Back to Journals » Neuropsychiatric Disease and Treatment » Volume 14
Effectiveness of high-frequency repetitive transcranial magnetic stimulation in patients with depression and Parkinson’s disease: a meta-analysis of randomized, controlled clinical trials
Authors Qin B, Chen H, Gao W, Zhao LB, Zhao MJ, Qin HX, Yang MX
Received 10 November 2017
Accepted for publication 4 December 2017
Published 11 January 2018 Volume 2018:14 Pages 273—284
DOI https://doi.org/10.2147/NDT.S156695
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Wai Kwong Tang
Bin Qin,1,* Hong Chen,1,* Wen Gao,1,* Li-Bo Zhao,2,* Ming-Jun Zhao,3 Hui-Xun Qin,1 Ming-Xiu Yang1
1Department of Neurology, Affiliated Liuzhou People’s Hospital of Guangxi University of Science and Technology/Liuzhou People’s Hospital, Liuzhou, 2Department of Neurology, Yongchuan Hospital of Chongqing Medical University, Chongqing, 3Department of Pharmacy, The Second Affiliated Hospital of Xinxiang Medical University (Henan Mental Hospital), Xinxiang, China
*These authors contributed equally to this work
Aim: This meta-analysis aimed to assess the effect of high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) for the treatment of depression in patients with Parkinson’s disease (PD).
Methods: The design was a meta-analysis of randomized controlled trials (RCTs). The participants were patients with PD who suffered from depression. The interventions were HF-rTMS alone or in combination with other treatments compared with sham-rTMS, placebo, and antidepressant treatments. The primary outcome measure was changes in depressive symptoms, defined as the mean change in the total depression score. The secondary outcome was changes in motor symptoms, defined by Unified Parkinson’s Disease Rating Scale part III scores, and the acceptability, defined as the risk of all-cause discontinuation. These were expressed as mean differences (MDs), standardized mean differences (SMDs), or risk ratios (RRs) with 95% confidence intervals (CIs).
Results: We identified nine suitable trials, with data from 332 participants. For the patients with depression in PD, HF-rTMS was not better than sham-rTMS (SMD =-0.33, 95% CI -0.83 to 0.17) or selective serotonin re-uptake inhibitors (SSRIs) (SMD =0.07, 95% CI -0.52 to 0.18) for the treatment of depressive symptoms. However, the motor benefits after treatment with HF-rTMS might be better than sham-rTMS (MD =-2.80, 95% CI -5.45 to -0.15) and SSRIs (MD =-2.70, 95% CI -4.51 to -0.90).
Conclusion: This meta-analysis provides some evidence that in patients with PD with depression, HF-rTMS may lead to improvement in motor function but not in depression compared with sham-rTMS or SSRIs.
Keywords: sham-repetitive transcranial magnetic stimulation, selective serotonin re-uptake inhibitors, depressive symptoms, motor symptoms
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease, associated with major disability issues and increased mortality.1,2 Non-motor symptoms in PD, such as depressive symptoms, major depressive disorder (MDD), and dysthymia, affect 13%–51.7% of all PD patients.2–4 However, there is evidence that overlapping symptoms of the two disorders may confound the diagnosis of depression in patients with PD.5,6 Moreover, mild depression is an especially common symptom in the early stages of PD and is associated with increased disability, rapid progression of motor symptoms, and increased mortality.7
As depression results in disability and poor quality of life, recognition and sufficient treatment of its symptoms are important objectives in the management of PD.8 To date, antidepressants, such as selective serotonin reuptake inhibitors (SSRIs) are the most commonly used antidepressant treatment used in patients with depression and PD. However, the lack of strong evidence supporting the efficacy of antidepressants, the side-effects of antidepressants potentially worsening some pre-existing non-motor problems, and the burden of polypharmacy have led many clinicians to seek non-pharmacological treatments for depressive symptoms and MDD in PD patients.9,10
As a noninvasive procedure, repetitive transcranial magnetic stimulation (rTMS) does not require surgery or anesthesia. It is a well-tolerated technique for brain stimulation based on electromagnetic induction.11 There is evidence that high frequency (HF)-rTMS of the left dorsolateral prefrontal cortex (DLPFC) can ameliorate depression.12–14 Furthermore, two of the largest meta-analyses on the topic showing that HF-rTMS favored sham-rTMS in patients with MDD.15,16
Based on its favorable side-effect profile and tolerability, rTMS seems to be a useful tool for treating concurrent PD and depression.17 Several previous controlled clinical trials indicated that low frequency (LF) (<1 Hz)-rTMS was superior to sham-rTMS, and that it could successfully be applied as a potential therapy in PD.18–20 Moreover, one meta-analysis published in 2015 also confirmed the benefit of LF-rTMS on depression in PD, and HF-rTMS had similar antidepressant efficacy as SSRIs.10 However, a systematic review and meta-analysis of randomized controlled trials suggest that SSRIs are no more effective than placebo in treating depression in PD patients.21 Moreover, there are contradictory data available on the efficacy of HF-rTMS in this special depression. For example, Pal et al reported that HF-rTMS had better efficacy than sham stimulation for depressive symptoms in PD.22 However, recently a multicenter, double-blind, randomized controlled trial (RCT) demonstrated that HF-rTMS and sham stimulation had comparable efficacy for depressive symptoms in PD.23 Therefore, the effectiveness of HF-rTMS is still unknown due to the small sizes of studies comparing HF-rTMS and sham-rTMS.
