Back to Journals » Neuropsychiatric Disease and Treatment » Volume 10
Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer's disease
Authors Hager K, Baseman A, Nye JS, Brashear HR, Han J, Sano M, Davis B, Richards H
Received 20 November 2013
Accepted for publication 15 January 2014
Published 21 February 2014 Volume 2014:10 Pages 391—401
DOI https://doi.org/10.2147/NDT.S57909
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Klaus Hager,1 Alan S Baseman,2 Jeffrey S Nye,2 H Robert Brashear,2 John Han,2 Mary Sano,3 Bonnie Davis,4 Henry M Richards2
1Clinic for Medicine of the Elderly, Hannover, Germany; 2Janssen Research and Development, LLC, Raritan, NJ, USA; 3The Mount Sinai Medical Center, New York, NY, USA; 4Synaptec Inc., Palm Beach Gardens, FL, USA
Background: Currently available treatments for Alzheimer's disease (AD) can produce mild improvements in cognitive function, behavior, and activities of daily living in patients, but their influence on long-term survival is not well established. This study was designed to assess patient survival and drug efficacy following a 2-year galantamine treatment in patients with mild to moderately severe AD.
Methods: In this multicenter, double-blind study, patients were randomized 1:1 to receive galantamine or placebo. One primary end point was safety; mortality was assessed. An independent Data Safety Monitoring Board monitored mortality for the total deaths reaching prespecified numbers, using a time-to-event method and a Cox-regression model. The primary efficacy end point was cognitive change from baseline to month 24, as measured by the Mini-Mental State Examination (MMSE) score, analyzed using intent-to-treat analysis with the 'last observation carried forward' approach, in an analysis of covariance model.
Results: In all, 1,024 galantamine- and 1,021 placebo-treated patients received study drug, with mean age ~73 years, and mean (standard deviation [SD]) baseline MMSE score of 19 (4.08). A total of 32% of patients (661/2,045) completed the study, 27% (554/2,045) withdrew, and 41% (830/2,045) did not complete the study and were discontinued due to a Data Safety Monitoring Board-recommended early study termination. The mortality rate was significantly lower in the galantamine group versus placebo (hazard ratio [HR] =0.58; 95% confidence interval [CI]: 0.37; 0.89) (P=0.011). Cognitive impairment, based on the mean (SD) change in MMSE scores from baseline to month 24, significantly worsened in the placebo (-2.14 [4.34]) compared with the galantamine group (-1.41 [4.05]) (P<0.001). Functional impairment, based on mean (SD) change in the Disability Assessment in Dementia score (secondary end point), at month 24 significantly worsened in the placebo (-10.81 [18.27]) versus the galantamine group (-8.16 [17.25]) (P=0.002). Incidences of treatment-emergent adverse events were 54.0% for the galantamine and 48.6% for the placebo group.
Conclusion: Long-term treatment with galantamine significantly reduced mortality and the decline in cognition and daily living activities, in mild to moderate AD patients.
Identification: This study is registered at ClinicalTrials.gov (NCT00679627).
Keywords: cholinesterase inhibitors, cognition, long-term treatment, mortality, nicotinic
Corrigendum for this article has been published
Introduction
Alzheimer’s disease (AD) is the sixth leading cause of death in the USA.1 Although there is extensive literature showing that the currently available treatments can produce mild improvements in cognitive function, behavior, and activities of daily living in patients with AD,2–4 the influence of antidementia drugs on long-term survival is not yet well established. Given that the mainstay of AD treatment remains cholinesterase inhibitors (ChEIs), determination of their impact on mortality remains important. The analyses examining the effect of ChEIs on survival have been largely retrospective or observational.2,5–7A recent retrospective, long-term observational study analysis showed decreased mortality with ChEIs in AD patients versus (vs) untreated patients.8 However, controlled data to date do not suggest that any of the antidementia drugs improves long-term survival.5,9
Galantamine HBr (Reminyl®; called Razadyne® in the USA [Janssen Pharmaceuticals, Inc., Titusville, NJ, USA]) is a reversible, competitive ChEI and a positive allosteric modulator of nicotinic receptors.10 It is approved for the treatment of mild to moderately severe dementia of Alzheimer type in the USA and for AD with cerebrovascular disease in certain other countries.11,12 Galantamine’s efficacy and safety have been documented in pivotal Phase III, double-blind, randomized controlled trials (RCTs) of ≤6 months duration; however, long-term RCTs have not been performed.13–16
A comprehensive post hoc analysis of a study for patients with mild cognitive impairment demonstrated no significant difference in survival for those on drug vs placebo, despite an initial impression of increased mortality in the galantamine group.17 Considering the potential risk raised in this mild cognitive impairment trial, we designed a trial to prospectively assess the long-term survival of patients and efficacy of galantamine, in a 2-year placebo-controlled, randomized study in mild to moderately severe AD patients.
