Back to Journals » Clinical Interventions in Aging » Volume 12
Effects of a giant exercising board game intervention on ambulatory physical activity among nursing home residents: a preliminary study
Authors Mouton A, Gillet N, Mouton F, Van Kann D, Bruyère O, Cloes M, Buckinx F
Received 15 February 2017
Accepted for publication 16 March 2017
Published 22 May 2017 Volume 2017:12 Pages 847—858
DOI https://doi.org/10.2147/CIA.S134760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Video abstract presented by Fanny Buckinx and Alexandre Mouton.
Views: 1127
Alexandre Mouton,1 Nicolas Gillet,1 Flore Mouton,1 Dave Van Kann,2,3 Olivier Bruyère,1,4 Marc Cloes,1 Fanny Buckinx4
1Department of Sport and Rehabilitation Sciences, Multidisciplinary Research Unit on Health and Society, University of Liège, Liège, Belgium; 2Department of Health Promotion, Maastricht University Medical Center (MUMC+), Maastricht, 3School of Sport Studies, Fontys University of Applied Sciences, Eindhoven, the Netherlands; 4Department of Public Health, Epidemiology and Health Economics, University of Liège Teaching Hospital (CHU), Liège, Belgium
Purpose: This study examined the effects of a giant (4×3 m) exercising board game intervention on ambulatory physical activity (PA) and a broader array of physical and psychological outcomes among nursing home residents.
Materials and methods: A quasi-experimental longitudinal study was carried out in two comparable nursing homes. Ten participants (aged 82.5±6.3 and comprising 6 women) meeting the inclusion criteria took part in the 1-month intervention in one nursing home, whereas 11 participants (aged 89.9±3.1 with 8 women) were assigned to the control group in the other nursing home. The giant exercising board game required participants to perform strength, flexibility, balance and endurance activities. The assistance provided by an exercising specialist decreased gradually during the intervention in an autonomy-oriented approach based on the self-determination theory. The following were assessed at baseline, after the intervention and after a follow-up period of 3 months: PA (steps/day and energy expenditure/day with ActiGraph), cognitive status (mini mental state examination), quality of life (EuroQol 5-dimensions), motivation for PA (Behavioral Regulation in Exercise Questionnaire-2), gait and balance (Tinetti and Short Physical Performance Battery), functional mobility (timed up and go), and the muscular isometric strength of the lower limb muscles.
Results and conclusion: In the intervention group, PA increased from 2,921 steps/day at baseline to 3,358 steps/day after the intervention (+14.9%, P=0.04) and 4,083 steps/day (+39.8%, P=0.03) after 3 months. Energy expenditure/day also increased after the intervention (+110 kcal/day, +6.3%, P=0.01) and after 3 months (+219 kcal/day, +12.3%, P=0.02). Quality of life (P<0.05), balance and gait (P<0.05), and strength of the ankle (P<0.05) were also improved after 3 months. Such improvements were not observed in the control group. The preliminary results are promising but further investigation is required to confirm and evaluate the long-term effectiveness of PA interventions in nursing homes.
Keywords: exercise, nursing home, elderly, ambulatory physical activity, autonomy, game
Background
The majority of nursing home residents are physically inactive.1,2 In the existing literature, physical activity (PA) levels among nursing home residents are much lower than existing recommended levels, which advocate a minimum of 3,000 steps/day.3,4 Most of their time is spent sleeping, doing nothing or watching TV in a lying or sitting position.5 Therefore, this population is among the most sedentary segment of the society, with an increased risk of physical and neurocognitive impairment leading to frailty and increased mortality.6–9 Promoting regular PA is considered to be an effective strategy in reducing all-cause mortality and improving quality of life among the elderly.10,11 Even a low dose of moderate-to-vigorous physical activity (MVPA) reduces mortality in the elderly by 22%, with the greatest risk reduction observed in those who change from doing no MVPA to doing some MVPA (1–499 metabolic equivalent task (MET)-minutes/week or ~15 minutes/day).12 Significant health benefits are also seen among older adults who became physically active in later life.13 Walking programs set up by ambulatory nursing home residents produced significant improvements in walk endurance capacity and distance.14 Moreover, improvements in physical and muscular performance among this population could counter the development of frailty and preserve the quality of life of nursing home residents.15 A recent review16 identified only eight randomized controlled studies in which the modification of PA behavior of nursing home residents was assessed as a clear outcome. However, six studies have reported significant increases in PA, supporting the feasibility and the promising effectiveness of interventions developed in this context. Interventions combining physical exercise and behavioral components seem to be more effective in this context and could lead to an autonomous form of motivation for PA through different strategies, including the satisfaction of exercise-related basic psychological needs for autonomy, competence, and relatedness as described in the self-determination theory (SDT).17,18 In order to move beyond the relatively monotonous lifestyle in nursing homes,19 making PA enjoyable and sociable could encourage residents to participate in activities more regularly.19 Playing board games is a common stimulating activity among the elderly and is considered as a possible protective factor against cognitive decline and dementia.20 Growing evidence indicates that gaming approaches for PA promotion, such as interactive video games, led to increased enjoyment and motivation in addition to positive cognitive and physical outcomes.21 However, active video games are not suitable for all elderly, as they mostly involve one-on-one supervision,22 and are not as effective as traditional real-life interventions.23 