Recently, several high-quality, sham-controlled, RCTs of HF-rTMS in treating depression in PD patients have been published.23–25 Therefore, there is an urgent need for additional systematic review to reassess the efficacy of HF-rTMS in treating depression in PD patients. We conducted a meta-analysis to review the literature systematically to evaluate the effects of HF-rTMS in the treatment of depression in PD patients.
Methods
A systematic review of peer-reviewed literature relating to the effect of HF-rTMS on depression in patients with PD was conducted in accordance with a study protocol registered on the PROSPERO database (record number CRD42017076693). The protocol was informed by the Cochrane Handbook for Systematic Reviews of Interventions,26 and reporting conformed to the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement.27
Eligibility criteria
Criteria for considering studies for this systematic review were:
Type of studies
All published RCTs, including cluster RCT, were systematically searched in this review. Controlled (non-randomized) clinical trials, prospective and retrospective cohort studies, case-control or nested case control studies, cross-sectional studies, case series, and case reports were excluded.
Types of participants
Subjects were diagnosed with idiopathic PD based on UK brain bank criteria28 and also had a clinical diagnosis of depression.
Types of interventions
HF-rTMS (>1 Hz) compared with sham-rTMS or antidepressant treatments. Antidepressant treatments includes tricyclic antidepressants (TCA), selective serotonin reuptake inhibitors (SSRI), serotonin and norepinephrine reuptake inhibitors (SNRI), and monoamine-oxidase inhibitors (MAOI).
Outcomes
The primary outcome measure was a change in depression rating scales, including the Hamilton Rating Scale for Depression (HRSD),29 Montgomery-Åsberg Depression Rating Scale (MADRS),30 and Beck Depression Inventory (BDI),31 after the completion of HF-rTMS treatment compared with baseline (pretreatment) scores.
Secondary outcomes measures included changes in motor symptoms with Unified Parkinson’s Disease Rating Scale part III (UPDRS-III)32 scores after the completion of HF-rTMS treatment compared with baseline (pretreatment) scores, and the acceptability of the treatment, defined as risk of all-cause discontinuation.
Search methods for identification of studies
Potentially eligible trials were identified by searching the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase, PsycINFO, and clinical trials websites. The reference lists of potential studies were also screened to identify other relevant studies. Keywords used in the searches were ‘parkin* AND (‘transcranial magnetic stimulation’ OR rTMS)’ (Box S1). The search was performed from inception until July 2017. There were no restrictions by study design, setting, or country.
Data collection and analysis
The review was performed in accordance with instructions given in the Cochrane Handbook for Systematic Reviews of Interventions.26
Selection of studies
Two reviewers, BQ and MXY, identified trials for inclusion independently of each other. Excluded studies and the reason for exclusion were recorded.
