Back to Journals » Clinical Ophthalmology » Volume 14
Effectiveness of 190 μg Fluocinolone Acetonide and 700 μg Dexamethasone Intravitreal Implants in Diabetic Macular Edema Using the Area-Under-the-Curve Method: The CONSTANT Analysis
Authors Zarranz-Ventura J , Mali JO
Received 10 March 2020
Accepted for publication 8 May 2020
Published 22 June 2020 Volume 2020:14 Pages 1697—1704
DOI https://doi.org/10.2147/OPTH.S253370
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Javier Zarranz-Ventura, 1, 2 Joshua O Mali 3
1Clinical Institute of Ophthalmology (ICOF), Hospital Clinic, Barcelona, Spain; 2August Pi i Sunyer Biomedical Research Institute (IDIBAPS), Barcelona, Spain; 3The Eye Associates, Sarasota, FL, USA
Correspondence: Javier Zarranz-Ventura
Clinical Institute of Ophthalmology (ICOF), Hospital Clinic, Barcelona, Spain
Tel +34 932 275 400
Email [email protected]
Purpose: Calculations of area-under-the-curve (AUC) provide the average letters gained across the entire treatment period, which may be a better estimate of long-term effectiveness than single time-point outcomes, particularly when it comes to sustained-release therapies.
Materials and Methods: The AUC method was used to compare the efficacy of the 0.2 μg/day fluocinolone acetonide (total dose of 0.19 mg; FAc) and dexamethasone (DEX) 700 μg implants based on published data from their respective Phase 3 FAME (Fluocinolone Acetonide for Macular Edema) and MEAD pivotal clinical trials in diabetic macular edema (DME). Best-corrected visual acuity (BCVA) letter scores were collated from the FAME trial and compared with those reported in MEAD. The trapezoidal rule was then used to calculate AUC, based on BCVA letter score, from baseline to Month 36 (FAME)/Month 39 (MEAD) and presented as an overall mean visual acuity change per day.
Results: Treatment with either the FAc or DEX implant resulted in an improved BCVA over the treatment period compared with sham. This effect was statistically greater (p=0.029) for the FAc implant than the DEX implant (5.2 vs 3.5 letters/day, respectively) and even greater in the recurrent DME subgroup (p< 0.001; 6.9 vs 3.5 letters/day, respectively).
Conclusion: Although direct comparisons between trial cohorts cannot be performed, this analysis indicated that, in their respective pivotal clinical trial cohorts, treatment with the FAc implant provides better long-term visual acuity outcomes and a lower treatment burden than achieved with the DEX implant.
Keywords: area-under-the-curve, best-corrected visual acuity, diabetic macular edema, corticosteroid, fluocinolone acetonide implant, dexamethasone implant
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Plain Language Summary
The area-under-the-curve method was used to demonstrate greater 3-year visual outcomes in the FAME trial (pivotal trial for the fluocinolone acetonide implant) than the MEAD trial (pivotal trial for the dexamethasone implant).
Introduction
Diabetic macular edema (DME) is a severe, vision-limiting stage of diabetic retinopathy.1 The American Academy of Ophthalmology Preferred Practice Pattern recommends anti-vascular endothelial growth factor (anti-VEGF) treatments to provide better outcomes than focal/grid laser alone.2 However, there may be a substantial impact on the outcomes of anti-VEGF treatment from missed treatments and suboptimal response to intravitreal injections of anti-VEGF.3,4 Consequently, continuous therapy options may be particularly beneficial in the treatment of DME.