Therefore, taking into consideration the encouraging evidence about the implementation of PA interventions in nursing homes, this preliminary study investigated the effects of a giant exercising board game intervention on ambulatory PA among nursing home residents. A primary objective was to examine the effects of this intervention on the ambulatory PA of residents by recording the number of steps/day and the time spent in sedentary, light, or MVPA. Secondary objectives were related to the assessment of the impact of the intervention on a broader array of physical and psychological outcome measurements, including measuring physical and muscular performance, health and cognitive status, and motivation for PA. We hypothesized that a life-size board game based on exercising activities could impact both physical and psychological components of the health of nursing home residents. Such an innovative intervention might elicit the satisfaction of exercise-related activities by older adults in promoting social interactions (relatedness), providing adapted physical exercises (competence), and encouraging regular voluntary participation in the game (autonomy).
Materials and methods
Design and sample
A quasi-experimental longitudinal study was performed in two Belgian nursing homes in the Province of Liège, namely “Le Jardin des Chantoirs” and “Le Prestige”. In order to prevent contamination between groups, subjects in one nursing home received the intervention and were designated as participants, and those in the other nursing home were designated as control group. The two nursing homes were selected according to their similarities in terms of number of beds (>90), services (nursing care, physical therapy, social and physical activities) and their environment, (semi-rural area), and were then randomized into one intervention and one control group. Prior to the baseline assessment, investigators met the director and staff of each nursing home to inform them of the study, the inclusion and exclusion criteria, and the study procedures and intervention. The selection criteria for the participants were: 1) to live in a nursing home that was included in this study; 2) to be aged 65 years or older; 3) to be oriented to provide informed consent and understand the questionnaires (mini mental state examination [MMSE]) score >18 out of 30;24 and 4) to be able to walk and stand, including with technical assistance (assessed by the physiotherapist in the nursing home). A first screening was performed by the medical staff (ie, physical therapists and nurses) to identify potentially eligible participants who were then approached by the researchers and asked if they were willing to participate in the study. The study was approved by the Ethics Committee of the University Teaching Hospital of Liège under number 2013/178. All participants gave written informed consent and an identification number was created for each participant to ensure anonymity.
Intervention
Prior to the intervention, a team comprising two public health specialists, two specialists in PA promotion, and a designer met several times to develop the design of the program. A giant exercising board game measuring 4×3 m was the central component of the intervention (Figure 1). The tarpaulin surface was printed with 24 numbered squares of 50×50 cm and surrounded by a walking lane. Each square was colored according to the component of physical fitness that was to be performed (ie, 6 squares/component): strength, flexibility, balance, and endurance. Systematically, an illustration explained the movements to be executed and any adaptations for participants with a lower or higher level of physical fitness. Different symbols were drawn on the walking lane in order to execute some balance exercises requested appropriately on the corresponding squares. Finally, ladders and snakes were used to link pairs of squares so that participants could move forward or backward faster in the game.
  | Figure 1 The giant exercising board game. |
The game included a foam ball (for some strengthening or balance exercises) and a wheel with an arrow that was randomly turned to one of the four colors of the board game. The rules were simple and made available to the participants in a folder adjacent to the mat. Taking turns, participants turned the wheel and had reach the next square with the color targeted by the arrow. After completing the requested exercises, participants were expected to do systematically two laps on the walking lane. Participants made their way through the squares until the finish line after the 24th square. The intervention took place in the living room of the nursing home and was supervised by a specialist in PA. The playing time of a session ranged between 30 and 60 minutes and the game requires a minimum of 2 participants. In order to progressively incite nursing home residents to participate independently in the giant exercising board game, the assistance provided by the supervisor was decreased gradually during the 1-month intervention period: 4 supervised exercising sessions were planned on the board game during the first week and then 3, 2, and 1 sessions were planned during the second, third, and fourth week of the intervention. Some participants came to the sessions by themselves, but for most of them, the supervisor was required to take them from their room and to the living room where the giant exercising board was placed. The pedagogical strategy was also autonomy oriented: the supervisor helped the participants to play during the first sessions (eg, miming the body movements) but encouraged them to play as much as possible by themselves. Residents were encouraged to register their participation in the game on a nearby logbook after each session was performed, with or without the supervisor. During the 3-month follow-up period, the giant exercising board game remained accessible in the living room but without any planned supervision. Participants in the control group were requested neither to change their lifestyle during the study nor to get involved in any new type of PA.