Data extraction
The two authors independently screened the search output to identify records of potentially eligible trials examining the targeted outcomes, the full texts of which were retrieved and assessed for inclusion. A standardized form was used to extract data from the included studies for assessment of study quality and evidence synthesis. Extracted information included the following: 1) the first author, year and language of publication, sample size, sex ratio (male/female), mean age of subjects, and diagnostic criteria for depression and PD; 2) study design, depression scale used, and duration of study; and 3) intervention characteristics of the trial groups (stimulation site and intensity and number of treatment sessions) and control groups. The two reviewers extracted data independently; if any disagreements on the eligibility of studies occurred these were discussed with another specialized investigator to resolve the dispute. Missing data were requested from study authors. If there was doubt as to whether trials completely or partially shared participants (with common authors and centers), we contacted the study authors to ascertain whether the study report had been duplicated.
Risk of bias
Two reviewers (BQ and HC) independently assessed the validity of the included studies, with provisions for moderation from a third reviewer. The risk of bias of RCTs was assessed independently using the Cochrane Handbook for Systematic Reviews of Interventions.26 This tool classifies the studies as having low, moderate, or high risk of bias across six domains: sequence generation, allocation concealment, blinding, missing data, selective reporting, and sources of funding biases.
Assessment of reporting bias
Where 10 or more studies were identified for each outcome, we assessed publication bias by visual assessment of funnel plots and Egger’s test.26
Measures of treatment effect
For dichotomous variables, we calculated the risk ratios (RRs) with 95% confidence intervals (CIs). For continuous variables, we calculated the mean differences (MDs) with 95% CIs for outcomes such as UPDRS-III scores, and standardized mean differences (SMDs) with 95% CIs for the depression rating scales (when different scales were used).
Assessment of heterogeneity
We explored heterogeneity within each meta-analysis using a χ2 test with significance set at p=0.10, and expressed the percentage of heterogeneity due to variation rather than to chance as I2.33 We defined heterogeneity as follows: (1) I2, 0–40%: no or mild heterogeneity; (2) I2, 40%–80%: moderate heterogeneity; (3) I2, >80%: severe heterogeneity. In the presence of severe heterogeneity, meta-analysis was not performed.
Data synthesis
We utilized a random-effects model because it took into account the fact that the true treatment effects had likely varied between the RCTs included. For continuous variables, pooled SMDs or MDs and 95% CIs were calculated. For dichotomous variables, pooled ORs and 95% CIs were calculated. When necessary, sensitivity and subgroup analysis was conducted. All analyses were performed using RevMan version 5.3, and p-values ≤0.05 were considered significant.
Results
Description of studies
Search results
A total of 466 citations were identified through database searches. After screening the titles and abstracts, 439 were excluded because they failed to meet the inclusion criteria. No additional references were identified by reference searching. By scanning the full text of the remaining 27 studies, 18 studies were removed according to the inclusion criteria. In total, nine publications describing nine RCTs fulfilled the inclusion and exclusion criteria and provided the quantitative data for this review (Figure 1).20,34–37
  | Figure 1 Flow diagram of the selection of the studies. |
Study characteristics
Table 1 describes these nine RCTs, which contained 332 PD patients with depression, composed of 148 patients receiving sham-rTMS or SSRIs (mean age range 59.05±6.8 to 67.5±7.9 years) and 184 patients receiving HF-rTMS (mean age range 59.6±12.6 to 69±13.5 years). The number of participants in each study included in this meta-analysis ranged from 21 to 61 and the duration of treatment ranged from 10 days to 8 weeks. Focal rTMS of the left DLPFC were reported in eight studies, while the remaining three studies reported that of the primary motor cortex (M1) and M1 + left DLPFC. Five RCTs used 5 Hz, two RCTs used 10 Hz, and two RCTs used 15 Hz. In the control groups, there are four SSRIs studies (three fluoxetine studies and one paroxetine study), and five sham-rTMS trials. In most studies, depression was diagnosed according to Diagnostic and Statistical Manual of Mental Disorders criteria. In terms of outcome measures, seven studies used the HRSD to evaluate the severity of depression and the MADRS was used in two studies.