Materials and methods
Study design and participants
This was a randomized, double-blind, placebo-controlled, parallel-group, multicenter study, conducted from May 19, 2008 to May 20, 2012, of galantamine vs placebo in patients with mild to moderately severe AD. The study was conducted at 127 centers in Czech Republic, Estonia, France, Germany, Greece, Italy, Latvia, Lithuania, Romania, Russia, Slovakia, Slovenia, and Ukraine.
The major inclusion criteria were: 1) men or women outpatients, aged 45 to 90 years (inclusive), with mild to moderate, probable or possible AD;18 and 2) patient with or without cerebrovascular disease, having a computed tomography or magnetic resonance imaging of the head performed since the diagnosis of AD, and before inclusion in the study, a Mini-Mental State Examination (MMSE) score of 10–26, and a responsible caregiver.
Exclusion criteria were: 1) other neurodegenerative or major psychiatric disorders or other causes of dementia, including cerebral trauma, vascular dementia without AD, hypoxic cerebral damage, vitamin deficiency, central nervous system infections, transmissible diseases, primary or metastatic cerebral neoplasia, mental retardation, or significant endocrine or metabolic disease; and 2) epilepsy, hepatic, renal, or pulmonary disturbances, urinary outflow obstruction, or clinically significant cerebrovascular disease.
Concomitant medications
Memantine and other cognition-affecting medications, including psychotropic drugs, sedatives/hypnotics, antidepressants, antipsychotics, cough and cold remedies, cholinergic agents (disallowed 2 weeks before screening), antiemetics, and antihypertensives with known cognitive side effects, were allowed during the study, but not <1 day before scheduled visits, whenever possible. Galantamine products other than the study drug, experimental agents, or other ChEIs were prohibited.
Standard protocol approvals, registrations, and patient consents
The Independent Ethics Committee or Institutional Review Board at each study site approved the protocol, and the study was conducted in accordance with ethical principles having origin in the Declaration of Helsinki and consistent with Good Clinical Practice (GCP) and applicable regulatory requirements. All patients and caregivers (or legally acceptable representatives) provided written informed consent before entering the study.
Randomization and blinding
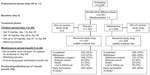
The study consisted of a pretreatment phase of up to 4 weeks, including the screening period, followed by a treatment phase (24 months), which included a 12-week titration period and a maintenance period of approximately 21 months, and a posttreatment follow-up phase (30 days) (Figure 1). Patients were assigned in a 1:1 ratio to either a galantamine or placebo treatment group, based on a computer-generated randomization schedule prepared at Janssen Research and Development, LLC. The randomization was balanced by using randomly permuted blocks and was stratified by study center. An Interactive Voice Response System (IVRS) assigned patients to the study treatment; the assignment codes were maintained within the IVRS to ensure that investigators, patients, and caregivers were blinded.
Galantamine was supplied as oral extended release capsules equivalent to 8 mg, 16 mg and 24 mg with matching placebo, and dosed according to the product labeling.
Outcomes
Safety
The primary safety analysis compared the rate of mortality between patients randomly assigned to receive galantamine or matching placebo over the 2-year study. Safety data were monitored during the study by a company-commissioned, external, independent, blinded Data Safety Monitoring Board (DSMB).
Secondary safety outcomes were the number of treatment-emergent adverse events (TEAEs), including serious TEAEs. Clinical laboratory test results, pregnancy tests, vital signs, body weight, physical and neurological examinations, and electrocardiograms were also assessed.