Outcome measurement
The assessments consisted of a battery of physical and muscular performance tests and anamnestic data gathered at baseline, after the intervention (1 month), and after the follow-up period (3 months; Figure 2). The study started in December 2015 in nursing home 1 and in January 2016 in nursing home 2. All subjects were tested and interviewed in their room by a clinical research assistant, with each interview lasting on an average of ~1 hour. Data were always collected in the same order as mentioned in the following paragraph. Data collection, according to the protocol was conducted by two different clinical research assistants.
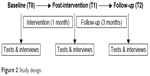  | Figure 2 Study design. |
Anamnestic data
PA level
PA was measured objectively using ActiGraph GT3X+ (ActiGraph, Pensacola, FL, USA) accelerometers. During 3 consecutive days, two clinical research assistants helped the participants place the accelerometer on an elastic strap around the ankle above the right lateral malleolus. According to Korpan, using the ankle placement for GT3X+ provides the most accurate step counts in the elderly.25 It should be noted that the device proved to have excellent reliability and validity using various settings.26–28
Participants were instructed to wear the accelerometer during waking hours until the research assistant came back to remove the device at the end of the day. Troiano’s wear time criteria were applied to define non-wear periods.29 A valid day of accelerometer data was defined as having non-missing counts for at least 80% of a measurement day. Differences in wear time above this value were not taken into account because research assistants were instructed to place and remove accelerometers from the participants in the same order and at the same hour each day. Average energy expenditure per day (kcal/day), calculated as the sum of all PA in counts per minute (cpm), was obtained by a derivation of cpm values to mean MET minutes values using the following regression equation: 1.439008+0.000795*counts/min.30 The number of steps per day was obtained by calculating the average number of steps walked during 3 days of recording.
Cognitive status
Cognitive skills were assessed with the MMSE, which consists of a 30-item questionnaire. A maximum score of 30 is attainable for a person without any neuropsychological impairment. A score ≥24 points indicates normal cognition and scores below this point reveal mild (19–23 points), moderate (10–18 points), or severe (≤9 points) cognitive impairment.31
Quality of life
Quality of life was assessed using EuroQol 5-dimensions (EQ-5D) that documents the level of self-reported health problems in five dimensions (mobility, self-care, usual activity, pain/discomfort, and anxiety/depression), each comprising three levels: no problems, some problems, and severe problems. Each health state was converted into a single summary index, providing a score ranging from 1 (perfect health) to 0 (death).32
Motivation for PA
The French version of the Behavioral Regulation in Exercise Questionnaire-2 (BREQ-2) was used to assess the participants’ motivation concerning exercise.33 The BREQ-2 consists of 19 items measured on a 5-point Likert-type scale ranging from 0 (not true for me) to 4 (very true for me), appraising an individual’s level of intrinsic motivation (eg, “I exercise because it’s fun”), identified regulation (eg, “I value the benefits of exercise”), introjected regulation (eg, “I feel guilty when I don’t exercise”), external regulation (eg, “I exercise because other people say I should”), and no motivation (eg, “I don’t see why I should have to exercise”). In order to assess motivation for all physical activities and not just for exercise in particular, the term “exercise” was replaced by the term “physical activity”. A similar change has been made and successfully applied in the previous research.34
Body balance, physical, and muscular performance
Tinetti test
The Tinetti test, or performance-oriented mobility assessment (POMA), was used to assess body balance and gait abnormalities. This assessment is one of the most widely used tests in this field.35 It consists of two subtests: a balance test (9 items scored on 16 points) and a gait test (7 items scored on 12 points). A total score <19 points indicates severe risk, a score between 19 and 24 points indicates moderate risk, and a score of more than 24 points indicates low risk of falls.36
Short Physical Performance Battery test
The Short Physical Performance Battery (SPPB) test is composed of three separate tests: balance, gait speed over 4 m, and a chair stand test. A score between 0 and 4 is assigned to each test, and the three tests are weighted equally. Therefore, the maximum score is 12 points. According to the European Working Group on Sarcopenia in Older People, the cut-off value used to assess poor physical performance is ≤8 points.37
Timed up and go test
The timed up and go test was used to assess the functional mobility of patients.38 From a sitting position, the subject is required to stand up, walk 3 m, turn around, walk back, and sit down again. The time needed to complete the task is recorded and used for analysis. A time of more than 30 seconds indicates a high level of dependence, a time between 20 and 30 seconds indicates uncertain mobility and a risk of falling, and a time <20 seconds indicates independence of the subject.38
Muscular isometric strength
Maximal isometric muscle strength of six lower limb muscle groups (knee extensors and flexors, hip abductors and extensors, ankle flexors and extensors) was measured according to the protocol defined by Buckinx39 with the MicroFET2 hand-held dynamometer (Hoggan Industries, Inc., West Jordan, UT, USA). High relative and moderate absolute reliability of the MicroFET2 has been observed among nursing home residents.40 Three consecutive maximal contractions of each muscle group were performed and the highest performance was considered for the analysis.