Risk of bias in included studies
The risk of bias in individual trials is shown in Figure 2, and the proportions of trials with low risk, unclear risk, and high risk of bias in each of the domains are shown in Figure 3. All studies included claimed randomization, and six articles described the method of random sequence generation (random number table and computer generated). Allocation concealment was unclear in all trials. Seven studies reported the blinding of participants and eight studies reported the blinding of assessors. Six trials reported completeness of follow-up for the outcome. Selective reporting was found in three trials. Sources of funding were reported in five trials, of which all were funded by independent sources.
  | Figure 2 Risk of bias summaries for individual studies. |
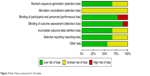  | Figure 3 Risk of bias summaries for all studies. |
Meta-analyses
Primary outcome
Depressive symptoms: seven studies reported HRSD scores and two studies reported MADRS scores as the outcome measures for 326 of the participants included in this analysis. Of these, five studies compared the outcomes with sham-rTMS and four studies compared the outcomes with SSRIs. The pooled SMD was calculated using direct weights defined as the inverse of the variance of SMD for each study, which was −0.33 (95% CI =−0.83 to 0.17, p=0.20, heterogeneity test: τ2=0.27, χ2=14.87, p=0.02, I2=60%, with moderate heterogeneity [Figure 4]) between the HF-rTMS compared with sham-rTMS groups. There were no significant statistical differences between the HF-rTMS and sham-rTMS groups or the HF-rTMS and SSRI groups (SMD =0.07, 95% CI −0.52 to 0.18, p=0.34; heterogeneity test: τ2=0.18, χ2=21.93, p=0.02, I2=54%, with moderate heterogeneity [Figure 4]).
Secondary outcomes
Eight studies reported UPDRS-III scores to reflect the motor symptoms in 308 participants included in this analysis. Of these, four studies compared the outcomes with sham-rTMS and four studies compared the outcomes with SSRIs. The effect size of UPDRS-III score was larger in HF-rTMS group than in the sham-rTMS group (MD =−2.80, 95% CI −5.45 to −0.15, p=0.04; heterogeneity test: τ2=0.00, χ2=0.81, p=0.98, I2=0%, with no heterogeneity [Figure 5]). Furthermore, HF-rTMS may also have an additional advantage with evidence of some improvement in UPDRS-III scores compared with SSRIs (MD =−2.70, 95% CI −4.51 to −0.90, p=0.003; heterogeneity test: τ2=0.00, χ2=1.62, p=1.00, I2=0%, with no heterogeneity [Figure 5]). For acceptability, seven studies reported the outcome of all-cause discontinuation, and there were no significant statistical differences between the HF-rTMS and sham-rTMS or SSRIs groups (Figure S1).
Subgroup analysis
We performed subgroup analysis according to rTMS site (left DLPFC, M1, left DLPFC + M1) (Figure S2). Among the nine RCTs included, four performed focal rTMS of the left DLPFC compared with sham-rTMS (SMD =−0.22, 95% CI: −0.82 to 0.37, p=0.46), four performed focal rTMS of the left DLPFC compared with SSRIs (SMD =0.07, 95% CI: −0.27 to 0.41, p=0.68), two performed focal rTMS of the M1 compared with sham-rTMS (SMD =−0.80, 95% CI: −1.89 to 0.29, p=0.15), and one performed focal rTMS of the left DLPFC + M1 compared with sham-rTMS (SMD =0.27, 95% CI: −0.72 to 1.26, p=0.60). Overall, in this subgroup, there were no significant differences (p=0.39).
Discussion
The objective of our meta-analysis was to assess the efficacy and acceptability of HF-rTMS in patients with depression and PD. In this meta-analysis of nine studies involving 332 patients, we revealed no significant benefit of HF-rTMS in the treatment of depression in the context of PD. Compared with sham-rTMS, the HF-rTMS findings indicated no improvement of depressive symptoms in patients with depression and PD. While compared to SSRIs treatment, our results showed no significant effects of HF-rTMS in improving depressive symptoms. Interestingly, we found that HF-rTMS may be a more effective treatment for motor symptoms than sham-rTMS or SSRIs. In terms of acceptability, there was no significant difference between HF-rTMS and sham-rTMS, or between HF-rTMS and SSRIs.
Although rTMS has been focused upon as the promising therapeutic tool in patients with PD, previous rTMS studies of patients with PD have mainly targeted motor symptoms rather than non-motor symptoms.10,14 Although, HF-rTMS was well tolerated and found to be statistically and clinically effective in patients with treatment-resistant depression.38,39 However, it was unclear whether HF-rTMS could produce superior effects in patients with depression and PD, as RCTs that have explored the relative efficacies of this method and sham stimulation have shown inconsistent results.22–25 Moreover, Xie et al’s meta-analysis showed that HF-rTMS has the same antidepressant efficacy as SSRIs, although unfortunately, none of the RCTs included targeted sham-rTMS.10 Our meta-analysis is in agreement with a previous study showing that HF-rTMS has no significant antidepressant effects in patients with depression and PD, when compared with SSRIs. Additionally, our results show that the pooled effect of HF-rTMS for depression in PD is not significantly different to sham-rTMS. Therefore, based on the results of our study and the previous study, there is insufficient evidence to support the hypothesis that HF-rTMS can produce superior effects in patients with depression and PD, with no significant difference between HF-rTMS and SSRIs on the treatment of depressive symptoms. In future, large-scale and multicenter RCTs directly comparing the antidepressant efficacy of HF-rTMS and sham-rTMS are needed to verify the hypothesis.