Efficacy
The MMSE scale (in which the maximum score is 30, with lower scores indicating greater impairment) was used for the primary efficacy variable;19 assessment was conducted by trained site staff.
The secondary efficacy measures included the score on the Disability Assessment in Dementia (DAD),20 a 20-item scale measuring activities of daily living (with a higher score representing better performance). The DAD was administered by the caregiver, who was trained in study processes.
Analyses sets
The intent-to-treat (ITT) analysis set included all randomized patients who had at least one postbaseline MMSE measure. The per protocol analysis set was a subset of the ITT analysis set and excluded patients with major protocol deviations. The safety analysis set included all randomized and treated patients. After the exclusion of patients from two sites with GCP noncompliance, the ITT data analysis set included 1,812 patients (906 patients in each group), and the per protocol analysis set included 1,729 patients (869 in the galantamine and 860 in the placebo group); all sites’ data were included in the safety analyses.
Statistical evaluations
Safety analyses
The primary safety end point was the mortality data (all deaths) analyzed by the time-to-event (death) analysis method, using Cox proportional hazards regression model with treatment as covariate. A log-rank test was performed to compare the estimates of the hazard functions of the two groups.
Kaplan–Meier survival curves, relative risk (RR), confidence interval (CI), and summary statistics were also provided. A group sequential method was used to monitor mortality when the total number of deaths reached 16, 32, 48, 64, and 80. If <80 deaths occurred when all patients had completed the study, then the final analysis was to be based on <80 events. If ≥80 events occurred before all enrolled patients had completed the study, the study continued as planned unless the DSMB recommended stopping the study early. The final analysis was to be based on the total number of events. Additional analyses of the mortality data included a survival analysis of death cases that occurred within 30 days of the last dose of study drug; a Cox proportional hazards regression model that provided a point estimate; and a 95% CI for the hazard ratio (HR) comparing mortality between the treatment groups. An analysis of deaths by baseline characteristics (age, MMSE, time-dependent covariates) was conducted. The TEAEs, vital signs and clinical examinations were monitored. The TEAE terms were coded using MedDRA® version 15.0.
Efficacy analyses
The primary efficacy analysis used the change in MMSE score from baseline to month 24. The secondary end points included MMSE change from baseline to month 6 and change in the DAD scores from baseline to month 24.
An analysis of the ITT cohort with the last observation carried forward (LOCF) approach was used for the primary analysis. For MMSE scores, an analysis of covariance with a two-sided significance level of 0.05 was used to compare the two treatment groups, including treatment and study site as fixed factors and MMSE baseline values as covariates. A similar statistical approach was used for the DAD scores. A mixed-effect modeling analysis was used for the primary end point, as a sensitivity analysis to address missing data and the time course of treatment effect; analysis was based on the observed case data, with no imputation of missing values.
The MMSE scores were also analyzed by use vs nonuse of memantine.
Sample size determination
Based on 6-month studies and with a two-sided logrank test and a proportional HR assumption, a 3% placebo mortality rate was estimated: a sample size of 1,000 patients per group was to be used to ensure ≥80% power to detect an RR of 2.0. A sample size of 1,000 patients per group would achieve a >99% power to detect 0.8 point difference in MMSE between the two treatment groups.
Results
A total of 2,225 patients were enrolled in the study, and 2,051 were randomized to galantamine or placebo. Of the 2,045 patients who received the study drug (galantamine [n=1,024] or placebo [n=1,021]), 32% (661/2,045) completed the study, while 27% (554/2,045) withdrew; 41% (830/2,045) did not complete the study and were discontinued as a result of the DSMB-recommended study termination when the prespecified number of deaths was reached and a significant difference in death rate between treatments was observed (Figure 1).
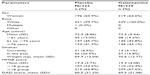
The demographic and baseline characteristics were comparable between the galantamine and placebo groups (Tables 1 and S1), as were medical histories (Tables S2 and S3). The majority of patients were women (64.8%) and white (99.9%), and the mean (standard deviation [SD]) age of the patients was 73 (8.77) years. The mean (SD) baseline MMSE score was 19.0 (4.08) and DAD score was 61.4 (21.35), suggesting few disabilities in activities of daily living. The majority of patients (>80%) were on concomitant medications that were balanced between the groups. Less than 1% patients (n=8 [0.8%] placebo-treated and n=4 [0.4%] galantamine-treated patients) had received prior ChEIs. Memantine or memantine hydrochloride, was taken by 21.8% of galantamine- and 21.1% of placebo-treated patients.
