
Kurita D, Miura K, Nakagawa M, et al. Dose-intensified CHOP with rituximab (R-Double-CHOP) followed by consolidation high-dose chemotherapies for patients with advanced diffuse large B-cell lymphoma. Int J Hematol. 2015;101(6):585–593. | ||
Chen AI, Leonard JT, Okada CY, et al. Outcomes of DA-EPOCH-R induction plus autologous transplant consolidation for double hit lymphoma. Leuk Lymphoma. 2018;59(8):1884–1889. | ||
Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124(15):2354–2361. | ||
Greer JP, Ds M, Li S. Role of aggressive chemotherapeutic regimens in double hit lymphoma- can alternate aggressive induction regimens overcome the poor prognosis of diffuse large B cell lymphoma? Blood. 2013;122:4361–4361. | ||
Munoz J, Vekaria M, Hanbali A, Janakiraman N. Progression of double-hit lymphoma in the midst of R-hyper CVAD. Am J Hematol. 2013;88(1):87–88. | ||
Sun H, Savage KJ, Karsan A, et al. Outcome of patients with double-hit lymphomas treated with CODOX-M/IVAC + R followed by hematopoietic stem cell transplantation in British Columbia. Blood. 2013;122:1788. | ||
Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted epoch and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26(16):2717–2724. | ||
Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328(14):1002–1006. | ||
Mangasarova YK, Magomedova AU, Nesterova ES, et al. Therapy for primary mediastinal large B-cell lymphoma in accordance with the R-DA-EPOCH-21 program: the first results. Ter Arkh. 2016;88(7):37–42. | ||
Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369(18):1681–1690. | ||
Chiappella A, Martelli M, Angelucci E, et al. Rituximab-dose-dense chemotherapy with or without high-dose chemotherapy plus autologous stem-cell transplantation in high-risk diffuse large B-cell Lymphoma (DLCL04): final results of a multicentre, open-label, randomised, controlled, phase 3 study. Lancet Oncol. 2017;18(8):1076–1088. | ||
Pejša V, Prka Ž, Lucijanić M, et al. Rituximab with dose-adjusted epoch as first-line treatment in patients with highly aggressive diffuse large B-cell lymphoma and autologous stem cell transplantation in selected patients. Croat Med J. 2017;58(1):40–48. | ||
Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. | ||
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. | ||
Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI sponsored international working group. J Clin Oncol. 1999;17(4):1244–1244. | ||
Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. | ||
Herrera AF, Mei M, Low L, et al. Relapsed or refractory double-expressor and double-hit lymphomas have inferior progression-free survival after autologous stem-cell transplantation. J Clin Oncol. 2017;35(1):24–31. | ||
Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166(6):891–901. | ||
Cohen JB, Geyer SM, Lozanski G, et al. Complete response to induction therapy in patients with Myc-positive and double-hit non-Hodgkin lymphoma is associated with prolonged progression-free survival. Cancer. 2014;120(11):1677–1685. | ||
Tomita N, Tokunaka M, Nakamura N, et al. Clinicopathological features of lymphoma/leukemia patients carrying both Bcl2 and Myc translocations. Haematologica. 2009;94(7):935–943. | ||
Savage KJ, Slack GW, Mottok A, et al. Impact of dual expression of myc and Bcl2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. 2016;127(18):2182–2188. | ||
Howlett C, Snedecor SJ, Landsburg DJ, et al. Front-line, dose-escalated immunochemotherapy is associated with a significant progression-free survival advantage in patients with double-hit lymphomas: a systematic review and meta-analysis. Br J Haematol. 2015;170(4):504–514. | ||
Récher C, Coiffier B, Haioun C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell Lymphoma (LNH03-2B): an open-label randomised phase 3 trial. The Lancet. 2011;378(9806):1858–1867. | ||
Sun H, Savage KJ, Karsan A, et al. Outcome of patients with non-Hodgkin lymphomas with concurrent myc and Bcl2 rearrangements treated with CODOX-M/IVAC with rituximab followed by hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk. 2015;15(6):341–348. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.