A previous study has provided some evidence that HF-rTMS presumably increases the excitability of the stimulated cortex.40 Moreover, several controlled clinical trials and systematic reviews have shown that high-frequency rTMS can be successfully applied as a potential therapy for Parkinsonian motor function.41–43 Therefore, HF-rTMS might be an acceptable treatment for motor symptoms in depression in the context of PD. However, these previous rTMS studies of Parkinsonian motor symptoms have mainly targeted patients with PD alone rather than the patients with comorbid depression. A current network meta-analysis also found that SSRIs had efficacy for the treatment of depression in PD patients and could improve patients’ motor function, but with significant adverse effects.44 Additionally, our study provides evidence that HF-rTMS may be superior not only to sham-rTMS but also SSRIs, in treating motor functioning in PD with comorbid depression. Our results also show that HF-rTMS has good acceptability with few reports of dropout rates.
Limitations
Several limitations of this study are worth noting. First, our meta-analysis included a limited number of studies and the sample sizes were also small in these studies. Only nine articles met the inclusion criteria. Sample sizes of the individual studies were small, and represented a combined total of 332 patients. Four of the studies had fewer than 40 patients. Given this limitation, this review should be viewed with care when applying results to clinical practice. However, small sample size was also a problem with previous studies examining the effects of HF-rTMS or LF-rTMS on depression in PD patients. This small sample size is at least partially explained by the relatively low prevalence of depression in patients with PD. Second, with a limited number of studies reporting the outcomes of long-term antidepressant effects, this factor and the cost-effectiveness of HF-rTMS could not be assessed here.45 Third, due to the heterogeneity of the included studies, our findings might be restricted in coming to a robust conclusion. Fourth, some of the trials recruited in our analysis did not report adequate concealment of the randomization sequence and blinding of the investigators and outcome assessors, which further weakened the validity of the conclusions. Large multicenter and double-blind studies are needed to obtain more conclusive evidence on the efficacy of HF-rTMS in the treatment of depression in patients with PD.
Conclusion
In summary, our meta-analysis failed to demonstrate a beneficial effect of HF-rTMS for the treatment of depression in PD patients, but it may have the advantage of producing some improvement in motor function. However, several factors, such as a limited number of studies, the small sample size, and little knowledge about the long-term side-effects, undermine the validity of our findings. There is an urgent need for more high-quality research on HF-rTMS for the treatment of depression in PD patients.