The mean duration of study drug exposure was similar in patients receiving galantamine (16.1 months) and placebo (15.9 months). A total 1,247/2,045 (60.98%) patients received >12 months exposure to the study drug (Table 2).
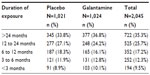  | Table 2 Extent of exposure to galantamine and placebo |
Safety and tolerability findings
At the final interim mortality analysis (conducted when the study reached the prespecified milestone of 80 deaths), the DSMB recommended early termination due to a statistical difference in deaths between the blinded treatment groups. Subsequent unblinding revealed significantly lower mortality in patients treated with galantamine (3.1%) vs placebo (4.9%) (P=0.021). The subsequent final analysis of 89 deaths showed that the 33 (3.2%) deaths in the galantamine group were significantly lower than the 56 (5.5%) deaths in the placebo group (HR =0.58; 95% CI: 0.37; 0.89) (P=0.011). Patients receiving galantamine had a significantly higher survival rate than did the placebo-treated patients (Figure 2). Prespecified analyses of the mortality data in the subgroups, defined by age (P=0.798) and MMSE (P=0.341), revealed no significant difference. However, the death rate (per 100 patient years) in patients with MMSE <18 was slightly higher (placebo: 7.01, galantamine: 4.82) than those with MMSE ≥18 (placebo: 2.63, galantamine: 1.15), consistent with the primary analysis results.
  | Figure 2 Time from randomization to death (safety analysis set). |
Overall, 54% galantamine-treated patients and 49% placebo-treated patients reported one or more TEAEs, most of which were mild or moderate in severity. The most commonly reported TEAEs in the galantamine group vs the placebo group were nausea (8.4% vs 2.4%) and headache (5.7% in both the groups) (Table 3). Serious TEAEs occurred in similar proportions in both groups (galantamine: 12.6%, placebo: 12.0%), with 11% hospitalizations and 3.0% mortality in the galantamine group, and 8.6% hospitalizations and 4.6% mortality in the placebo group. The TEAEs leading to treatment discontinuation were higher in the galantamine- (n=87 [8.5%]) vs placebo-treated patients (66 [6.5%]) and included nausea (2.0% vs 0.3%) and vomiting (1.0% vs 0.2%).
Of the total 89 deaths, 78 were due to TEAEs; the proportion of TEAEs leading to death tended to be lower in the galantamine (n=31 [3.0%]) vs placebo group (47 [4.6%]). The remaining 11 deaths (two in the galantamine-treated and nine in the placebo-treated group) were due to events that occurred more than 30 days after last study drug intake and hence, not classified as TEAEs. The most frequent TEAEs (galantamine vs placebo) leading to death were cardiac failure (n=4 vs n=3, respectively), cardiopulmonary failure (n=3 vs n=4, respectively), Alzheimer-type dementia worsening (n=3 vs n=5, respectively), and pneumonia (n=3 vs n=2, respectively). Pulmonary or cardiovascular events occurred in 265 patients (n=139 in the galantamine-treated vs n=126 in the placebo-treated group). These resulted in the hospitalization of 27 galantamine- and 13 placebo-treated patients, with 17 deaths in galantamine-treated and 24 deaths in placebo-treated patients.
No clinically meaningful changes were observed in vital signs, weight, physical and neurological examination findings, or laboratory tests. No clear pattern or category of TEAEs or adverse events or TEAEs leading to mortality differentiated the galantamine and placebo groups.
Efficacy findings
Primary efficacy
At 24 months, MMSE scores declined significantly less in the galantamine than in the placebo group (−1.41 vs −2.14) (P<0.001) (Figure 3). The analyses including the two noncompliant GCP sites also showed similar results; exclusion of the noncompliant GCP sites did not affect the results. Similar results were observed with analysis of the per protocol analysis set (MMSE scores at 24 months declined less in the galantamine vs the placebo groups [−1.39 vs −2.00; P<0.001]). The results of the sensitivity analysis were consistent with the primary LOCF analysis.