There are currently two corticosteroid implants approved for the treatment of DME; both have been validated in their respective pivotal clinical trials. OZURDEX® (dexamethasone intravitreal implant, Allergan USA, Inc., Irvine, CA, USA) contains 700 µg dexamethasone (DEX) that is released into the vitreous over a 6-month period,5 and there are a growing number of studies reporting its use across a number of macular diseases.6–11 The MEAD trial included two Phase 3 studies (ClinicalTrials.gov identifiers NCT00168337 and NCT00168389) conducted in patients with DME in the United States and Europe. The primary efficacy measure in the European study was the mean change in best-corrected visual acuity (BCVA) from baseline and calculated using the area-under-the-curve (AUC) approach.12 At 39 months, there was a statistically significant mean improvement in DEX-treated patients with DME versus the sham group.12–14 ILUVIEN® (fluocinolone acetonide intravitreal implant, Alimera Sciences Inc., Alpharetta, GA, USA) continuously treats DME with a submicrogram dose (daily release of 0.2 µg and containing a total dose of 0.19 mg) of fluocinolone acetonide (FAc) for up to 36 months.15 The primary endpoint measure in the Phase 3 FAME trial (ClinicalTrials.gov identifier NCT00344968) was the proportion of patients achieving ≥15-letter improvement in BCVA from baseline compared with sham; the endpoint was met in both FAME A and B studies at 24 months and the outcome was sustained for 36 months.16 AUC calculations for BCVA did not form part of the analysis plan in the FAME trial, even though the AUC analysis17 provides a more holistic clinical comparison than single time-point measurements and has previously been used to evaluate the effect of longer duration drugs.18
The AUC method may provide a better means of comparing outcomes between studies than other efficacy measures,19 and the objective of the current study was to assess AUC outcomes in the FAME trial and to indirectly compare these with the outcomes achieved in the MEAD trial.
Materials and Methods
Study designs for the MEAD and FAME trials have been reported previously.14,20 In both FAME and MEAD, the study drug (at the licensed total dose of 0.19 mg for the FAc implant and 0.7 mg for the DEX implant) was compared with sham treatment, which consisted of the use of a needle-free drug-delivery system without medication. In the FAME trial, sham-treated patients were eligible for the same rescue treatments as the treated study arms. In the MEAD trial, patients had to leave the study in order to be eligible for rescue treatment.14
Data Sources
Data from the MEAD trial was extracted from the data published in the assessment report that was conducted by the European Medicines Agency.12 The FAME trial was conducted by Alimera Sciences and was available to the authors during the current analyses.
AUC Calculation
The calculation of AUC has been described previously.17,18 AUC was based on BCVA as this was the primary outcome in both FAME16 and MEAD trials.3 AUC for MEAD and FAME trials were based on BCVA letter score from baseline through to final visit (36 months in the FAME trial and 39 months in the MEAD trial). AUC was calculated using the trapezoidal rule in which each section of the BCVA versus time curve was treated as an individual trapezoid, and its area calculated using the formula: 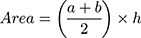 .17 The sum of the areas of the trapezoids was divided by the number of days in the study to give a summary value in letters per day. Mean AUC was then calculated using individual patient AUC values.18
.17 The sum of the areas of the trapezoids was divided by the number of days in the study to give a summary value in letters per day. Mean AUC was then calculated using individual patient AUC values.18
AUC for DEX was obtained from published data.12–14 In the FAME trial, the observed change in BCVA letter score from baseline through to Month 36 was used to calculate AUC using the trapezoidal rule.17 For each subject, the summarized variable represented total AUC divided by the total number of days in the study.18
Full-Group Analysis
AUC outcomes represented the combined study population from the FAME and MEAD trials, respectively. AUC was captured for both the treated (0.2 µg/day FAc in the FAME trial and 700 µg DEX in the MEAD trial) and sham arms.
Subgroup Analysis
To enable comparison of patients with the most severe DME from the different studies, a “persistent or recurrent DME” population was defined. For the FAME population, patients with DME duration of ≥3 years were considered to have persistent or recurrent DME. For the MEAD population, patients who had received any prior treatment were considered to have persistent or recurrent DME.13
Statistical Analysis
DEX and FAc groups were compared using the between treatment difference, 95% confidence interval (CI), and p-values are based on an analysis of variance model. Data are reported as mean ± standard deviation (SD) unless otherwise stated. A p-value less than 0.05 was taken to define a statistically significant difference between groups.
Results
Study Populations
A total of 956 patients were enrolled in the FAME trial, with a mean age of 62.5 years. In the MEAD trial, 1048 patients were enrolled, and patients had a mean age of 62.4 years. Baseline characteristics for the FAME and MEAD trials are further described in Table 1 (full DME population) and Table 2 (persistent or recurrent population). In general, notable differences existed between the trials, with the FAME trial showing a higher percentage of patients pre-treated with laser, the inclusion of no treatment-naïve patients, and a lower percentage of eyes with a phakic lens.
 |
Table 1 Baseline Characteristics of Patients in the FAME and MEAD Trials (Full DME Group) |
 |
Table 2 Baseline Characteristics of Patients with Persistent or Recurrent DME in the FAME and MEAD Trials |
Number of FAc and DEX Treatments in the FAME and MEAD Trials
The number of FAc implants administered during the FAME trial was 1.3±0.6 implants, and 4.1±2.0 implants were administered in the MEAD trial.