Acknowledgment
We thank Wendy Brooks, PhD, from Liwen Bianji, Edanz Group China, for editing the English text draft of this manuscript.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Rao SS, Hofmann LA, Shakil A. Parkinson’s disease: diagnosis and treatment. Am Fam Physician. 2006;74:2046–2054. | ||
Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology. 2009;72:S1–S136. | ||
Hsu HY, Huang TT, Weng YH, et al. The inclination to depressive mood and related factors among patients with Parkinson’s disease. Journal of Evidence-Based Nursing. 2007;3:195–204. Available from: http://www.airitilibrary.com/Publication/alDetailedMesh?DocID=18142869-200709-3-3-195-204-a. Accessed December 14, 2017. | ||
Tagliati M, Chaudhuri K, Pagano G. Prevalence of non-motor symptoms in Parkinson’s disease: A systematic review with meta-analysis (P2.053). Neurology. 2014;82 (10 Supple). | ||
Dissanayaka NN, Sellbach A, Silburn PA, O’Sullivan JD, Marsh R, Mellick GD. Factors associated with depression in Parkinson’s disease. J Affect Disord. 2011;132:82–88. | ||
Pachana NA, Egan SJ, Laidlaw K, et al. Clinical issues in the treatment of anxiety and depression in older adults with Parkinson’s disease. Mov Disord. 2013;28:1930–1934. | ||
Wu PL, Lee M, Huang TT. Effectiveness of physical activity on patients with depression and Parkinson’s disease: A systematic review. PLoS One. 2017;12:e0181515. | ||
Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;69:308–312. | ||
Bomasang-Layno E, Fadlon I, Murray AN, Himelhoch S. Antidepressive treatments for Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2015;21:833–842. | ||
Xie CL, Chen J, Wang XD, et al. Repetitive transcranial magnetic stimulation (rTMS) for the treatment of depression in Parkinson disease: a meta-analysis of randomized controlled clinical trials. Neurol Sci. 2015;36:1751–1761. | ||
Wagle Shukla A, Vaillancourt DE. Treatment and physiology in Parkinson’s disease and dystonia: using transcranial magnetic stimulation to uncover the mechanisms of action. Curr Neurol Neurosci Rep. 2014;14:449. | ||
George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–516. | ||
O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–1216. | ||
Lefaucheur JP, Andre-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125:2150–2206. | ||
Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44:225–239. | ||
Kedzior KK, Azorina V, Reitz SK. More female patients and fewer stimuli per session are associated with the short-term antidepressant properties of repetitive transcranial magnetic stimulation (rTMS): a meta-analysis of 54 sham-controlled studies published between 1997–2013. Neuropsychiatr Dis Treat. 2014;10:727–756. | ||
Dowd S, Rado J, Welch MJ, et al. Transcranial magnetic stimulation for depression. Curr Psychiatry. 2008;7:27–35. Available from: https://www.mdedge.com/sites/default/files/Document/September-2017/0712CP_Pipeline.pdf. Accessed December 22, 2017. | ||
Feng JM, Li SM, Guo W, et al. [Research of optimize rTMS treatment for depression in Parkinson’s disease]. HaiNan Med. 2012;23:35–38. Chinese. Available from: http://www.hainanyixue.cn/zadmin/auploadfile/zzspdf/20122316/20122316-13.pdf. Accessed December 22, 2017. | ||
Niu XP, Gou YC. [Observation of rTMS treatment for depression in Parkinson’s disease]. Chin J Prac Nerv Dis. 2012;15:11–13. Chinese. Available from: http://hnsj.cbpt.cnki.net/WKE/WebPublication/paperDigest.aspx?paperID=8dea936c-0b7b-49d0-b92d-1a3f65d05fb0#. Accessed December 22, 2017. | ||
Wu ZH, Cen HH, Cui LQ, et al. Efficacy studies of high and low frequency repetitive transcranial magnetic stimulation joint sertraline in the treatment of Parkinson’s disease patients with depression. Mod Diagn Treat. 2013;24:2164–2167. Available from: http://en.cnki.com.cn/Article_en/CJFDTOTAL-XDZD201310005.htm. Accessed December 22, 2017. | ||
Skapinakis P, Bakola E, Salanti G, Lewis G, Kyritsis AP, Mavreas V. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurol. 2010;10:49. | ||
Pal E, Nagy F, Aschermann Z, Balazs E, Kovacs N. The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson’s disease: a randomized, double-blind, placebo-controlled study. Mov Disord. 2010;25:2311–2317. | ||
Brys M, Fox MD, Agarwal S, et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: A randomized trial. Neurology. 2016;87:1907–1915. | ||
Shin HW, Youn YC, Chung SJ, et al. Effect of high-frequency repetitive transcranial magnetic stimulation on major depressive disorder in patients with Parkinson’s disease. J Neurol. 2016;263:1442–1448. | ||
Makkos A, Pál E, Aschermann Z, et al. High-frequency repetitive transcranial magnetic stimulation can improve depression in Parkinson’s disease: A randomized, double-blind, placebo-controlled study. Neuropsychobiology. 2016;73:169–177. | ||