Secondary efficacy
The mean (SD) change in MMSE from baseline at month 6 improved significantly in the galantamine (0.15 [2.73]) vs placebo group (−0.28 [2.94]) (P<0.001). The mean (SD) change from baseline in DAD scores showed significant worsening in the placebo vs galantamine group at both prespecified secondary end points: at month 12 LOCF, was −6.50 (16.17) vs −4.55 (14.68) (P=0.009), and at month 24 LOCF, it was −10.81 (18.27) vs −8.16 (17.25) (P=0.002) (Figure 4).
Additional analyses
In memantine nonusers, galantamine reduced MMSE decline by 1.03 points, nearly half of the decline in placebo patients (galantamine: –1.12 [3.87], placebo: –2.15 [4.41]). Galantamine did not appear to affect MMSE decline in memantine users (memantine + placebo: –2.10 [4.12], memantine + galantamine: –2.35 [4.48]). In placebo patients, the decline in the MMSE at 24 months was similar in memantine users and nonusers (memantine+placebo: –2.10 [4.14], placebo: –2.15 [4.41]) (Table 4).
Discussion
In patients with mild to moderate AD, 2-year treatment with galantamine resulted in a significantly lower mortality rate, a significant reduction of 34% from baseline in cognitive decline, and less decline in activities of daily living by 24% vs placebo. Due to the significant mortality difference between treatment arms, the DSMB recommended early study termination. This is the largest placebo-controlled, randomized, prospective, 2-year study of a member of the class of anticholinesterases in AD to be reported and the first to establish a significantly lower mortality vs placebo.
The reduction in deaths was not attributable to any particular organ system. Except for gastrointestinal galantamine-associated adverse effects, the TEAE and serious TEAE incidences were similar to placebo, and the results corroborate those of other galantamine studies.5,13–16,21 However, in this study, numerically more frequent hospitalizations were noted in galantamine- vs placebo- treated patients. The improved cognitive benefits, measured in part by the MMSE and DAD, possibly enabled patients to communicate their symptoms better and thus receive timely treatment and may underlie the improved survival that occurred with galantamine treatment. These results are also consistent with data suggesting that worsening functional abilities was the only time-dependent covariate detected that significantly decreased survival in AD patients.9
In a separate mild cognitive impairment study, galantamine reduced cortical atrophy by 34% in a subset of the patients who underwent magnetic resonance imaging (MRI) scans at baseline and after 2 years, consistent with the reduced MMSE deterioration in the current study.22 In the in vitro studies, galantamine, via positive allosteric modulation of nicotinic receptors, has been shown to protect neurons against amyloid-β (Aβ) and glutamate toxicity, and to promote microglial uptake of Aβ at therapeutic concentrations.23–25 As late-onset AD, which affected the majority of patients in this study, is characterized by an Aβ-clearance deficit, galantamine’s nicotinic mechanism could have contributed to the observed effects.26
Our efficacy results corroborate those of previous shorter-term (up to 6 months) RCTs wherein galantamine treatment demonstrated significant improvement in cognitive and global function.13–16 Although the change in MMSE (0.73) appears small in our study, it is significant and was persistent over 2 years. Galantamine patients’ MMSE scores at 24 months were similar to placebo patients’ interpolated scores at about 15 months. In memantine nonusers, galantamine MMSE scores at 24 months were approximately those of placebo patients at 13 months. In contrast, memantine appeared to oppose the effects of galantamine so that MMSE declined similarly to placebo in memantine users. Possibly, patients were on memantine in our study because they had failed on ChEIs previously and were not responsive to them. Only 0.4% of galantamine patients had previous ChEI treatment. Alternatively, as memantine is a powerful antagonist of α4β2 and α7 nicotinic receptors, our findings may illustrate the importance of nicotinic enhancement in galantamine’s mechanism of action.27,28 In another study in moderate to severe AD, the effects of donepezil, a ChEI without nicotinic modulatory properties, were not reduced by concomitant memantine administration.29
The limitations of this study included a lack of information concerning medication compliance, an exclusively white European cohort, and a requirement for a responsible caregiver. As with all clinical trial results, these findings may not be broadly applicable to more diverse sets of patients or treatment conditions experienced in actual clinical practice settings.