BCVA Over Time in the FAME and MEAD Trials
In both the FAME and MEAD trials, 0.19 mg FAc and 0.7 mg DEX-treated eyes (n=375 and n=351, respectively) experienced improved BCVA compared with sham-treated eyes (n=185 and n=350). At Month 36 in the FAME trial, there was an improvement of 5.3 letters in the FAc-treated eyes versus 2.0 letters in the sham control (a difference of 3.3 letters). At Month 39 in the MEAD trial, the improvement in DEX-treated eyes was 3.5 letters versus 2.0 letters in the sham control (a difference of 1.5 letters) (Figure 1).
AUC Analysis of BCVA Over Time in the FAME and MEAD Trials
In both FAME and MEAD trials, patients in the treatment arms experienced significantly greater improvements in AUC than observed in the sham-treated arms (Table 3).
 |
Table 3 Mean Visual Acuity Outcomes Based on AUC Calculations in the FAME and MEAD Trials |
In the FAME trial, FAc-treated eyes experienced a mean improvement in BCVA of 5.17 letters/day in the full group and 6.92 letters/day in the persistent or recurrent group over the 36-month period. This compares with 3.5 letters/day and 3.2 letters/day over the 39-month period in the MEAD trial (Table 3).
Comparison between trials revealed that the FAc-treated eyes experienced significantly greater improvements in BCVA compared with DEX-treated eyes both in the full DME group (mean difference of 1.67 letters/day, 95% CI, 0.17–3.17; p=0.029) and in the persistent or recurrent DME subgroups (mean difference of 3.42 letters/day, 95% CI, 1.56–5.28; p<0.001) (Table 3; Figure 2).
Effect of Treatment with FAc or DEX on Retinal Thickness
Both FAc and DEX resulted in reduced retinal thickness—and therefore an improved control of edema—compared with sham. In the overall population of the FAME trial, the change in mean foveal thickness from baseline was −181.1 µm for FAc-treated patients compared with −142.7 µm for sham-treated patients (difference, −38.4 µm; p=0.015) at Month 36. In the overall population of the MEAD study, the mean change in central retinal thickness from baseline over 36 months was −111.6 µm for DEX-treated patients and −41.9 µm for sham-treated patients (difference, −69.7 µm; p<0.001).14
Adverse Events
FAc-treated patients experienced a smaller or equivalent difference from sham compared with DEX-treated eyes in almost all adverse events (AEs) related to intraocular pressure (IOP) and cataracts. The only AEs in which DEX-treated eyes experienced a smaller difference from sham compared with FAc were an IOP increase to ≥35 mm Hg and the use of IOP-lowering surgery. However, both AEs had very low incidences in the subgroups studied (Figure 3).
 |
Figure 3 Difference between percentage of actively treated and sham control patients experiencing adverse events. The MEAD trial reported the efficacy and safety outcomes from a total of 1048 patients with DME, and the FAME trial reported outcomes from a total of 956 subjects. Data from Hall J. Hall. Correspondence. Retina. 2017;37(3):e34–e37.21Note: *Requiring IOP-lowering drops. Abbreviations: AE, adverse event; DME, diabetic macular edema; FAME, Fluocinolone Acetonide for Macular Edema; IOP, intraocular pressure. |
Discussion
The AUC method provides a summary value of the treatment effect over the entire follow-up period, rather than relying on a single time point. The AUC method also provides a superior comparison between two long-term formulations, as it assesses the overall treatment effect and is not skewed by the time taken for the treatment to reach maximum efficacy. The current analysis of the FAME and MEAD trial data favors the use of FAc for the treatment of DME in these specific clinical trial conditions.
In this post-hoc analysis of published data from the FAME and MEAD Phase 3 clinical trials, the AUC method revealed significantly superior visual gains over 36 months for the 0.2 µg/day FAc implant compared with the 700 µg DEX implant, both in the overall and persistent or recurrent DME populations. This may be attributable to the method of drug delivery from the FAc implant (continuous microdosing over 36 months) compared with DEX (single injection lasting up to 6 months with repeated injections needed).