Statistical analysis
A Shapiro–Wilk test verified the normal distribution for all parameters. When data were normally distributed, one-way analysis of variance (ANOVA) and one-way ANOVAs with repeated measures were performed to assess differences between groups and within groups at the three different data collection points, respectively. Post-hoc tests using the Bonferroni correction were applied for multiple comparisons. Nonparametric statistics were used when data were not normally distributed (Kruskall–Wallis test: between groups’ differences; Wilcoxon test: withingroups’ differences). For qualitative variables, a Pearson’s Chi-square test was performed. Analyses were adjusted for baseline variables that were significantly different between intervention and control groups by means of multiple regression. Quantitative variables that were normally distributed were expressed as the mean ± standard deviation (SD), and quantitative variables that were not normally distributed were reported as the median and percentiles (P25–75). Qualitative variables were reported as absolute and relative frequencies (%). Results were considered statistically significant when 2-tailed P-values were <0.05. Analyses were executed using Statistica 13 software on an intention-to-treat basis: data for dropouts who returned for follow-up measurements were also included in the analyses.
Results
Population
The selection of participants for the present study is summarized in Figure 3. Respectively, 50% and 24% of the population of the intervention and control nursing homes were eligible for the study according to the medical staff.
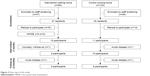  | Figure 3 Flow chart of the study. |
Subsequently, a number of residents in the intervention (74%) and the control (63%) nursing homes declined to participate in the study after the initial screening operated by the medical staff, and a further two of them did not reach the required MMSE. The main reasons for refusal were associated with their lack of interest in the study, with their reluctance to take part in an unknown intervention, or with their lack of motivation to change their habits. Acute diseases that occurred during the study (n=3) were not associated with the intervention components. All this resulted in a baseline number of 10 participants in the intervention group and 11 participants in the control group. Table 1 shows the baseline characteristics of the participants included in the study. The two group of participants did not differ significantly in any of the characteristics measured. Women represented the majority of the participants in both the intervention (60%) and control (73%) groups.
Anamnestic data
The primary outcome of this study was related to the recording of the number of steps/day in both groups at baseline, after the 1 month intervention, and after the follow-up period of 3 months. The evolution of the average steps/day during the study is presented in Figure 4.
  | Figure 4 Evolution of the average steps per day for both groups. |
In the intervention group, significant increases of 437 (+14.9%, P=0.04) and 1,162 (+39.8%, P=0.03) steps/day were observed after the intervention and the follow-up period, respectively. Meanwhile, average steps per day in the control group decreased significantly after the intervention (−817, −24.1%, P=0.02), but not after the follow-up period (−280, −8.3%, P=0.22). Detailed evolution of the anamnestic characteristics for both groups is presented in Table 2. Among the other anamnestic data, we can highlight the significant increases of the mean energy expenditure per day in the intervention group after the intervention (+110 kcal/day, +6.3%, P=0.01) and the follow-up period (+219 kcal/day, +12.3%, P=0.02), whereas significant decreases were observed in the control group (−53 kcal/day, −3.2%, P=0.03; and −239 kcal/d, −14.5%, P=<0.01; respectively). Scores from EQ-5D increased significantly in the intervention group between baseline and follow-up (+6.1%, P=0.04).