Conflicting views exist regarding the class of ChEIs in AD treatment; some favor their long-term use, while others question the clinically meaningful benefits. A recent retrospective observational study analysis from the Swedish Dementia Registry showed decreased mortality in AD patients with ChEI treatment for an average of 500 days, compared with untreated patients; the authors noted that it would be worthwhile if these results could be confirmed in RCTs.8 Findings of RCTs like the AD2000 study have not confirmed long-term reduced mortality with ChEI-treated as compared with placebo-treated patients.30 Due to limited controlled-data availability, more long-term ChEI placebo-controlled RCTs are warranted to focus on these outcomes, although ethical concerns are a challenge.
Conclusion
This study represents the longest placebo-controlled RCT of a ChEI that addresses the issue of mortality. Conducted in over 2,000 patients with mild to moderate AD, it establishes that galantamine treatment resulted in a significantly decreased mortality rate and showed benefits in cognition and activities of daily living that persisted for 2 years.
Acknowledgments
We thank Dr Madhavi Patil (SIRO Clinpharm Pvt. Ltd.) for providing writing assistance and Dr Wendy P Battisti (Janssen Research and Development, LLC) for providing expert editorial assistance. The authors wish to thank Dr Walter Neto, formerly employed by Janssen Research and Development, LLC for his contribution as Project Physician in the early stages of the study. The authors thank the study participants, without whom this study would not have been accomplished, as well as the following investigators for their participation in this study:
Czech Republic: Bartova Petra, MD; Brunovsky Martin, MD, PhD; Cernohorsky Dusan, MD; Latalova Klara, MD; Pietrucha Slavomir, MD; Topinkova Eva, MD, PhD; and Vaclavik Daniel, MD. Estonia: Andresen Kadri, MD; Ennet Jüri, MD; Gross-paju Katrin, MD, PhD; Linnamagi Ulla, MD, PhD; Schults Marje, MD; and Talvik Pille, MD. France: Dantoine Thierry, PhD. Germany: Bittkau Simon, MD; Bodenschatz Ralf, MD; Böhringer Johannes, MD; Diery Hans D, MD; Dorn Brita, MD; Glatzel Dirk, MD; Hager Klaus, MD; Horn Rolf, MD; Hundt Wolfgang, MD; Hüntemann Reinhard, MD; Käfferlein Wolfgang, MD; Koppai-reiner Joachim, MD; Krug Reinhard, MD; Müller Friedemann, MD; Müller Thomas, MD; Niklewski Guenter, MD; Oldenburg Wolfgang, MD; Peltz Jörg, MD; Sallach Klaus MD; Schoell Irma, MD; Sigel Karl-Otto, MD; Springub Joachim, MD; Vollmuth Markus, MD; Wiswedel Henning, MD; and Zerr Inga, MD. Greece: Papanastasiou Ioannis, MD; Spinaki Cleanthe, MD, PhD; and Tsolaki Magda, MD, PhD. Italy: Barbanti Piero, MD; Lorusso Sebastiano, MD; Mazzei Bruno, MD; Onofrj Marco, MD; Postacchini Demetrio, MD; Scarpino Osvaldo, MD; and Stracciari Andrea, MD. Latvia: Sarkane Rudite, MD. Lithuania: Budrys Valmantas, MD and Liesiene Vanda, MD. Romania: Badescu Alexandra, PhD; Chirita Vasile, PhD; Dan Irina-Ana, PhD; Fodoreanu Liana, PhD; Gabos-Grecu Iosif, PhD; Giurgiuca Liana, PhD; Lapadat Mihaela, MD; Marinescu Victor, PhD; Pantu Camelia Mihaela, MD; Podea