The current analysis showed that, while the absolute change in the central retinal thickness in the treated arms was greater in FAME than MEAD (−181.1 µm vs −111.6 µm, respectively), the difference in treated eyes compared with sham appeared more substantial in MEAD compared with FAME (−69.7 µm vs −38.4 µm, respectively). The more marked improvement in central retinal thickness in treated eyes versus sham in MEAD may be explained by the MEAD study protocol. Patients in MEAD were required to leave the study before receiving rescue treatment, while no such condition was placed on patients in the FAME trial. Therefore, sham-treated patients in the FAME trial may be expected to experience an improvement in central retinal thickness due to treatment received, unlike sham-treated patients in the MEAD trial.
An indirect comparison of the FAME and MEAD trials also revealed that, with the exception of IOP-lowering surgery, eyes treated with the FAc implant appeared to experience similar (ie cataract extraction, change in IOP ≥10 mm Hg) and in some cases numerically fewer AEs (ie cataract-related AEs, elevated IOP, change in IOP ≥25 mm Hg) compared with DEX-treated eyes.21 This may also be attributable to reduced daily exposure to corticosteroid in FAc-treated eyes because of continuous microdosing.
The relevance of the current findings to real-world practice needs to be considered. The analysis is important to current practice as currently there are no clinical trials that directly compare outcomes achieved with DEX and FAc implants. However, that being said, there are a growing number of studies that report outcomes with the DEX implant6–11 and some that even report the use of a FAc implant after prior therapy with DEX implants, in both vitrectomised22–24 and non-vitrectomised eyes.25,26 Others have also compared outcomes achieved with the FAc implant after prior therapy with anti-VEGF.25 Few studies report a standardised approach to changing therapy, as studies seem to report outcomes in patients that have been heavily pre-treated before being treated with the FAc implant.27–29 That said, the study by Rehak et al25 reported switching from anti-VEGF therapy to the FAc implant after a mean of 8.37 injections. In a second group, this number was 5.1 injections of anti-VEGF followed by 1.27 injections of the DEX implant. Other studies also suggest that the best outcomes were achieved with the FAc implant in patients with short-standing chronic DME as opposed to long-standing chronic DME.28
Limitations
The post-hoc nature of this analysis of clinical trial data is a limitation, as is the analysis of two different clinical trials. Whilst conclusive statements cannot be made based on meta-type analyses, the authors believe these comparisons are still valuable, as there are no head-to-head clinical trials comparing FAc and DEX implants, even though both are approved to treat DME in the US and in Europe. Furthermore, limitations relate to the differences in study protocols and populations. For example, patient demographics were similar (Table 1) but not identical in the two studies, with lens status being notably different at baseline (62.7% of eyes were phakic in the 0.2 µg/day FAc-treated arm of the FAME trial as opposed to 75.5% in the DEX 0.7-mg treated arm of the MEAD trial). It was also apparent that prior therapies differed significantly, with 100% of patients in the FAME trial being treated with laser as opposed to 65.8% of patients in the DEX 0.7-mg treated arm of the MEAD trial. In addition, there was no equivalent “severe” DME subgroup in the two studies, necessitating the identification of the “persistent or recurrent DME” subgroup in this analysis to highlight that these subgroups had been previously treated and were not treatment naïve. These factors were not accounted for in the post-hoc analysis and it is unclear as to their effect on reported outcomes. The optical coherence tomography (OCT) devices used, as well as the parameters measured, differed between MEAD and FAME trials and may have had a potential confounding effect on reported outcomes. In the FAME trial, center point thickness was measured using the Fast Macular Scan protocol on a Stratus 3 OCT system (Carl Zeiss Meditec Inc., Dublin, CA) and patients were eligible if center point thickness was ≥250 µm. This differs from the MEAD trial and may be a potential confounder because, in this study, central retinal thickness was measured in the 1-mm central macular subfield of the study eye and was required to be >300 µm using the Stratus 2 or 3 OCT system (Carl Zeiss Meditec Inc., Dublin, CA). Finally, the results may not reflect experiences in routine clinical care; differences between clinical trial and real-life conditions may prove beneficial or detrimental. For example, the outcomes of treatment with the DEX implant may improve with more frequent re-injections (<6 month intervals), if feasible in real-world clinical practice. Alternatively, the FAc implant may be less affected by lack of patient adherence (such as missed appointments and delayed injections) due to the implant’s constant and near zero-order drug release kinetics.15
Conclusion
The comparison of visual acuity outcomes provided by the AUC method represents the treatment effect over time as opposed to single point measurement effects. This is important for both longer duration treatments, such as sustained-release implants, and larger studies where individual time points for comparison may vary considerably. The current analysis showed the FAc implant had a more favorable benefit on visual acuity and a lower treatment burden in the treatment of DME.