Physical and muscular performance
Evolution of the physical and muscular performance of both groups between each data collection period is summarized in Table 3. Tinetti scores increased significantly after the follow-up period (+9.1%) in the intervention group, whereas values remained fairly stable in the control group. For muscular performance, significant improvements in the strength of the ankle were measured in the intervention group. After the intervention and the follow-up period, the strength of ankle extensors (+20.3% and +37.6%, respectively) and flexors (+48.2% and +32.1%, respectively) increased significantly. No other significant changes were observed for the evolution of physical performance.
Discussion
The life-size board game intervention, based on exercising activities, seemed to have had a positive impact on the ambulatory PA of nursing home residents. Even though caution needs to be used due to limitations that will be discussed later, the fact that values tend to continue to increase after the intervention supports the view that progressively withdrawing expert assistance might encourage autonomy among participants. From an average 2,921 steps/day at baseline, participants in the intervention group reached an average of 4,083 steps/day after the follow-up period. In the control group, the relative increase in the number of steps/day during the follow-up period could be partially explained by a seasonal effect, and thus the weather, on levels of PA.41 Post-intervention data collection occurred during the winter (between December and January), whereas follow-up measurements took place in the spring (between March and April). Even if residents are living in nursing homes, participants in the study were those with the best level of autonomy, and were thus perfectly able to go for walks outside the institution. Winter conditions (cold, frost, or snow) have been identified as barriers to participate in PA among various populations.41 This large difference is partly concealed by the large SD observed for this variable. Evaluations were performed at the same time in the two nursing homes, but a seasonal effect, which would be masked by the intervention in the intervention group, may explain this result in the control group. In a more guided walking program (each walk was assisted by a research assistant), MacRae et al,14 found similar results with an increase of 52% for the maximal walking time after a 12-week intervention, with additional improvements (8%) after 10 weeks of extra intervention. Energy expenditure provides another argument in favor of the intervention. Outcomes are even clearer for this variable at each data collection period: significant increases were observed in the intervention group, and significant decreases were observed in the control group. Simmons and Schnelle42 have highlighted significant improvements of daily PA after 32 weeks of an intervention provided by research staff and encouraging residents to walk four times/day, 5 days/week. In a comparable 12 weeks multicomponent intervention comprising prompts for walking, Schnelle et al,43 reported an average increase of 10.6 minutes of PA in the intervention group versus a decrease of 10.9 minutes in the control group. A major limitation in those protocols is related to their dependence on the research team, which cannot be extended on a long-term basis. To our knowledge, this study is the first to implement a PA intervention driven by the SDT among nursing home residents.17,18 Previous studies have already emphasized good evidence for the value of SDT in understanding long-term maintenance of exercise behavior,17,44 even among older adults.45 We hypothesized that the social support experienced during the game, associated with the pedagogical approach oriented towards progressive autonomy with adapted activities, had contributed to the significant outcomes observed in the intervention group. Schutzer and Graves clearly identified these factors as clear predictors of exercise adherence in older adults.46 Overall, the experience was positive for the participants. They expressed appreciation for the intervention at 8.91 cm ±1.03 on a 10-cm visual analogue scale. In addition, the attendance rate was high (77.8%).
Outcomes related to other anamnestic data are also rather encouraging. Quality of life (EQ-5D) increased slightly but significantly in the intervention group. This could mean that participation in the giant exercising board game could lead to improvements in, or at least the maintenance of, physical states that prevent further frailty and associated diseases among nursing home residents. Indeed, exercise has been shown to improve outcomes of mobility and functional ability in two systematic reviews of home-based and group-based exercise interventions for frail elderly people.47,48 In the same way, motivation for PA tends to decrease with age.49 However, embodied in the Relative Autonomy Index (BREQ-2), motivation for PA only decreased significantly in the control group. Finch50 has demonstrated that older adults report motivation for PA for the purpose of enjoyment, pleasure, fitness, and to reduce the effects of aging. In light of such motives, the game-oriented intervention developed in this study may have played a crucial role.
Outcomes concerning the physical and muscular performance of the participants are less obvious. The Tinetti test revealed significant improvements in the intervention group from baseline to follow-up. Both components of this assessment (gait and balance) increased during the intervention and follow-up periods. When participants played the giant exercising board game, they systematically had to do two laps on the walking lane after each round.