Delia, MD, PhD; Rosca Mihaela Cleopatra, MD; and Tudose Catalina, PhD. Russia: Agarkov Alexey, MD; Balunov Oleg, MD, PhD; Belova Anna, MD; Burdukovsky Mikhail, MD; Dobrovolskaya Natalya, MD; Gavrilova Svetlana, MD, PhD; Gribanov Andrey, MD; Gustov Alexander, MD; Ivlieva Irina, MD; Khasanova Dina, MD; Kolchev Alexander, MD, PhD; Lebedeva Anna, MD, PhD; Malakhova Anna, MD; Maslova Natalya, MD, PhD; Neznanov Nikolay, MD, PhD; Odinak Miroslav, MD, PhD; Pizova Natalya, MD, PhD; Sherman Mikhail, MD; Sheyfer Mikhail, MD, PhD; Shiryaev Oleg, MD; Sitchikhin Pavel, MD; Sluchevskaya Sofia, MD, PhD; Suchkov Yuri, MD; Tadtaev Vitaly, MD; Vacula Irina, MD; Vaulin Sergey, MD, PhD; Yakhin Kausar, MD, PhD; Zagoruyko Elena, MD; and Zayka Vladimir, MD, PhD. Slovakia: Dvorak Miloslav, MD, PhD; Janikova Eva, MD; Mateffy Izabela, MD; Milichovska Daniela, MD; Molcan Peter, MD; Perichtova Magda, MD; Sarissky Peter, MD; Turcani Peter, MD, PhD; Vavrusova Livia, MD, PhD; and Vavrusova Livia, MD, PhD. Slovenia: Flisar Dusan, MD, PhD; Pirtosek Zvezdan, MD, PhD; and Stare Lidija, MD. Ukraine: Abramov Volodymyr, MD, PhD; Bachinskaya Natalia, MD; Bitenskyy Valery, MD, PhD; Blazhevych Yulia, MD; Demchenko Vladislav, MD, PhD; Dubenko Andriy, MD, PhD; Kulyk Bohdan, MD, PhD; Kushnir Grygory, MD, PhD; Litvinenko Nataliya, MD, PhD; Lytovchenko Tetyana, MD, PhD; Maruta Natalia, MD, PhD; Maryenko Lidiya, MD, PhD; Moroz Olena, MD; Moroz Svitlana, MD, PhD; Palamarchuk Pavlo, MD; Pashkovskyy Valeryy, MD, PhD; Romaniv Olexander, MD; Skrypnikov Andrii, MD; Smolko Nadiya, MD, PhD; Statinova Olena, MD; Studzinskyy Oleg, MD; and Verbenko Viktoriya, MD, PhD.
Author contributions
KH was an investigator for this study. JSN and HRB were involved in the original study design and implementation. HMR and ASB were the (responsible) Medical Officers supervising the conduct of the study. JH was the study statistician. MS and BD contributed to the analysis and interpretation and critical revision of the manuscript for important intellectual content.
All authors contributed to the development of the manuscript and approved the final manuscript for submission. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors met the International Council of Medical Journal Editors’ criteria for authorship, and anyone who met those criteria is listed as an author.
Disclosure
The study was sponsored by Janssen Research and Development LLC, Raritan, New Jersey, USA.
ASB, JSN, JH, and HMR are employees of Janssen Research and Development, LLC; and HRB is an employee of Janssen Alzheimer Immunotherapy, LLC (both are Johnson and Johnson companies). All these authors hold stocks in Johnson and Johnson. MS is employed by The Mount Sinai Medical Center. She has provided consultation to Janssen Pharmaceuticals, Eli Lilly, Esai, Medivation, Sanofi Aventis, and Takeda. She has also consulted for Merck and Nutricia without any compensation. BD is the inventor of the use of galantamine to treat Alzheimer’s disease and is an employee and stockholder in Synaptec Inc., which licensed use of galantamine to Janssen. The authors report no other conflicts of interest in this work.