Higgins JPT, Green S (editors), Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK; Hoboken, NJ: Wiley-Blackwell, 2008. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. | ||
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. | ||
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. | ||
Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. | ||
Beck AT, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. | ||
Fahn S, Elton RL, UPDRS program members. Unified Parkinson’s Disease Rating Scale. Florham Park, NJ: Macmillan Healthcare Information, 1987. | ||
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. | ||
Boggio PS, Fregni F, Bermpohl F, et al. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson’s disease and concurrent depression. Mov Disord. 2005;20:1178–1184. | ||
Cardoso EF, Fregni F, Martins Maia F, et al. rTMS treatment for depression in Parkinson’s disease increases BOLD responses in the left prefrontal cortex. Int J Neuropsychopharmacol. 2008;11:173–183. | ||
Chen SD, Yu SW, Zhao JF, et al. [The effect of rTMS treatment for depression in Parkinson’s disease]. Chin J Gerontol. 2011;31:3482–3484. Chinese. Available from: http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFD2011&filename=ZLXZ201118021&v=MTUzMjIxTHV4WVM3RGgxVDNxVHJXTTFGckNVUkwyZVplUm5GQ25oVzczQVB5SFRkTEc0SDlETnA0OUhaWVI4ZVg&=. Accessed December 22, 2017. | ||
Fregni F, Santos CM, Myczkowski ML, et al. Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1171–1174. | ||
Tarhan N, Sayar FG, Tan O, Kagan G. Efficacy of high-frequency repetitive transcranial magnetic stimulation in treatment-resistant depression. Clin EEG Neurosci. 2012;43:279–284. | ||
Eche J, Mondino M, Haesebaert F, Saoud M, Poulet E, Brunelin J. Low- vs. high-frequency repetitive transcranial magnetic stimulation as an add-on treatment for refractory depression. Front Psychiatry.2012;3:13. | ||
Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. | ||
Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Hamdy A. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson’s disease. Mov Disord. 2006;21:2201–2205. | ||
Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov Disord. 2006;21:325–331. | ||
Elahi B, Elahi B, Chen R. Effect of transcranial magnetic stimulation on Parkinson motor function – systematic review of controlled clinical trials. Mov Disord. 2009;24:357–363. | ||
Zhuo C, Xue R, Luo L, et al. Efficacy of antidepressive medication for depression in Parkinson disease: a network meta-analysis. Medicine (Baltimore). 2017;96:e6698. | ||
Wassermann EM, Zimmermann T. Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacol Ther. 2012;133:98–107. |
Supplementary materials
  | Box S1 Search strategy |
References
Brys M, Fox MD, Agarwal S, et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: A randomized trial. Neurology. 2016;87:1907–1915. | ||
Makkos A, Pál E, Aschermann Z, et al. High-frequency repetitive transcranial magnetic stimulation can improve depression in Parkinson’s disease: A randomized, double-blind, placebo-controlled study. Neuropsychobiology. 2016;73:169–177. | ||
Pal E, Nagy F, Aschermann Z, Balazs E, Kovacs N. The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson’s disease: a randomized, double-blind, placebo-controlled study. Mov Disord. 2010;25:2311–2317. | ||
Shin HW, Youn YC, Chung SJ, et al. Effect of high-frequency repetitive transcranial magnetic stimulation on major depressive disorder in patients with Parkinson’s disease. J Neurol. 2016;263:1442–1448. | ||
Wu ZH, Cen HH, Cui LQ, et al. Efficacy studies of high and low frequency repetitive transcranial magnetic stimulation joint sertraline in the treatment of Parkinson’s disease patients with depression. Mod Diagn Treat. 2013;24:2164–2167. Available from: http://en.cnki.com.cn/Article_en/CJFDTOTAL-XDZD201310005.htm. Accessed December 22, 2017. | ||
Chen SD, Yu SW, Zhao JF, et al. [The effect of rTMS treatment for depression in Parkinson’s disease]. Chin J Gerontol. 2011;31:3482–3484. Chinese. Available from: http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFD2011&filename=ZLXZ201118021&v=MTUzMjIxTHV4WVM3RGgxVDNxVHJXTTFGckNVUkwyZVplUm5GQ25oVzczQVB5SFRkTEc0SDlETnA0OUhaWVI4ZVg=. Accessed December 22, 2017. | ||
Fregni F, Santos CM, Myczkowski ML, et al. Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1171–1174. | ||
Boggio PS, Fregni F, Bermpohl F, et al. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson’s disease and concurrent depression. Mov Disord. 2005;20:1178–1184. | ||
Cardoso EF, Fregni F, Martins Maia F, et al. rTMS treatment for depression in Parkinson’s disease increases BOLD responses in the left prefrontal cortex. Int J Neuropsychopharmacol. 2008;11:173–183. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