Moreover, adapted balance exercises were proposed throughout the entire game. In a meta-analysis, Sherrington et al,51 have shown that a walking program could lead to a 10% reduction in falls, whereas a combination with balance training could imply a 21% reduction. Because falls are an important independent marker of frailty, it is crucial to put in place interventions to limit such events.52,53 Improvements in the strength of the ankle in the intervention group are also likely to be associated with gait and balance training. Sufficient strength and flexibility of the musculature is needed to ensure extension (dorsiflexion) and flexion (plantar flexion) of the ankle during walking. Nevertheless, these results were not confirmed by other physical (SPPB and time up and go) or muscular assessments. We could hypothesize that improvements in those tests would need a longer and more intensive training, such as those implemented in existing literature.16
A limitation in the autonomy-oriented approach is that, when exercising is not supervised by an exercise specialist, participants are not always practicing at an optimal intensity level. A training intensity of 70%–79% of the one-repetition maximum is recommended, but evidence-based dose–response relationships regarding exercise characteristics (type, frequency, duration, setting, combinations) are still unclear in older adults.54,55 Supervision by exercise specialists or by trained physiotherapists would be preferable, but will generally involve additional charge for the institution. A review by Shakeel et al,56 suggest that effective group-based exercise programs can be implemented in nursing homes with trained staff members (nurses or volunteers).
Raising awareness among facility staff members about PA and educating them to promote movement would probably result in long-term, cost-effective improvements among residents. In our study, staff members were not directly implicated, limiting the potential durability of the intervention. The present study was subject to other limitations. First, results were based on a limited number of participants from two nursing homes. This means that our conclusions should be interpreted and generalized with particular caution. However, even if the two groups of participants were rather small, significant results were found, encouraging further broader scale investigations. In addition, the recruitment strategy could have caused a selection bias. We might have introduced a bias by enrolling patients who were sufficiently oriented and able to walk or stand with technical assistance. Eligible participants represented a minority of the nursing home population, which might not be representative of the general population of such homes. However, no baseline differences between groups were observed for the outcomes measured. We assume that this autonomy-oriented approach could not be applicable to residents with severe functional or cognitive limitations. Even so, it should be noted that some residents, even in a wheelchair, took part in some gaming sessions even if they were not eligible or declined to participate at baseline. Because there is no one-size-fits-all approach to PA in older adults,46 some residents expressed the need to observe this new intervention before deciding to join the game. Longer intervention and follow-up periods would contribute to further understanding of such behavior. Another limitation is related to the process control during the follow-up of the intervention. Residents were encouraged to register each participation in the game in a logbook placed nearby, but this information turned out to be unusable because of the numerous failures to complete this task. The small sample size in this preliminary study would not allow using the data in further analyses, so we decided to rely on the comprehensive assessment achieved at each data collection period. Finally, participants were not assigned to intervention conditions at random such as in a randomized controlled trial. This quasi-experimental design was chosen because contamination between groups is very likely to occur in a nursing home, even more with an autonomy-oriented approach. Efforts were made to recruit two similar nursing homes in terms of number of beds, services, and geographical situation.
Conclusion
After a 3-month follow-up period, results of this study showed that a giant board game intervention led to a significant increase in ambulatory PA among nursing home residents. This original intervention, combining enjoyable exercising activities and behavioral strategies with respect to the SDT, resulted in significant improvements in participants’ daily energy expenditure, quality of life (EQ-5D), balance and gait (Tinetti), and strength of the ankle. These improvements were not observed in the control group, but several limitations such as the limited sample size should encourage a cautious interpretation of the results. In future interventions, efforts should be made to include a larger proportion of residents and to engage more directly trained staff members. Further investigation is required to confirm these preliminary results and to evaluate the long-term effectiveness of PA interventions in nursing homes.
Acknowledgment
The authors would like to thank all the participants who participated in this study. We also thank directory and healthcare staff from the “Le Jardin des Chantoirs” and “Le Prestige” for their collaboration in the study. We are also grateful for the free loan of accelerometers by the Department of Health Promotion of the University of Maastricht.