Data Sharing Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Acknowledgments
Medical writing assistance was provided by Helios Medical Communications, Alderley Edge, Cheshire, UK, and supported by Alimera Sciences.
Author Declaration
The manuscript has not been published elsewhere and that it has not been submitted simultaneously for publication elsewhere.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article. This study was funded by Alimera Sciences. The FAME trial was funded by Alimera Sciences Inc. (Alpharetta, GA, USA) who also provided financial support for the statistical and editorial assistance for this paper. The authors were responsible for the data development, presentation, review and approval of the manuscript. The sponsors reviewed the manuscript for accuracy of the statistical and scientific information.
Disclosure
The authors wish to acknowledge the following financial interests:
JZ-V: Alcon: Speaker, travel grant; Alimera Sciences: Consultant, speaker, travel grant; Allergan: Consultant, speaker, travel grant, grant recipient; Bausch and Lomb: Speaker, travel grant; Bayer: Consultant, speaker, travel grant; Brill Pharma: Consultant, speaker; D.O.R.C: Travel grant; Novartis: Consultant, speaker, travel grant, grant recipient; Topcon: Speaker, travel grant; Zeiss: Speaker. JM: Alimera Sciences: Consultant, Speaker, Stock Shareholder; Allergan: Consultant, Research Funding/Grant. The authors report no other conflicts of interest in this work.
References
1. Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi:10.2337/dc11-1909
2. American Academy of Ophthalmology (AAO). About Preferred Practice Patterns (PPPs) - American academy of ophthalmology. Available from: https://www.aao.org/about-preferred-practice-patterns.
3. Boyer DS, Nguyen QD, Brown DM, Basu K, Ehrlich JS. Outcomes with as-needed ranibizumab after initial monthly therapy. Ophthalmology. 2015;122(12):2504–2513.e1. doi:10.1016/j.ophtha.2015.08.006
4. Bressler SB, Liu D, Glassman AR, et al. Change in diabetic retinopathy through 2 years. JAMA Ophthalmol. 2017;135(6):558. doi:10.1001/jamaophthalmol.2017.0821
5. Chang-Lin J-E, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Investig Opthalmology Vis Sci. 2011;52(1):80. doi:10.1167/iovs.10-5285
6. Zur D, Iglicki M, Loewenstein A. The role of steroids in the management of diabetic macular edema. Ophthalmic Res. 2019;62(4):231–236. doi:10.1159/000499540
7. Iglicki M, Zur D, Fung A, et al. TRActional DIabetic reTInal detachment surgery with co-adjuvant intravitreal dexamethasONe implant: the TRADITION study. Acta Diabetol. 2019;56(10):1141–1147. doi:10.1007/s00592-019-01357-y
8. Mello Filho P, Andrade G, Maia A, et al. Effectiveness and safety of intravitreal dexamethasone implant (Ozurdex) in patients with diabetic macular edema: a real-world experience. Ophthalmologica. 2019;241(1):9–16. doi:10.1159/000492132
9. Iglicki M, Zur D, Busch C, Okada M, Loewenstein A. Progression of diabetic retinopathy severity after treatment with dexamethasone implant: a 24-month cohort study the ‘DR-Pro-DEX study’. Acta Diabetol. 2018;55(6):541–547. doi:10.1007/s00592-018-1117-z
10. Iglicki M, Busch C, Zur D, et al. Dexamethasone implant for diabetic macular edema in naive compared with refractory eyes. Retina. 2019;39(1):44–51. doi:10.1097/IAE.0000000000002196
11. Zur D, Iglicki M, Sala‐Puigdollers A, et al. Disorganization of retinal inner layers as a biomarker in patients with diabetic macular oedema treated with dexamethasone implant. Acta Ophthalmol. 2020;98(2). doi:10.1111/aos.14230.