References
Alzheimer’s Association. 2013 Alzheimer’s Disease. Facts and Figures. Washington, DC: Alzheimer’s Association; 2013. Available from: http://www.alz.org/alzheimers_disease_facts_and_figures.asp. Accessed March 25, 2013. | |
Lopez OL, Becker JT, Wisniewski S, Saxton J, Kaufer DI, DeKosky ST. Cholinesterase inhibitor treatment alters the natural history of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;72(3):310–314. | |
Atri A, Shaughnessy LW, Locascio JJ, Growdon JH. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):209–221. | |
Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med. 1998;158(9):1021–1031. | |
Feldman HH, Pirttila T, Dartigues JF, et al. Analyses of mortality risk in patients with dementia treated with galantamine. Acta Neurol Scand. 2009;119(1):22–31. | |
Doody RS, Dunn JK, Clark CM, et al. Chronic donepezil treatment is associated with slowed cognitive decline in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2001;12(4):295–300. | |
Kavanagh S, Van Baelen B, Schäuble B. Long-term effects of galantamine on cognitive function in Alzheimer’s disease: a large-scale international retrospective study. J Alzheimers Dis. 2011;27(3):521–530. | |
Nordström P, Religa D, Wimo A, Winblad B, Eriksdotter M. The use of cholinesterase inhibitors and the risk of myocardial infarction and death: a nationwide cohort study in subjects with Alzheimer’s disease. Eur Heart J. 2013;34(33):2585–2591. | |
Rountree SD, Chan W, Pavlik VN, Darby EJ, Doody RS. Factors that influence survival in a probable Alzheimer disease cohort. Alzheimers Res Ther. 2012;4(3):16. | |
Geerts H, Grossberg GT. Pharmacology of acetylcholinesterase inhibitors and N-methyl-D-aspartate receptors for combination therapy in the treatment of Alzheimer’s disease. J Clin Pharmacol. 2006;46(7 Suppl 1):8S–16S. | |
Razadyne ER® and Razadyne® (galantamine hydrobromide) extended-release tablets, tablets, and oral solution [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2013. Available from: http://www.razadyneer360.com/full-prescribing-information.html. Accessed November 21, 2013. | |
Small G, Erkinjuntti T, Kurz A, Lilienfeld S. Galantamine in the treatment of cognitive decline in patients with vascular dementia or Alzheimer’s disease with cerebrovascular disease. CNS Drugs. 2003;17(12):905–914. | |
Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54(12):2261–2268. | |
Rockwood K, Mintzer J, Truyen L, Wessel T, Wilkinson D. Effects of a flexible galantamine dose in Alzheimer’s disease: a randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2001;71(5):589–595. | |
Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology. 2000;54(12):2269–2276. | |
Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ. 2000;321(7274):1445–1449. | |
Winblad B, Gauthier S, Scinto L, et al. GAL-INT-11/18 Study Group. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70(22):2024–2035. | |
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. | |
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. | |
Gélinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther. 1999;53(5):471–481. | |
Pirttilä T, Wilcock G, Truyen L, Damaraju CV. Long-term efficacy and safety of galantamine in patients with mild-to-moderate Alzheimer’s disease: multicenter trial. Eur J Neurol. 2004;11(11):734–741. | |
Scheltens P, van de Pol L. Impact commentaries. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 2012;83(11):1038–1040. | |
Takada-Takatori Y, Kume T, Sugimoto M, et al. Neuroprotective effects of galanthamine and tacrine against glutamate neurotoxicity. Eur J Pharmacol. 2006;549(1–3):19–26. | |
Takata K, Kitamura Y, Saeki M, et al. Galantamine-induced amyloid-{beta} clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. J Biol Chem. 2010;285(51):40180–40191. | |
Kihara M, Kubo T. Immunocytochemical localization of glutamate containing neurons in the ventrolateral medulla oblongata and the nucleus tractus solitarius of the rat. J Hirnforsch. 1991;32(1):113–118. | |
Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010; 330(6012):1774. | |
Buisson B, Bertrand D. Open-channel blockers at the human alpha4beta2 neuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1998;53(3):555–563. | |
Aracava Y, Pereira EF, Maelicke A, Albuquerque EX. Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. J Pharmacol Exp Ther. 2005;312(3):1195–1205. | |
Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012;366(10):893–903. | |
Courtney C, Farrell D, Gray R, et al; AD2000 Collaborative Group. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet. 2004; 363(9427):2105–2115. |
Supplementary materials
  | Table S2 Medical history (safety analysis set) |
  | Table S3 Medical history (completer analysis set) |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.