Authors’ contributions
AM, MC, OB, and FB conceived the project and developed the study hypotheses and the protocol. AM, FB, DVK, NG, and FM were responsible for the data collection, data management and data analyses. DVK ran the accelerometer analyses. AM wrote the first draft of the article under the supervision of FB, DVK, OB and MC. All authors read, revised, and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
MacRae PG, Schnelle JF, Simmons SF, Ouslander JG. Physical Activity Levels of Ambulatory Nursing Home Residents. J Aging Phys Act. 1996;4(3):264–278. | ||
Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada’s Physical Activity Guidelines. Int J Behav Nutr Phys Act. 2010;7:38. | ||
Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80–80. | ||
Buckinx F, Mouton A, Reginster JY, et al. Relationship between ambulatory physical activity assessed by activity trackers and physical frailty among nursing home residents. Gait Posture. 2017;54:56–61. | ||
den Ouden M, Bleijlevens MH, Meijers JM, et al. Daily (In)activities of nursing home residents in their wards: an observation study. J Am Med Dir Assoc. 2015;16(11):963–968. | ||
Hallal P, Andersen L, Bull F, Guthold R, Haskell W, Ekelund U; Lancet Physical Activity Series Working Group. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838):247–257. | ||
Sun F, Norman I, While A. Physical activity in older people: a systematic review. BMC Public Health. 2013;13(1):449. | ||
Weening-Dijksterhuis E, de Greef MH, Scherder EJ, Slaets JP, van der Schans CP. Frail institutionalized older persons: a comprehensive review on physical exercise, physical fitness, activities of daily living, and quality-of-life. Am J Phys Med Rehabil. 2011;90(2):156–168. | ||
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. | ||
Brown WJ, McLaughlin D, Leung J, et al. Physical activity and all-cause mortality in older women and men. Br J Sports Med. 2012;46(9):664–668. | ||
Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose-response meta-analysis of cohort studies. Int J Epidemiol. 2011;40(5):1382–1400. | ||
Hupin D, Roche F, Gremeaux V, et al. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥60 years: a systematic review and meta-analysis. Br J Sports Med. 2015;49(19):1262–1267. | ||
Hamer M, Lavoie KL, Bacon SL. Taking up physical activity in later life and healthy ageing: the English longitudinal study of ageing. Br J Sports Med. 2014;48(3):239–243. | ||
MacRae PG, Asplund LA, Schnelle JF, Ouslander JG, Abrahamse A, Morris C. A walking program for nursing home residents: effects on walk endurance, physical activity, mobility, and quality of life. J Am Geriatr Soc. 1996;44(2):175–180. | ||
Buckinx F, Reginster JY, Petermans J, et al. Relationship between frailty, physical performance and quality of life among nursing home residents: the SENIOR cohort. Aging Clin Exp Res. 2016;28(6):1149–1157. | ||
Jansen CP, Claβen K, Wahl HW, Hauer K. Effects of interventions on physical activity in nursing home residents. Eur J Ageing. 2015;12(3):261–271. | ||
Teixeira PJ, Carraça EV, Markland D, Silva MN, Ryan RM. Exercise, physical activity, and self-determination theory: a systematic review. Int J Behav Nutr Phys Act. 2012;9:78–78. | ||
Deci EL, Ryan RM. The “What” and “Why” of goal pursuits: human needs and the self-determination of behavior. Psychol Inquiry. 2000;11(4):227–268. | ||
Chen YM, Li YP. Motivators for physical activity among ambulatory nursing home older residents. ScientificWorldJournal. 2014;2014:329397. | ||
Dartigues JF, Foubert-Samier A, Le Goff M, et al. Playing board games, cognitive decline and dementia: a French population-based cohort study. BMJ Open. 2013;3(8):e002998. | ||
Bleakley CM, Charles D, Porter-Armstrong A, McNeill MDJ, McDonough SM, McCormack B. Gaming for health: asystematic review of the physical and cognitive effects of interactive computer games in older adults. J Appl Gerontol. 2015;34(3):NP166–NP189. | ||
Bieryla KA, Dold NM. Feasibility of Wii Fit training to improve clinical measures of balance in older adults. Clin Interv Aging. 2013;8:775–781. | ||
Molina KI, Ricci NA, de Moraes SA, Perracini MR. Virtual reality using games for improving physical functioning in older adults: a systematic review. J Neuroeng Rehabil. 2014;11(1):1–20. | ||
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. JPsychiatrRes. 1975;12(3):189–198. | ||
Korpan SM, Schafer JL, Wilson KC, Webber SC. Effect of actiGraph GT3X+ position and algorithm choice on step count accuracy in older adults. J Aging Phys Act. 2015;23(3):377–382. | ||
O’Neil ME, Fragala-Pinkham MA, Forman JL, Trost SG. Measuring reliability and validity of the ActiGraph GT3X accelerometer for children with cerebral palsy: a feasibility study. J Pediatr Rehabil Med. 2014;7(3):233–240. | ||