12. European Medicines Agency (EMA). Ozurdex. Assessment Report. 2014. ozurdex-epar-summary-public_en.pdf.
13. Augustin AJ, Kuppermann BD, Lanzetta P, et al. Dexamethasone intravitreal implant in previously treated patients with diabetic macular edema: subgroup analysis of the MEAD study. BMC Ophthalmol. 2015;15(1):150. doi:10.1186/s12886-015-0148-2
14. Boyer DS, Yoon YH, Belfort R, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. doi:10.1016/j.ophtha.2014.04.024
15. Campochiaro PA, Nguyen QD, Hafiz G, et al. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 2013;120(3):583–587. doi:10.1016/j.ophtha.2012.09.014
16. Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–2132. doi:10.1016/j.ophtha.2012.04.030
17. Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy. JAMA. 2015;314(20):2137. doi:10.1001/jama.2015.15217
18. Singer MA, Miller DM, Gross JG, et al. Visual acuity outcomes in diabetic macular edema with fluocinolone acetonide 0.2 μg/day versus ranibizumab plus deferred laser (DRCR protocol I). Ophthalmic Surg Lasers Imaging Retina. 2018;49(9):698–706. doi:10.3928/23258160-20180831-08
19. Singer MA, Kermany DS, Waters J, Jansen ME, Tyler L. Diabetic macular edema: it is more than just VEGF. F1000Research. 2016;5:1019. doi:10.12688/f1000research.8265.1
20. Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626–635.e2. doi:10.1016/j.ophtha.2010.12.028
21. Hall J. Correspondence. Retina. 2017;37(3):e34–e37. doi:10.1097/IAE.0000000000001547
22. Singh P, Chedid A, Deuchler SK, et al. The efficacy and safety outcomes of the 0.19 mg fluocinolone acetonide implant after prior treatment with the 0.7 mg dexamethasone implant in patients with diabetic macular edema. Int Med Case Rep J. 2018;11:265.
23. Vaz-Pereira S, Castro-de-Sousa J, Martins D, et al. The outcomes of switching from short- to long-term intravitreal corticosteroid implant therapy in patients with diabetic macular edema. Ophthalmic Res. 2020;63(2):114–121. doi:10.1159/000503036
24. Coelho J, Malheiro L, Melo Beirão J, Meireles A, Pessoa B. Real-world retrospective comparison of 0.19 mg fluocinolone acetonide and 0.7 mg dexamethasone intravitreal implants for the treatment of diabetic macular edema in vitrectomized eyes. Clin Ophthalmol. 2019;13:1751–1759. doi:10.2147/OPTH.S201611
25. Rehak M, Busch C, Unterlauft J-D, Jochmann C, Wiedemann P. Outcomes in diabetic macular edema switched directly or after a dexamethasone implant to a fluocinolone acetonide intravitreal implant following anti-VEGF treatment. Acta Diabetol. 2020;57(4):469–478. doi:10.1007/s00592-019-01439-x
26. Elbarky AM. Rapid structural and functional improvements with the 0.19 mg fluocinolone acetonide intravitreal implant for patients with DME and low visual acuity: 6-month data from the UAE. Clin Ophthalmol. 2020;14:823–830. doi:10.2147/OPTH.S238740
27. Bailey C, Chakravarthy U, Lotery A, et al. Real-world experience with 0.2 μg/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom. Eye. 2017;31(12):1707–1715. doi:10.1038/eye.2017.125
28. Chakravarthy U, Taylor SR, Koch FH, et al. Changes in intraocular pressure after intravitreal fluocinolone acetonide (ILUVIEN): real-world experience in three European countries. Br J Ophthalmol. 2018:1–6.
29. Augustin AJ, Atorf J, McGahan MC. The first European report of real-life clinical outcomes after 3 years of treatment with ILUVIEN® (fluocinolone acetonide) in patients with chronic diabetic macular edema (DME). Invest Ophthalmol Vis Sci. 2017;58(2):928. doi:10.1167/iovs.16-20610
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