Pulakka A, Cheung YB, Ashorn U, et al. Feasibility and validity of the ActiGraph GT3X accelerometer in measuring physical activity of Malawian toddlers. Acta paediatr. 2013;102(12):1192–1198. | ||
Aadland E, Ylvisaker E. Reliability of the actigraph GT3X+ accelerometer in adults under free-living conditions. PLoS One. 2015;10(8):e0134606. | ||
Troiano RP. Large-scale applications of accelerometers: new frontiers and new questions. Med Sci Sports Exerc. 2007;39(9):1501. | ||
Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. | ||
Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. | ||
Dolan P. Modeling valuations for EuroQol health states. MedCare. 1997;35(11):1095–1108. | ||
Markland D, Tobin V. A modification to the behavioural regulation in exercise questionnaire to include an assessment of a motivation. J Sport Exercise Psychol. 2004;26(2):191–196. | ||
Verloigne M, De Bourdeaudhuij I, Tanghe A, et al. Self-determined motivation towards physical activity in adolescents treated for obesity: an observational study. Int J Behav Nutr Phys Act. 2011;8:97. | ||
Perell KL, Nelson A, Goldman RL, Luther SL, Prieto-Lewis N, Rubenstein LZ. Fall risk assessment measures: an analytic review. J Gerontol A Biol Sci Med Sci. 2001;56(12):M761–M766. | ||
Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119–126. | ||
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. | ||
Schoppen T, Boonstra A, Groothoff JW, de Vries J, Goeken LN, Eisma WH. The Timed “up and go” test: reliability and validity in persons with unilateral lower limb amputation. Arch Phys Med Rehabil. 1999;80(7):825–828. | ||
Buckinx F, Croisier JL, Reginster JY, Petermans J, Goffart E, Bruyere O. Relationship between isometric strength of six lower limb muscle groups and motor skills among nursing home residents. J Frailty Aging. 2015;4(4):184–187. | ||
Buckinx F, Croisier JL, Reginster JY, et al. Reliability of muscle strength measures obtained with a hand-held dynamometer in an elderly population. Clin Physiol Funct Imaging. Epub 2015 Oct 30. | ||
Tucker P, Gilliland J. The effect of season and weather on physical activity: a systematic review. Public Health. 2007;121(12):909–922. | ||
Simmons SF, Schnelle JF. Effects of an exercise and scheduled-toileting intervention on appetite and constipation in nursing home residents. J Nutr Health Aging. 2004;8(2):116–121. | ||
Schnelle JF, Leung FW, Rao SS, et al. A controlled trial of an intervention to improve urinary and fecal incontinence and constipation. J Am Geriatr Soc. 2010;58(8):1504–1511. | ||
Friederichs SAH, Bolman C, Oenema A, Lechner L. Profiling physical activity motivation based on self-determination theory: a cluster analysis approach. BMC Psychology. 2015;3(1):1. | ||
McAuley E, Jerome GJ, Elavsky S, Marquez DX, Ramsey SN. Predicting long-term maintenance of physical activity in older adults. Prev Med. 2003;37(2):110–118. | ||
Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Prev Med. 2004;39(5):1056–1061. | ||
Theou O, Stathokostas L, Roland KP, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res. 2011;2011:569194. | ||
de Vries NM, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Staal JB, Nijhuis-van der Sanden MW. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community-dwelling older adults with impaired mobility, physical disability and/or multi-morbidity: a meta-analysis. Ageing Res Rev. 2012;11(1):136–149. | ||
Brunet J, Sabiston CM. Exploring motivation for physical activity across the adult lifespan. Psychol Sport Exerc. 2011;12(2):99–105. | ||
Finch H, Health Education A. Physical Activity “at Our Age”: Qualitative Research Among People Over the Age of 50. London, UK: Health Education Authority; 1997. | ||
Sherrington C, Tiedemann A, Fairhall N, Close JC, Lord SR. Exercise to prevent falls in older adults: an updated meta-analysis and best practice recommendations. N S W Public Health Bull. 2011;22(3–4):78–83. | ||
Schultz M, Rosted E, Sanders S. Frailty is associated with a history with more falls in elderly hospitalised patients. Dan Med J. 2015;62(6).pii:A5058. | ||
Soriano TA, DeCherrie LV, Thomas DC. Falls in the community-dwelling older adult: a review for primary-care providers. Clin Interv Aging. 2007;2(4):545–554. | ||
Borde R, Hortobagyi T, Granacher U. Dose-response relationships of resistance training in healthy old adults: a systematic review and meta-analysis. Sports Med. 2015;45(12):1693–1720. | ||
Gine-Garriga M, Roque-Figuls M, Coll-Planas L, Sitja-Rabert M, Salva A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95(4):753–769.e753. | ||
Shakeel S, Newhouse I, Malik A, Heckman G. Identifying feasible physical activity programs for long-term care homes in the Ontario Context. Can Geriatr J. 2015;18(2):73–104. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



